Neuromyelitis optica spectrum disorder (NMOSD) is a rare but severe demyelinating condition that affects mainly adult patients. However, childhood onset has been reported and is related to a very active disease1 and poor outcome. Several evidence suggest that the crucial role of antiaquaporin-4 (AQP4) antibodies (Abs) in the pathogenesis of NMOSD justify the use of plasma exchanges and rituximab (RTX) as a treatment strategy targeting Ab and B-cells, respectively. Although RTX, a chimeric anti-CD20 monoclonal Ab, is associated with great efficacy in preventing NMOSD relapse, its use can be limited by severe infusion-related adverse event and infectious risk. Ofatumumab (OFA), a fully humanized anti-CD20 monoclonal antibody, has shown some efficacy in dysimmune diseases, including multiple sclerosis (MS).2 In pediatrics, OFA has been used in RTX-resistant nephrotic syndrome.3 We report here the case of a young girl with a very active AQP4-Ab NMOSD, with clinical worsening despite intensive immunosuppressant therapies. The use of OFA was associated to dramatic efficacy and great safety.
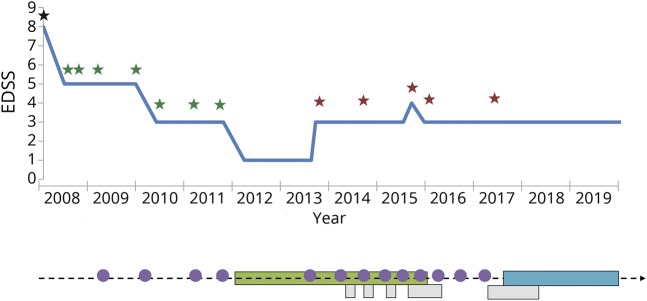
An 8-year-old girl born in Guyana, but living in France since 4 years, with unremarkable familial and medical history was admitted for a severe bilateral optic neuritis and longitudinally extensive transverse myelitis (LETM) in March 2008. After 3 infusions of methylprednisolone and oral tapering steroids, she fully recovered. Five months later, another LETM occurred, revealing the presence of AQP4-Ab in the serum tested as previously described,4 leading to the diagnosis of NMOSD. During the following 9 years, despite intensive immunosuppression by immunoadsorptions/plasma exchanges, mycophenolate mofetil, and RTX (figure), 9 severe relapses occurred, resulting in a permanent visual disability with right amblyopia (visual acuity using Snellen chart, with decimal equivalent [OD=0.1; OS=0.8, Expanded Disability Status Scale = 3]). RTX pediatric protocol was based on 2 infusions with an interval of 2 weeks (375 mg/m2 for each infusion): initially at each relapse (considering the very young age of the patient) and then every 6 months, since 2013. From September 2014, the patient began to suffer from RTX infusion-related reaction, culminating in July 2015, with a hospitalization in an intensive care unit for anaphylactic-like reaction clearly related to RTX. The following infusions were performed in an intensive care unit. Beyond the persistence of clinical activity, B-cells were still detected despite RTX. Moreover the patient experienced a severe sepsis related to the infection of a central catheter used for both immunoabsorption/plasma exchanges and RTX. Because of these different adverse events and the persistence of relapses, subcutaneous OFA was introduced in August 2017: one injection of 20 mg every week during 4 weeks and then one injection of 20 mg every 4 weeks. In 2018, detection of antibodies (immunoglobulin G) against RTX retrospectively validated the therapeutic change. After 2 years of follow-up and 30 injections of OFA, no further relapse occurred. In November 2019, clinical examination was normal, except visual disability (Expanded Disability Status Scale = 3). In January 2019, MRI revealed no radiologic activity but an atrophy of optic nerves and a persistent spinal cord lesion from C2 to T5. Tolerance was perfect with no anaphylactic-like nor infectious event. Apart from complete B-cell depletion, no lymphopenia or hypogammaglobulinemia was noticed.
Figure. Timeline of relapse frequency, disability course and different treatment regimes.
Stars indicate relapses: black star = optic neuritis and myelitis; red star = optic neuritis; green star = myelitis. Purple circles indicate infusions of rituximab. Green bar indicates treatment by MMF; blue bar indicates treatment by OFA; grey bars indicate treatment by PLEX. EDSS = Expanded Disability Status Scale; MMF = mycophenolate mofetil; OFA = ofatumumab; PLEX = plasma exchanges; RTX = rituximab.
RTX, a chimeric monoclonal antibody anti-CD20, is used off-label in NMOSD. CD20 is expressed at the membrane of the B-cell from the stage of pre-B-cells to mature B-lymphocyte but also in less than 5% of T lymphocytes. Major mechanism of action of RTX is the result of destruction of B-cells caused by antibody-dependent cell-mediated cytotoxicity involving natural killer cells and by complement-dependent cytotoxicity.5 Although RTX is associated to a good tolerance and safety profile in children with neuroinflammatory conditions, more than 10% of treated children presented infusion-related adverse event, suggesting the need for another B-cell-targeting treatment associated with a better tolerance.6 Moreover, RTX may induce immunization with anti-RTX antibodies associated with infusion-related adverse events and/or inefficacy.7
OFA, a fully humanized monoclonal antibody targeting CD20, binds to an epitope distinct from the one recognized by RTX and is a more potent activator of complement-dependent cytotoxicity in vitro. The fully humanized design prevents the risk of antidrug-antibodies production. Similar to other subcutaneous monoclonal antibodies, injection-related systemic reactions are less expected than infusion-related ones. Moreover, the subcutaneous way of administration allows a quick self-administration and limits the infectious risk of IV administration, as reported in our case.
Although emerging treatments have been proposed in AQP4-IgG NMOSD, this observation suggests that subcutaneous OFA may be a well-tolerated and effective alternative in refractory AQP4-IgG NMOSD or in case of intolerance of RTX. Further studies will be necessary to explore the safety and efficacy of OFA in NMOSD in a controlled clinical trial.
Classification of evidence
This case report provides Class IV evidence that OFA in patients with AQP4-IgG-NMOSD might be effective. This is a single observational study without controls.
Appendix. Authors
Study funding
No targeted funding reported.
Disclosure
E. Maillart reports personal fees from Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva and grants from Novartis and Roche, outside the submitted work. F. Renaldo reports no disclosures. C. Papeix reports personal fees from Biogen, MedDay, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva, outside the submitted work. K. Deiva has received consulting fees and travel grants from Novartis, Biogen, Sanofi, Merck, and Servier. J. Bonheur reports no disclosures. T. Kwon, O. Boespflug-Tanguy, and D. Germanaud report no disclosures. R. Marignier served on the scientific advisory board of MedImmune and received travel funding and speaker honoraria from Biogen, Genzyme, Novartis, Merck Serono, Roche, Sanofi-Aventis, and Teva. Go to Neurology.org/NN for full disclosures.
References
- 1.Absoud M, Lim MJ, Appleton R, et al. Paediatric neuromyelitis optica: clinical, MRI of the brain and prognostic features. J Neurol Neurosurg Psychiatry 2015;86:470–472. [DOI] [PubMed] [Google Scholar]
- 2.Hauser S. Efficacy and safety of ofatumumab versus teriflunomide in relapsing multiple sclerosis: results of the phase 3 ASCLEPIOS I and II trials. Paper presented at: 35th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); September 13, 2019; Stockholm, Sweden. [Google Scholar]
- 3.Basu B. Ofatumumab for rituximab-resistant nephrotic syndrome. N Engl J Med 2014;370:1268–1270. [DOI] [PubMed] [Google Scholar]
- 4.Marignier R, Bernard-Valnet R, Giraudon P, et al. Aquaporin-4 antibody-negative neuromyelitis optica: distinct assay sensitivity-dependent entity. Neurology 2013;80:2194–2200. [DOI] [PubMed] [Google Scholar]
- 5.Collongues N, Ayme-Dietrich E, Monassier L, de Seze J. Pharmacotherapy for neuromyelitis optica spectrum disorders: current management and future options. Drugs 2019;79:125–142. [DOI] [PubMed] [Google Scholar]
- 6.Dale RC, Brilot F, Duffy LV, et al. Utility and safety of rituximab in pediatric autoimmune and inflammatory CNS disease. Neurology 2014;83:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wincup C, Menon M, Smith E, et al. Presence of anti-rituximab antibodies predicts infusion-related reactions in patients with systemic lupus erythematosus. Ann Rheum Dis 2019;78:1140–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]