Ocrelizumab has been recently approved for relapsing-remitting MS, demonstrating a dramatic effect on MRI and clinical parameters in 2 pivotal phase III trials.1 However, long-term B-cell depletion might lead to an increased susceptibility to infections and/or to their increased severity (a case of fulminant hepatitis due to enterovirus infection has been recently reported by our group).2 Finally, depletion of B-cell compartment might impair acquisition of long-term humoral immunologic memory (i.e. production of antigen-specific class G immunoglobulins [IgG]) and might reduce T-lymphocyte response because of the absence of B lymphocyte–mediated activation. Indeed, humoral response to vaccination has been shown to be dampened or abolished in ocrelizumab-treated patients.3
To date, effective development of an antigen-specific immune response (either cell mediated or humoral) has not been reported in ocrelizumab-treated patients with MS after full-blown primary Varicella Zoster virus (VZV) infection.
The authors report the case of a 20-year-old woman with MS who developed chicken pox (due to primary VZV infection) 3 months after two 300 mg ocrelizumab infusions.
In July 2017, the patient developed optic neuritis; she was diagnosed with clinically definite MS in September 2017, following a clinical relapse. In October 2017, monthly natalizumab was started, despite a positive stratify John Cunningham virus (JCV) test. Natalizumab therapy was withdrawn after 15 infusions because of JCV-natalizumab-related risk of progressive multifocal leukoencephalopathy, and ocrelizumab was started in December 2018 after a 60-day washout period. Before ocrelizumab initiation, serum VZV IgG tested negative and the patient reported a negative history of chicken pox. As a standard procedure in our clinic, peripheral blood mononuclear cells (PBMCs) were collected by routine Ficoll-gradient centrifugation and stored on obtaining informed consent from the patient. VZV vaccine was not performed because of the tight schedule between natalizumab discontinuation and ocrelizumab initiation and to the risk of disease exacerbation after natalizumab withdrawal. In April 2019, she developed high fever with vesicular/pustular rash that rapidly disseminated throughout her body. Valaciclovir 1,000 mg 3 times daily was started 1 day after the appearance of skin eruption and continued up to 21 days. Patient fever abated on the third day and skin lesions healed within 10 days. No signs of organ damage were detected on routine laboratory tests. Four weeks after VZV infection onset, the patient tested positive for serum anti-VZV IgG, but negative for anti-VZV immunoglobulin M; PBMCs were collected to analyze the immunologic response after viral clearance.
Peripheral blood immune phenotype, T-lymphocyte proliferation, and cytokine production by VZV-stimulated cells were assessed by flow cytometry (fluorescence-activated cell sorting) and interferon-γ (IFN-γ) production assay. Samples analyzed included PBMCs obtained from the patient before and after VZV infection, PBMCs from a control patient with MS treated with ocrelizumab after natalizumab withdrawal (similarly to our index patient), and PBMCs from a healthy control (HC) (both controls had positive anti-VZV IgG test and VZV history); the T-cell mitogen (phytohaemagglutinin), was used as positive control for the proliferation and IFN-γ assays.
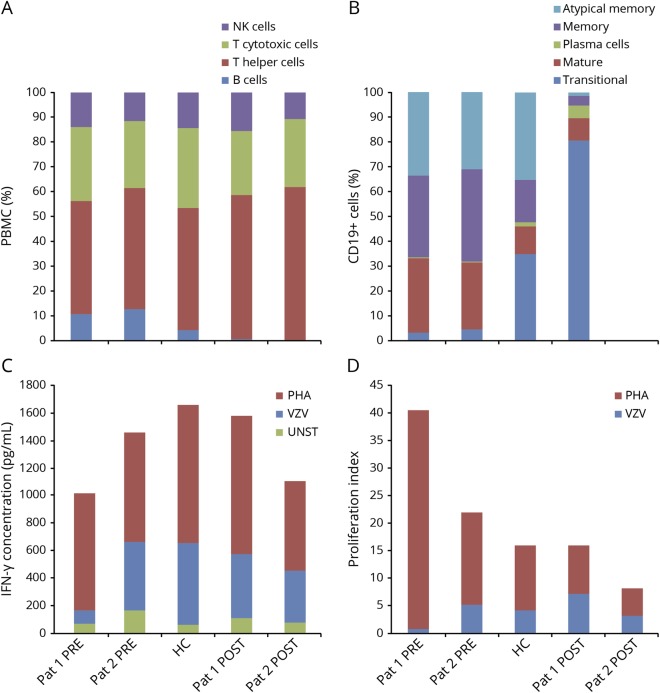
Peripheral blood immunophenotype showed that ocrelizumab-treated patients have a profound B-cell depletion with no alterations in the remaining lymphocyte subsets (figure, A). Interestingly, the few CD19+ B cells detected in the peripheral blood of our index case were represented by transitional B cells (CD24+ and CD38high), probably reflecting B cell migration from the bone marrow to secondary lymphoid organs that might have “escaped” from anti-CD20 antibody-mediated lysis (figure, B).
Figure. Ocrelizumab depletes B cells but does not impair T-cell response to primary VZV infection.
(A) Lymphocyte phenotype in the index case and control patient shows profound B-cell depletion after ocrelizumab treatment FACS analysis was performed on Fortessa LSR, Becton & Dickinson (BD) using the following BD antibodies: anti-CD3 (clone SK7, V500-C-conjugated), anti-CD4 (clone SK3, APC-H7-conjugated), anti-CD8 (clone SK1; PERCP-CY5.5-conjugated), anti-CD19 (clone SJ25C1, PE-CY7-conjugated), anti-CD56 (clone NCAM16.2, BV 421-conjugated), and anti-CD16 (clone 3 GB, PERCP-CY5.5-conjugated) antibodies. Lymphocyte phenotype was expressed as percentage of total PBMC, with NK cells identified as CD16+ and/or CD56+ cells, T cytotoxic cells as CD3+ CD8+ cells, T helper cells as CD3+ CD4+ cells, and B cells as CD19 + cells. Pat 1 PRE: index case before VZV infection and ocrelizumab treatment. Pat 2 PRE: control patient before ocrelizumab treatment. Pat 1 POST: index case after VZV infection and ocrelizumab treatment. Pat 2 POST: control patient after ocrelizumab treatment. (B) The B-cell phenotype of the index case after ocrelizumab and VZV exposure consists mostly of transitional cells. FACS analysis was carried out with BD antibodies including anti-CD19 as above, as well as anti-CD24 (clone ML5, PE-conjugated), and anti-CD38 (clone HB7, APC-conjugated) antibodies. B-cell phenotype was expressed as percentage of total CD19+ cells, with atypical B memory cells identified as CD24+, CD38− cells, B memory cells as CD24+, CD38low cells, plasma cells as CD24−, CD38high cells, mature B cells as CD24+, CD38intermediate cells, and transitional B cells as CD24+, CD38highcells. (C) After VZV infection, IFN-γ production in the index case is comparable with that of the control patient and HC after VZV stimulation IFN-γ production was assessed by ELISA (BioLegend, San Diego, CA), in basal condition (UNST), and on stimulation for 4 days with VZV lysate (VZV; 5 μg/mL, Microbix, Mississauga, Canada) or with PHA (1 μg/mL, Sigma, St. Louis, MO); values are expressed in pg/mL. (D) After VZV infection, T-cell proliferation in the index case is comparable with that of the control patient and HC after VZV stimulation. Proliferation index was assessed by FACS of carboxyfluorescein succinimidyl ester (CFSE)–labeled cells on stimulation for 4 days with VZV lysate (VZV) and PHA. Proliferation index: ratio of proliferated cells to unproliferated as a measure of CFSE response. HC = healthy control; IFN-γ = interferon-γ; FACS = fluorescence-activated cell sorting; PBMC = peripheral blood mononuclear cell; PHA = phytohaemagglutinin; UNST = unstimulated; VZV = Varicella Zoster virus.
Our analysis also indicates that T cells did proliferate and produce IFN-γ on VZV-antigen stimulation in our index case. Moreover, T-cell proliferation and IFN-γ production did not differ between samples obtained from our index patient and the other patient or HC (both previously exposed to VZV) (figure, C and D).
Incomplete depletion of B cells, probably within primary or secondary lymphoid organ compartments, might explain the development of a B- and T-cell response to VZV, probably effective in the clearance of the virus. In addition, the prompt treatment initiation with specific antiviral agents probably contributed to the lack of serious adverse events.
In conclusion, our results show that ocrelizumab does not impair B (humoral) and T (cell-mediated) immune response against primary VZV infection, circumstantially proving that secondary lymphoid organs are spared from drug-induced B-cell depletion.
Classification of evidence: this is an observational case report that provides class IV evidence.
Appendix. Authors
Study funding
No targeted funding reported.
Disclosure
G. Novi: received speaker honoraria from Merck, Novartis and Roche. F. Ivaldi, E. Sbragia, M. Mikulska, and G. Pesce report no disclosures relevant to the manuscript. M. Inglese: received honoraria or consultation fees from Roche, Biogen, Merck-Serono, Genzyme and research grants from NIH, NMSS, FISM, Novartis and Teva Neuroscience. N. Kerlero de Rosbo reports no disclosures relevant to the manuscript. A. Uccelli: received honoraria or consultation fees from Biogen, Roche, Teva, Merck-Serono, Genzyme, Novartis. Written consent was obtained from both patients and healthy control. Go to Neurology.org/NN for full disclosures.
References
- 1.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017;376:221–234. [DOI] [PubMed] [Google Scholar]
- 2.Nicolini LA, Canepa P, Caligiuri P, et al. Fulminant hepatitis associated with echovirus 25 during treatment with ocrelizumab for multiple sclerosis. JAMA Neurol 2019;76:866–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Available at: clinicaltrials.gov/ct2/show/study/NCT02545868. Accessed October 12, 2019.