Abstract
Herein, we report a Cu-catalyzed enantioselective allylic alkylation using a γ-butyrolactone-derived silyl ketene acetal. Critical to the development of this work was the identification of a novel mono-picolinamide ligand with the appropriate steric and electronic properties to afford the desired products in high yields (up to 96%) and high ee (up to 95%). Aryl, aliphatic, and unsubstituted allylic chlorides bearing a broad range of functionality are well-tolerated. Spectroscopic studies reveal that a CuI species is likely the active catalyst, and DFT calculations suggest ligand sterics play an important role in determining Cu coordination and thus catalyst geometry.
Keywords: Copper, Allylic Alkylation, Enolate Nucleophiles, γ-butyrolactones, Asymmetric Catalysis
Graphical Abstract
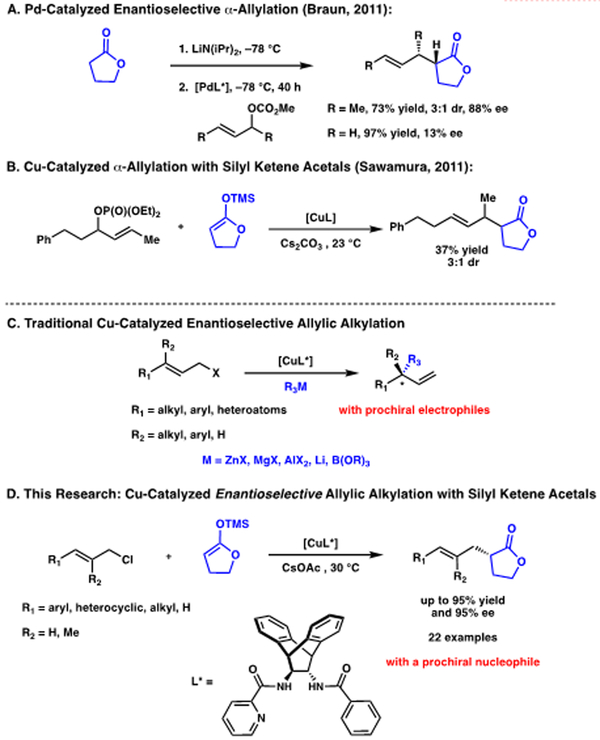
γ-Butyrolactones are important structural motifs present in numerous natural products and pharmaceutically-relevant molecules, and are also useful synthetic building blocks that serve as precursors for other highly functionalized molecules.1 Although there are a number of reports on the α-functionalization of γ-butyrolactones to form chiral quaternary centers, only a limited number of examples have demonstrated the construction of chiral α-tertiary γ-butyrolactones. Some previously disclosed strategies include chiral auxiliary-directed alkylation and subsequent cyclization1b, Claisen-type rearrangements1c and catalytic hydrogenation approaches.1d However, reports on the construction of these molecules via enantioselective α-functionalization remain limited, as the potential for racemization of the product necessitates the development of exceptionally mild reaction conditions.2
Since the seminal report by Tsuji,3 transition-metal catalyzed allylic alkylation of enolate-derived nucleophiles continues to be a powerful approach for α-functionalization of carbonyl-containing compounds, as the alkene may be easily converted to a diverse array of functional groups. Nonetheless, the use of γ-butyrolactones in this transformation remains underdeveloped.4
More specifically, there is only one example on the use of this transformation to produce enantioenriched α-tertiaryl lactones. 4c Although the desired product can be obtained in moderate diastereoselectivity and good ee when an electrophile possessing terminal substitution is used, the ee is extremely low when simple allyl carbonate is employed (Figure 1A). As part of our ongoing interest in exploring first row transition metals in enantioselective catalysis,5 we became interested in a system reported by Sawamura, wherein a Cu catalyst was used to obtain the desired α-allyl γ-butyrolactone in a racemic fashion by reaction of silyl ketene acetals with allylic phosphates (Figure 1B).6 It should be noted that Cu as a catalyst in allylic substitutions with hard, organometallic nucleophiles (pka >40) has been extensively studied, and the desired branched products can be accessed in high yields and enantioselectivities (Figure 1C).7 However, the use of Cu with softer, enolate derived nucleophiles (pka < 30), remains underdeveloped.8,9
Figure 1.
Transition-metal catalyzed α-allylation of γ-butyrolactones.
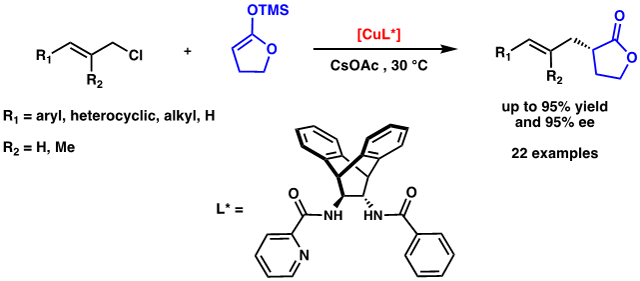
One challenge associated with the development of the enantioselective variant of this transformation is that it involves a prochiral nucleophile, which, to our knowledge, is unprecedented in Cu-allylic alkylations. For this reason, we believed that we would not be able to rely on chiral ligand scaffolds previously used in this type of transformation. Herein, we report a Cu-catalyzed asymmetric allylic alkylation with a γ-butyrolactone-derived enolate.
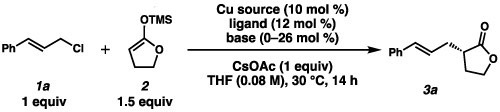
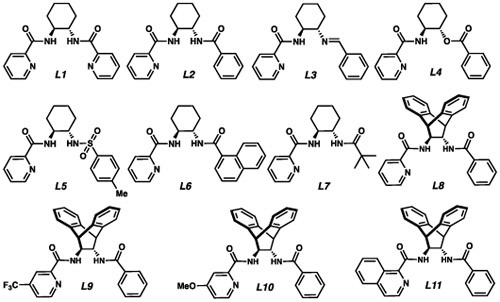
Upon examination of more than 20 commercially available ligands, we found cyclohexyl bis-picolinamide ligand L1 (Table 1, entry 1) was the only ligand to impart any degree of stereocontrol.10 Although encouraged by these results, we soon found that the reaction outcome was highly variable, and after careful examination of all the reaction parameters, we concluded that the source of variability was the ligand itself. As a consequence of the large number of coordinating atoms present and its high flexibility, we believed L1 could bind to Cu in a variety of conformations. As a result, small changes in the reaction set-up could alter the distribution of different Cu complexes in solution. We found that by employing mono-picolinamide L2 as the ligand, CuCl2 as the Cu source, and by deprotonation of both amide hydrogens on the ligand with n-BuLi, we were able to consistently obtain our product in moderate yields and ee (entry 2).
Table 1.
Evaluation of Ligands for Enantioselective Cu-Catalyzed Allylic Alkylationa
 | |||||
|---|---|---|---|---|---|
| entry | Cu source | base | ligand | yield (%)b | ee (%)c |
| 1 | [Cu(MeCN)4]PF6 | none | L1 | 74 | 22 |
| 2 | CuCl2 | n-BuLi (24 mol %) | L2 | 44 | 62 |
| 3 | CuCl2 | n-BuLi (12 mol %) | L3 | 60 | 11 |
| 4 | CuCl2 | n-BuLi (12 mol %) | L4 | 64 | 14 |
| 5 | CuCl2 | n-BuLi (24 mol %) | L5 | 44 | 40 |
| 6 | CuCl2 | n-BuLi (24 mol %) | L6 | 54 | 62 |
| 7 | CuCl2 | n-BuLi (24 mol %) | L7 | 33 | 62 |
| 8 | CuCl2 | LiHMDS (26 mol %) | L8 | 90 | 92 |
| 9 | CuCl2 | LiHMDS (26 mol %) | L9 | 73 | 91 |
| 10 | CuCl2 | LiHMDS (26 mol %) | L10 | 83 | 89 |
| 11 | CuCl2 | LiHMDS (26 mol %) | L11 | 80 | 87 |
| 12d | CuCl2 | LiHMDS (13 mol %) | L8 | 90 | 94 |
| 13d,e | CuCl2 | NaHMDS (13 mol %) | L8 | 94 | 87 |
| 14d,e | CuCl2 | KHMDS (13 mol %) | L8 | 95 | 85 |
 | |||||
0.1 mmol scale.
Determined by 1HNMR analysis of the crude reaction mixture using 1,3,5–trimethoxybenzene as a standard.
Determined by chiral SFC analysis.
5 mol% CuCl2, 6 mol% ligand, 0.042 M, 6 h.
3 h reaction time.
To determine whether the benzamide moiety was critical for reactivity and selectivity, we tested ligands bearing a mono-imine (L3), an ester moiety (L4) and a sulfonamide (L5). The anionic benzamide appears to be especially important for stereocontrol, as removing this moiety led to a drastic decrease in ee (L3 and L4, entries 3 and 4). In comparison, anionic sulfonamide- containing L5 performed most similarly to L2 (entry 5). Interestingly, we found the ee was insensitive to the identity of the organic substituent on the amide; although the use of naphthylamide L6 and pivalamide L7 resulted in very different yields, the ee’s were identical (entries 6 and 7).
After synthesizing and testing a number of chiral mono-picolinamide ligands, we found that a bulkier backbone (L8, entry 8) was crucial for consistently high levels of reactivity and selectivity. We also noted that altering the electronics at the position para- to the nitrogen (L9 and L10, entries 9 and 10) or increasing the steric bulk of the pyridine moiety (L11, entry 11) led to no improvement in yield or ee.
Upon more extensive optimization we found that lowering the reaction concentration from [0.08 M] to [0.042 M] led to a slight increase in ee, and with 5 mol % Cu, the reaction was complete in 6 h (entry 12).11 Furthermore, we noted that the counterion on the catalytic base did have an effect on the overall outcome of the reaction: when NaHMDS (entry 13) or KHMDS (entry 14) is used instead of LiHMDS, the ee is slightly reduced but the reaction is complete in only 3 hours.
With optimized conditions in hand, we examined the scope of the electrophile (Table 2). We were pleased to find 2-naphthyl 3c and ortho- methylphenyl (3d) substituents were well tolerated. Meta-phenyl substituted electrophiles (3b, 3e) also fared well, and even a sensitive triflate (3f) and a bulky 3,5-dimethoxy phenyl group (3g) led to products in high yield and ee. Electrophiles possessing substituents at the para-phenyl position such as halogens (3i, 3j, 3k) a trifluoromethyl (3l) and a nitrile (3m) were were also well-tolerated. Even heterocyclic compounds (3n and 3o), a diene substrate (3p), an aliphatic allylic chloride (3q) and unsubstituted allyl chloride (3r) led to product formation in good yields and ee’s.
Table 2.
Scope of the Allylic Chloridea
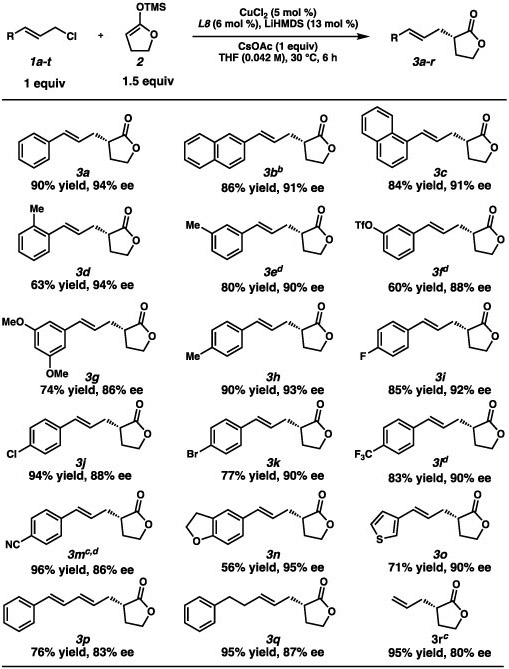 |
Isolated yields on 0.2 mmol scale. SFC analysis was used to determine ee.
Absolute stereochemistry was determined by X-ray diffraction.
With 10 mol % CuCl2, 12 mol % L8, and 26 mol % LiHMDS.
synthesized from a mixture of linear and branched allylic chlorides (see the SI for more details).
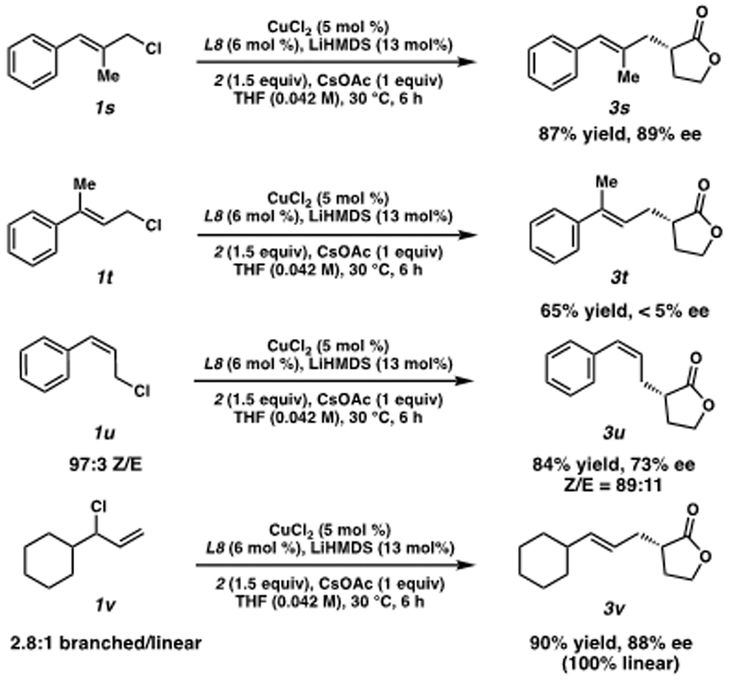
Although trisubstituted olefin 1s (Scheme 1) led to the desired product 3s in good selectivity and yield, the product formed from α-,α-substituted olefin 1t was obtained in significantly lower yield and ee. Intrigued by this result, we exposed Z-olefin substrate 1u to our reaction conditions. Interestingly, we obtained the corresponding Z-olefin product 3u in good yield, but in only moderate ee. Furthermore, we also noted that even when a mixture of linear and branched electrophiles is used, only the linear product is obtained (3v). Taking these results into account, and also considering that other electrophiles such as benzyl or phenethyl chloride result in no product formation, we believe the reaction is proceeding through a CuIII[σ+π] allyl species (Scheme 3, D).12
Scheme 1. Importance of Olefin Substitution Patterna.
(a) Isolated yields on 0.2 mmol scale. SFC analysis was used to determine ee.
Scheme 2.
Proposed Reaction Mechanism
Given the novelty of the Cu/L8 complex, we chose to examine it using continuous wave (CW) X-band electron paramagnetic resonance (EPR), UV-vis spectroscopies, and density functional theory (DFT) calculations. Although the crystal structure of a planar CuII complex with a tetradentate, deprotonated L1 has been reported,13 it was unclear how exclusion of one of the pyridine moieties, and the presence of a large, bulky backbone would affect the binding mode of the ligand. For this reason, we aimed to determine whether our best performing ligand L8 coordinates through the two amide nitrogens, or if it adopts an alternative binding mode through the benzamide oxygen, similarly to the previously disclosed Mo/L2 complex.14
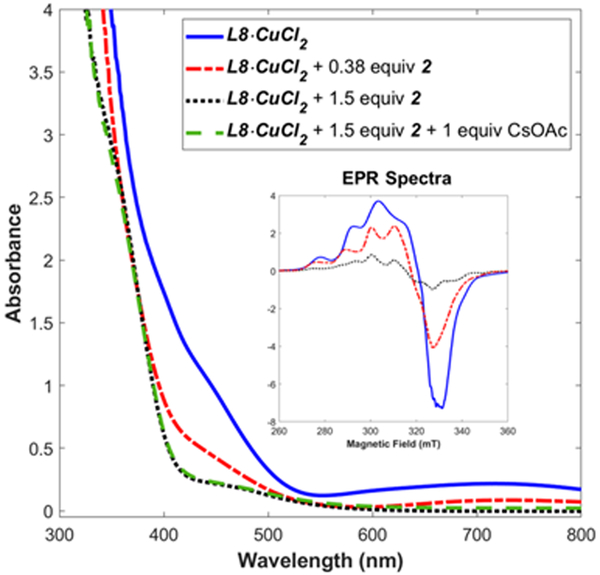
Preparing the pre-catalyst in THF afforded a green solution with low intensity d-d transitions ranging from 560 to 1400 nm (Figure 2 and S4). Spin quantification of the 77 K X-band EPR spectra revealed a monomeric CuII center was the major species at the reaction concentration.15 Negative ion mode ESI-MS generated product ions and isotope patterns consistent with the ionization of the fully deprotonated [L8•CuCl2]2− species, indicating that the Cl-counter-ions may be playing a role in the generation of a consistent complex.
Figure 2.
Room temperature UV-vis and 77 K X-band EPR (inset) spectroscopic monitoring of the reaction of 5 mol% of L8•CuCl2 with silyl ketene acetal 2.
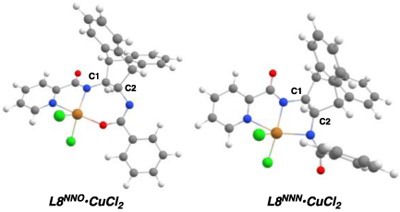
DFT calculations indicate L8 preferentially binds to Cu in a tridentate fashion, via Npyridine, Npicolinamide, and Obenzamide atoms (L8NNO•CuCl2, Table 3, entry 1). Regardless of the level of theory employed, the alternative tridentate binding mode via the benzamide nitrogen (L8NNN•CuCl2) is disfavored by 14-23 kcal/mol (entry 2 and Table S2). This large energy difference renders the thermal interconversion between the two binding modes unlikely; for this reason, we postulate only one conformer of the pre-catalyst accounts for the observed asymmetric catalytic activity.
Table 3.
DFT Calculations on L8 and L8•CuCl2a
 | |||||
|---|---|---|---|---|---|
| entry | ligand binding mode |
Egas phase (kcal/mol) |
Esolvent corrected (kcal/mol)c |
τ4’C1 | τ4’C2 |
| with CuCl2 | |||||
| 1 | L8NNO•CuCl2 | 0 | 0 | 0.93 | 0.95 |
| 2 | L8NNN•CuCl2 | 22.5 | 19.5 | 0.86 | 0.85 |
| without CuCl2b | |||||
| 3 | relaxed L82− | 0 | 0 | 0.96 | 0.97 |
| 4 | L8NNO | 18.7 | 13.6 | 0.93 | 0.95 |
| 5 | L8NNN | 39.5 | 30.0 | 0.86 | 0.85 |
Obtained using B3LYP density functional with 38% Hartree-Fock exchange.
Ligand coordination geometry was obtained by removing CuCl2 from the optimized geometry of the corresponding complex.
To further probe the origin for this binding preference, we performed single-point calculations on L8 in the absence of CuCl2 (entries 3-5). Interestingly, we found the energy gap between the two conformations L8NNO and L8NNN is still retained (entries 4 and 5), implying ligand sterics affect the binding preference and tune the metal coordination geometry. As demonstrated by the geometry index τ4’,16 the two sp3 ring carbons C1 and C2 exhibit a stronger deviation from the ideal tetrahedral geometry (τ4’ = 1) in L8NNN relative to L8NNO.17 Thus, in addition to providing steric bulk to assist in stereocontrol, the rigid backbone of L8 likely plays an important role in enforcing NNO binding.
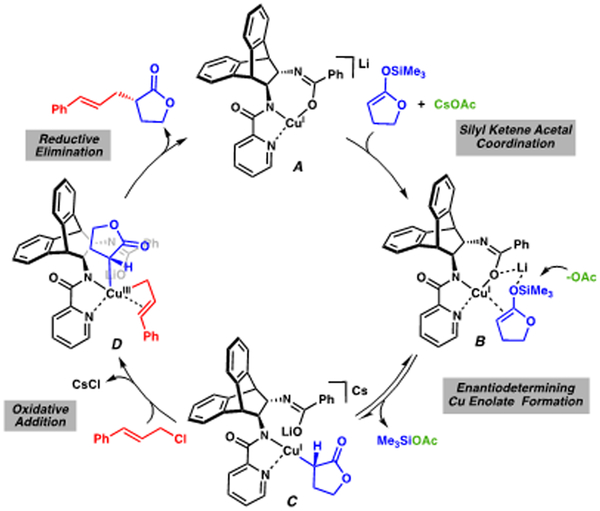
The characteristic d-d transitions and 77 K X-band EPR signal of the starting L8•CuCl2 complex disappear at the start of the reaction, signifying the CuII precatalyst is reduced to a CuI complex (Figure 2).18 We found reduction occurs even in the absence of CsOAc, indicating the silyl ketene acetal 2 is the reductant.19 Consequently, we propose the reaction follows a CuI-CuIII catalytic cycle as shown in Scheme 2.20
We believe the catalytic cycle commences with coordination of the electron-rich olefin of the silyl ketene acetal to CuI bis-amidate complex A, forming B. The Si-O bond is then cleaved via outer sphere attack by the acetate anion21 to afford the desired C-bound Cu enolate C.2d We envision this could be the enantiodetermining step, during which the lithium counter-ion may assist through electrostatic interactions, preventing rotation of the silyl ketene acetal. Dissociation of the benzamide then allows for the coordination and subsequent oxidative addition of the allyl chloride, generating CuIII[σ+π] species D.12,22 The sterically encumbered CuIII species can then undergo reductive elimination to generate the desired α-allyl γ-butyrolactone and the starting CuI species A.
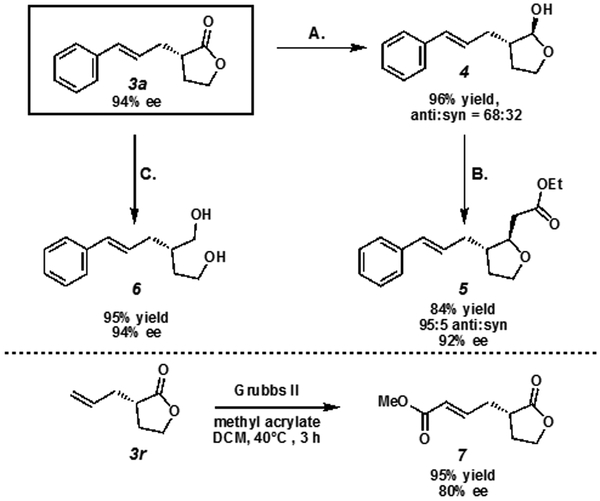
In order to demonstrate the synthetic utility of these products, we subjected α-allyl γ-butyrolactone 3a and 3r to a number of transformations (Scheme 3).23 Lactone 3a was reduced to the corresponding lactol 4 using DIBAL-H. Lactol 4 was then converted to the unsaturated ester, which spontaneously cyclized to the corresponding tetrahydrofuran 5 in high yield and high diastereoselectivity. Lactone 3a was also converted to the corresponding chiral diol 6 in good yields using lithium aluminum hydride. We also found 3r could be swiftly converted to the corresponding methyl acrylate species via cross metathesis.
Scheme 3. Derivatization of Allyl γ-Butyrolactone Products.
A. DIBAL-H, CH2Cl2, −78 °C, 30 min. (b). NaH, triethylphosphonoacetate, THF, 0 °C to 23 °C, 3 h. C. LiAlH4, Et2O, reflux, 3 h.
In conclusion, we have developed a Cu-catalyzed enantioselective allylic alkylation with a non-stabilized enolate-derived nucleophile. Critical to the development of this reaction was the identification of a novel mono-picolinamide ligand that led to product formation in high yields (up to 96%) and ee (up to 95%). A number of electrophiles with a broad range of functionality were well-tolerated in this reaction, including aryl, heteroaryl, and aliphatic allylic chlorides. DFT calculations suggest ligand sterics play an important role in determining metal coordination geometry, leading to tri-dentate NNO Cu coordination by the ligand. Preliminary mechanistic investigations indicate that a CuI species is likely the active catalyst, and that the reaction may proceed through a CuIII[σ+π] intermediate. Future work will focus on gaining a deeper understanding of the reaction mechanism and expanding the scope of this reaction to include other enolate derived nucleophiles.24
Supplementary Material
Acknowledgements
The NIH-NIGMS (R01GM080269) and Caltech are thanked for support of our research program. Financial support from Caltech and the Dow Next Generation Educator Fund is gratefully acknowledged (R. G. Hadt). C. I. Jette thanks the National Science Foundation for a predoctoral fellowship. Alexander Q. Cusumano is thanked for assistance and helpful discussions. Dr. Scott Virgil is thanked for instrumentation and SFC assistance. We thank Lawrence Henling for assistance with X-Ray Analysis. Dr. Mona Shahgholi is acknowledged for mass spectrometry assistance. Dr. Paul H. Oyala is thanked for his assistance with EPR spectroscopy. We acknowledge Prof. H. B. Gray for the use of the Cary 500 UV-vis-NIR spectrophotometer.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1. (a).Seitz M; Reiser O 2005, 9, 285–292. [DOI] [PubMed] [Google Scholar]; (b) Meyers AI; Yamamoto Y; Mihelich ED; Bell RA J. Org. Chem 1980, 45, 2792–2796. [Google Scholar]; (c) Madelaine C; Valerio V; Maulide N Angew. Chem. Int. Ed 2010, 49, 1583–1586, [DOI] [PubMed] [Google Scholar]; Angew. Chem 2010, 122, 1628–1631. [Google Scholar]; (d) Mao B; Fañanás-Mastral M; Feringa BL Chem. Rev 2017, 117, 10502–10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. (a).Evans DA; Kozlowski MC; Murry JA; Burgey CS; Campos KR; Connell BT; Staples RJ J. Am. Chem. Soc 1999, 121, 669–685. [Google Scholar]; (b) Evans DA; Murry JA; Kozlowski MC J. Am. Chem. Soc 1996, 118, 5814–5815. [Google Scholar]; (c) Saaby S; Nakama K; Lie MA; Hazell RG; Jørgensen KA Chem. Eur. J 2003, 9, 6145–6154. [DOI] [PubMed] [Google Scholar]; (d) Huang Z; Chen Z; Lim LH; Quang GCP; Hirao H; Zhou J Angew. Chem. Int. Ed 2013, 52, 5807–5812, [DOI] [PubMed] [Google Scholar]; Angew. Chem 2013, 125, 5919–5924. [Google Scholar]
- 3.Tsuji J;Takahashi H; Morikawa M Tetrahedron Lett., 1965, 6, 4387–4388. [Google Scholar]
- 4. (a).James J; Guiry PJ ACS Catal. 2017, 7, 1397–1402. [Google Scholar]; (b) Nascimiento de Oliveira M; Fournier J; Arseniyadis S; Cossy JA Org. Lett 2017, 19, 14–17. [DOI] [PubMed] [Google Scholar]; (c) Meletis P; Patil M; Thiel W; Frank W; Braun M Chem. Eur. J 2011, 17, 11243–11249. [DOI] [PubMed] [Google Scholar]; (d) Jiang X; Hartwig JF Angew, Chem. Int. Ed 2017, 56, 8887–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew, Chem. 2017, 129, 9013–9017. [Google Scholar]
- 5. (a).Ngamnithiporn A; Jette C; Bachman S; Virgil S; Stoltz BM Chem. Sci 2018, 9, 2547–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hayashi M; Bachman S; Hashimoto S; Eichman CC; Stoltz BM J. Am. Chem. Soc 2016, 138, 8997–9000. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Han S–J; Doi R; Stoltz BM Angew. Chem. Int. Ed 2016, 55, 7437–7440, [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem 2016, 128, 7563–7566. [Google Scholar]
- 6.Li D; Ohmiya H; Sawamura MJ Am. Chem. Soc 2011, 133, 5672–5675. [DOI] [PubMed] [Google Scholar]
- 7. (a).Falciola CA; Alexakis A Eur. J. Org. Chem 2008, 3765–3780. [Google Scholar]; (b) Alexakis A; Bäckvall JE; Krause N; Pàmies O; Diéguez M Chem. Rev 2008, 108, 2796–2823. [DOI] [PubMed] [Google Scholar]; (c) Harutyunyan SR; den Hartog T; Geurts K; Minnaard AJ; Feringa BL Chem. Rev 2008, 108, 2824–2852. [DOI] [PubMed] [Google Scholar]
- 8.For Cu(II) catalysts with 1,3-dicarbonyls see (a) Trillo P; Baeza A Adv. Synth. Catal 2017, 359, 1735–1741. Deng Q–H; Wadepohl H; Gade LH J. Am. Chem. Soc 2012, 134, 2946–2949. Given the large difference in sterics and reactivity between stabilized and non-stabilized enolates, it is unlikely that γ-butyrolactones would undergo allylation via a similar pathway.
- 9.The use of first row transition metals in allylation is mainly limited to 1,3-dicarbonyls: (a) Alexakis A; Begouin JM; Crawley ML; Guiry PJ; Kammerer-Pentier C; Kleimark J; Klein JEMN; Langlois J–B; Liron F; Liu W–B; Milhau L; Moberg C; Norrby P–O; Plietker B; Poli G; Prestat G; Trost BM Weickmann D; Xia J–B; You S–L Transition Metal Catalyzed Enantioselective Allylic Substitution in Organic Synthesis; Kazmeier U, Eds.; Springer, New York, 2012, vol. 38, pp1–341. [Google Scholar]; To our knowledge, there is only one report with Ni: (b) Wang J; Wang P; Wang L; Li D; Wang K; Wang Y; Zhu H; Yang D; Wang R Org. Lett. 2017, 19, 4826–4829. [DOI] [PubMed] [Google Scholar]
- 10.For other ligands, bases, and copper salts tested, see S6-8, 22.
- 11.With LiHMDS as the base, the complexation could be carried out at room temperature, allowing for an operationally simpler reaction set-up. For Cu/L complexation using different bases, see S8.
- 12. (a).Harada A; Makida Y; Sato T; Ohmiya H; Sawamura MJ Am. Chem. Soc 2014, 136, 13932–13939. [DOI] [PubMed] [Google Scholar]; (b) Yoshikai N; Zhang S-L; Nakamura EJ Am. Chem. Soc 2008, 130, 12862–12863. [DOI] [PubMed] [Google Scholar]; (c) Yamanaka M; Kato S; Nakamura EJ Am. Chem. Soc 2004, 126, 6287–6293. [DOI] [PubMed] [Google Scholar]; (d) Yoshikai N; Nakamura E Chem. Rev 2012, 112, 2339–2372. [DOI] [PubMed] [Google Scholar]
- 13. (a).Mulqi M; Stephens FS; Vagg RS Inorg. Chim. Acta, 1981, 51, 9–14. [Google Scholar]; (b) Mulqi M; Stephens FS; Vagg RS Inorg. Chim. Acta, 1981, 52, 177–182. [Google Scholar]; (c) Fan X; Zhang X; Li C; Gu Z ACS Catal. 2019, 9, 2286–2291. [Google Scholar]; (d) Carlo Sambiagio. Investigations on the use of Amidic Ligands in Copper-Catalyzed Arylation Reactions, Ph.D. Dissertation, University of Leeds, Leeds, West Yorkshire, England, 2015. [Google Scholar]
- 14. (a).Trost BM; Dogra K; Hachiya I; Emura T; Hughes DL; Krska S; Reamer RA; Palucki M; Yasuda N; Reider P Angew. Chem. Int. Ed 2002, 41, 1929–1932, [PubMed] [Google Scholar]; Angew. Chem 2002, 114, 2009–2012. [Google Scholar]
- 15.See S51 for more information.
- 16.Okuniewski A; Rosiak D; Chojnacki J; Becker B Polyhedron. 2015, 90, 47–57. [Google Scholar]
- 17.Although the energy gap between the two binding modes of L2 was smaller than that of L8, the energy difference was still too large for thermal interconversion (Table S3).
- 18.We also observed a diamagnetic species by 1HNMR (S234)
- 19. (a).Ito Y; Konoike T; Saegusa TJ Am. Chem. Soc 1975, 97, 2912–2914. [Google Scholar]; (b) Evans RW; Zbieg JR; Zhu S; Li W; MacMillan DW C. J. Am. Chem. Soc 2013, 135, 16074–16077. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rathke M; Lindert AJ Am. Chem. Soc 1971, 93, 4605–4606. [Google Scholar]; (d) Kochi JK J. Am. Chem. Soc 1955, 77, 5724–5728. [Google Scholar]; (e) Kosower EM; Cole WJ; Wu G–S, Cardy DE; Meisters G J. Org. Chem 1963, 28, 630–633. [Google Scholar]; (f) Kosower EM; Wu G–S J. Org. Chem 1963, 28, 633–638. [Google Scholar]
- 20.The NNO binding preference was retained in a number of CuII and CuI intermediates (Table S4).
- 21.IR data of our CuI catalyst indicates that the benzamide dissociates upon exposure to 2. DFT data indicates that a C-bound CuI enolate is lower in energy than the O-bound enolate (S50).
-
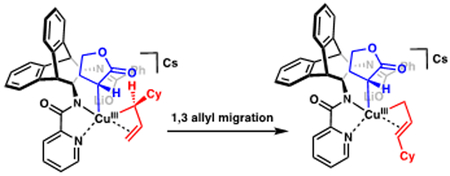
22.We believe that oxidative addition to 1v generates a sterically congested CuIII[σ+π] allyl species which undergoes a 1,3 allyl migration:12a
- 23.For other potential applications of chiral α-allyl γ-butyrolactones, see S76.
- 24.For other nucleophiles tested, see S23.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.