Abstract
Objective
To investigate whether aging differentially affects neural activity serving visuospatial processing in a large functional neuroimaging study of HIV-infected participants and to determine whether such aging effects are attributable to differences in the duration of HIV infection.
Methods
A total of 170 participants, including 93 uninfected controls and 77 HIV-infected participants, underwent neuropsychological assessment followed by neuroimaging with magnetoencephalography (MEG). Time-frequency analysis of the MEG data followed by advanced image reconstruction of neural oscillatory activity and whole-brain statistical analyses were used to examine interactions between age, HIV infection, and cognitive status. Post hoc testing for a mediation effect of HIV infection duration on the relationship between age and neural activity was performed using a quasi-Bayesian approximation for significance testing.
Results
Cognitively impaired HIV-infected participants were distinguished from unimpaired HIV-infected and control participants by their unique association between age and gamma oscillations in the parieto-occipital cortex. This relationship between age and gamma was fully mediated by the duration of HIV infection in cognitively impaired participants. Impaired HIV-infected participants were also distinguished by their atypical relationship between alpha oscillations and age in the superior parietal cortex.
Conclusions
Impaired HIV-infected participants exhibited markedly different relationships between age and neural responses in the parieto-occipital cortices relative to their peers. This suggests a differential effect of chronological aging on the neural bases of visuospatial processing in a cognitively impaired subset of HIV-infected adults. Some of these relationships were fully accounted for by differences in HIV infection duration, whereas others were more readily associated with aging.
HIV-associated neurocognitive disorders (HAND) are a persistent concern for those living with HIV and include substantial impairments to visuospatial processing.1–4 A greater understanding of the neural dynamics underlying these impairments has the potential to enhance diagnosis of HAND and inform prognoses and further delineation of HAND subtypes. Previous structural and functional imaging research has reported neural aberrations in visual-perceptual networks in HIV-infected adults.5–10 Importantly, only recently have researchers focused on differences in these visual circuits between HIV-infected adults with and without HAND.2,4,7,11 Furthermore, no studies to date have investigated the potential for interactions between age, HIV infection, and cognitive status on these neural dynamics. This is essential because functional brain networks affected by HAND overlap with networks that exhibit slow deterioration with healthy aging.2,4,12
Using magnetoencephalographic (MEG) neuroimaging and a visuospatial paradigm that elicits multispectral neural activity in the occipito-parietal cortices,2,12–14 we examined interactions between HIV infection, HAND status, and aging on the neural dynamics of visuospatial processing in a large sample of 170 participants. We hypothesized that HAND and aging would interact on higher-order patterns of neural activity such that the effect of aging on the occipital neural dynamics of unimpaired HIV-infected participants would closely approximate the same effect observed in uninfected controls. In contrast, we expected that those with HAND would display differences in the nature of this relationship compared with their unimpaired peers, indicating that these visuospatial neural circuits follow a distinct age-related trajectory in those with HAND.
Methods
Participants
We enrolled a total of 251 participants for this study, including 133 uninfected adult controls (74 males), 75 unimpaired HIV-infected adults (44 males), and 43 adults with HAND (26 males). Exclusion criteria for enrollment included any medical illness affecting CNS function (other than HIV infection), any neurologic disorder (other than HAND), known cognitive impairment (in the control sample), history of head trauma, current illicit substance use, and known clinically significant depression (i.e., a Beck Depression Inventory-II score of ≥20). Current substance use was assessed through a private interview and self-report questionnaires. Following consent and assessment, a portion of enrolled participants were excluded from the study, and these numbers are reported in figure 1. The final analysis cohort included a total of 93 uninfected controls (51 males), 48 unimpaired HIV-infected adults (27 males), and 29 adults with HAND (19 males). All 3 groups were matched on age, sex, handedness, average alcohol consumption, and ethnicity. Group demographics and neuropsychological scores are reported in table 1. All HIV-infected participants were receiving effective combined antiretroviral treatment (cART) and had documented undetectable plasma viremia. All participants completed the same experimental protocol.
Figure 1. Participant exclusions and final cohort sizes.
Of the 251 total participants originally enrolled, 81 were excluded due to drug use, depression, cognitive impairment, or unusable MEG/MRI data.
Table 1.
Group-wise demographic and neuropsychological profiles
Standard protocol approvals, registrations, and patient consents
The Institutional Review Board at the University of Nebraska Medical Center reviewed and approved this investigation. Written informed consent was obtained from each participant following detailed description of the study.
Neuropsychological testing
Participants underwent a comprehensive battery of neuropsychological assessments, which were chosen based on the Frascati consensus,15 with raw scores for each participant being converted to demographically adjusted z-scores using published normative data.16 The battery assessed multiple functional domains, including fine motor (grooved pegboard), language (Wide Range Achievement Test 4 reading), verbal learning and memory (Hopkins Verbal Learning Test–Revised), speed of processing (Trailmaking-A, digit symbol, Stroop color), attention (Symbol Search, Stroop word), and executive functioning (verbal fluency, semantic fluency, Stroop interference, and Trailmaking-B). Composite scores for each of these functional domains were computed using standardized z-scores from the assessment sets comprising the domain. Using these composites and activities of daily living, patients were rated by a neuropsychologist on a scale of 0–3 as having no cognitive impairment (0), HIV-associated asymptomatic neurocognitive impairment (ANI; 1), HIV-associated mild neurocognitive disorder (MND; 2), or HIV-associated dementia (HAD; 3) according to the Frascati guidelines. HIV-infected individuals with an impairment rating of 1 or higher were included as a part of the HAND group, whereas those with a rating of zero were included as a part of the unimpaired HIV-infected cohort. Control participants with an impairment rating of 1 or higher were excluded from the study.
MEG experimental paradigm
We used a visuospatial discrimination task, termed Vis-Attend (figure 2, top), to engage visual attention circuitry. During this task, participants were seated in a magnetically shielded room and told to fixate on a crosshair presented centrally. After a variable inter-stimulus interval (range: 1900–2100 ms), an 8 x 8 grid was presented for 800 ms at one of 4 positions relative to the fixation: above right, below right, above left, or below and to the left. The left/right orientations were defined as a lateral offset of 75% of the grid from the center of fixation. Participants were instructed to respond via button press with their right hand as to whether the grid was positioned to the left (index finger) or right (middle finger) of the fixation point on presentation of the grid. Each participant performed 240 repetitions of the task concurrent with MEG recording.
Figure 2. Task design and effects of group and age on task performance.
An illustration of the visuospatial task paradigm (top). Participants were instructed to indicate the laterality of the checkerboard stimulus using their right index (left-lateralized) and middle (right-lateralized) fingers. Task performance as a function of group and age (bottom). Generally, participants with HAND performed worse on the visuospatial task than those without cognitive impairment. In addition, older participants tended to respond slower on the task than younger participants. Of interest, none of these relationships remained significant when age and group were entered into a single statistical model. HAND = HIV-associated neurocognitive disorder. *p < 0.05.
MEG data acquisition, coregistration, and preprocessing
MEG signals were sampled at 1 kHz with an acquisition bandwidth of 0.1–330 Hz using a 306-sensor Elekta MEG system (Helsinki, Finland) equipped with 204 planar gradiometers and 102 magnetometers. All data were corrected for head movement, subjected to a noise reduction method,17 and coregistered to participant-specific high-resolution structural MRI. For a detailed account of the data acquisition, coregistration, and preprocessing steps used in this study, please refer to our previous publications.2,4
MEG preprocessing, time-frequency transformation, and sensor-level statistics
The continuous magnetic time series was divided into 2,700 ms epochs, with the baseline extending from −400 to 0 ms before stimulus onset. Epochs containing artifacts were rejected using a fixed threshold method, supplemented with visual inspection. An average of 205.99 (SD = 12.91) trials per participant were used for further analysis, and the mean number of trials per participant did not significantly differ between groups (control: 206.27; unimpaired HIV: 203.52; HAND: 206.96). To examine spectrally specific neural responses to the visuospatial discrimination task, the artifact-free epochs were transformed into the time-frequency domain, and the resulting spectral power estimations per sensor were averaged over trials to generate time-frequency plots of mean spectral density. These sensor-level data were normalized using the mean baseline data ((active-baseline)/baseline)*100) per frequency bin, which was defined as the mean power during the −400 to 0 ms time period. The specific time-frequency windows used for subsequent imaging were determined by a stringent statistical analysis of the sensor-level spectrograms across the entire array of gradiometers. Briefly, each data point in the spectrogram was initially evaluated using a mass univariate approach based on the general linear model. To reduce the risk of false-positive results while maintaining reasonable sensitivity, a 2-stage procedure was followed to control for type 1 error. In the first stage, paired-sample t-tests against baseline were conducted on each data point, and the output spectrogram of t-values was thresholded at p < 0.05 to define time-frequency bins containing potentially significant oscillatory deviations across all participants. In stage 2, the time-frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also above the threshold (p < 0.05), and a cluster value was derived by summing all the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster values, and the significance level of the observed clusters (from stage 1) was tested directly using this distribution.18,19 For each comparison, 1,000 permutations were computed to build a distribution of cluster values. Based on these analyses, the time-frequency windows that contained significant oscillatory events across all participants were subjected to an advanced image reconstruction analysis. Importantly, only those significant time-frequency events that began before the mean reaction time (RT) across all participants were included. This was done to focus on responses serving visuospatial attention and perception, rather than other processes important for this task (e.g., motor plan execution and response/error checking).
MEG image reconstruction and statistical analysis
Cortical networks were imaged through an extension of the linearly constrained minimum variance vector beamformer, named dynamic imaging of coherent sources,20 which uses the sensor-level cross-spectral density data to calculate voxel-wise source power for the entire brain volume. Such images are typically referred to as pseudo-t maps, with units (pseudo-t) that reflect noise-normalized power differences (i.e., active vs passive) per voxel.
To initially investigate the spatial location of each spectrally specific neural response to the task, we computed grand-average maps across all participants for each time-frequency window identified in the sensor level analysis. These grand-average maps were used to discern the nature of each response (e.g., motor vs visual) and to target subsequent analyses toward responses originating from nonmotor regions, as investigating somato-motor processing was not an aim of this study (see Results). The spectrally specific maps of each participant were then used to compute whole-brain statistical representations of the interaction between age and group. This was done by first computing voxel-wise correlations between age and spectrally specific neural activity within each group and then comparing the resulting statistical maps group-wise using a whole-brain Fisher Z transformation.4,12,21 Individual participant-level maps that contained significant artifacts were excluded frequency-wise at this step; however, all statistical balancing of group, age, and group-by-age effects on demographic factors remained intact following these exclusions. From the resulting significant clusters of neural activity, pseudo-t values per participant were extracted from the peak voxel (i.e., the voxel with the highest statistical value per cluster), and these were used in post hoc testing of interaction effects and targeted hypotheses. To account for multiple comparisons, a significance threshold of p < 0.01 was used for the identification of significant clusters in all whole-brain statistical maps, accompanied with a cluster (k) threshold of at least 300 contiguous voxels. All whole-brain statistical analyses were computed using custom functions in Matlab (MathWorks; Natick, Massachusetts). All other statistical analyses were performed using SPSS, with the exception of the mediation analyses, which were performed in R using the mediation package. Mediation analyses were performed using quasi-Bayesian estimation of CIs and 10,000 Monte Carlo iterations. For all reported analyses, outliers were excluded per statistical comparison based on a fixed threshold of ±2.5 SDs from the mean of their respective group.
Data availability
All data are available via the corresponding author on reasonable request and are being made publicly available via the National NeuroAIDS Tissue Consortium database on completion of the study.
Results
Sample characteristics and behavioral results
Assessment of the neuropsychological and ADL functional data indicated that 29 HIV-infected participants met the diagnostic criteria for HAND and were eligible for the study (e.g., not significantly depressed). Of these 29 participants with HAND, 20 were classified as having ANI, 7 were classified as having MND, and 2 were classified as having HAD. Neither key demographic variables, nor current alcohol use, differed between groups, and measures of central tendency for these data are reported in table 1. To aid in characterization of our participant sample, we have additionally included patient cART regimens in table 2. Chi-square tests with Yates correction indicated that the frequency of use of each drug did not significantly differ between patients with and without cognitive impairment (all p's > 0.05).
Table 2.
Antiretroviral medications in HIV-infected participants
Similar to previous findings in our laboratory,2 1-way analysis of variance examining the effect of group on task performance revealed significant differences (RT: F(2,159) = 3.05, p = 0.050; accuracy: F(2,159) = 4.24, p = 0.016), such that uninfected controls were significantly faster (t(115) = −2.17, p = 0.032) and more accurate (t(115) = 3.13, p = 0.002) than participants with HAND. Furthermore, unimpaired HIV-infected adults were marginally more accurate (t(70) = 1.98, p = 0.051) than those with HAND (figure 2, bottom). Of interest, when age was added into these models, none of the group effects (RT: F(2,156) = 0.87, p = 0.422; accuracy: F(2,156) = 0.06, p = 0.936) nor the interactions between age and group (RT: F(2,156) = 0.22, p = 0.804; accuracy: F(2,156) = 0.19, p = 0.830) remained significant, and only a main effect of age persisted on RT (F(1,156) = 18.60, p < 0.001). A summary of the group-wise neuropsychological scores and task performance metrics can be found in table 1.
Oscillatory neural responses in occipital, parietal, and motor cortices
To identify the spectro-temporal windows where significant brain responses occurred during task performance, the sensor-level MEG data were transformed into the time-frequency domain and examined using a rigorous statistical approach. In line with previous studies of visuospatial perception,2,12–14 participants exhibited significant responses in 4 distinct windows (theta: 4–8 Hz, 25–275 ms; alpha: 8–14 Hz, 375–650 ms; beta: 16–24 Hz, 250–650 ms; gamma: 64–72 Hz, 350–500 ms; all p's < 0.01). The cortical origins of these responses were then determined using a beamformer, which revealed that the theta and gamma responses were generated by the bilateral primary visual cortices, the alpha response by the bilateral occipito-parietal cortices, and the beta response by the primary motor cortex contralateral to the movement (figure 3). Of note, we did not examine the beta response further, as examining motor dynamics was not within the scope of this study.
Figure 3. Spectrally specific neural responses to the task.
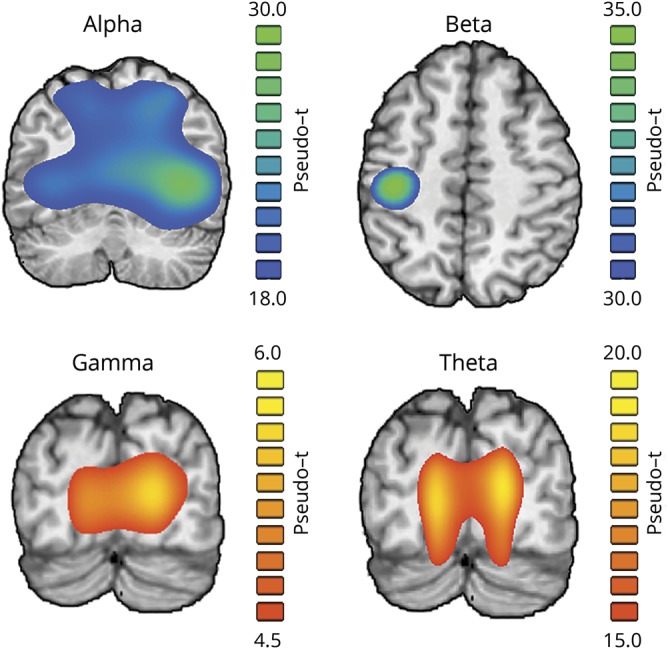
Brain slices show group mean beamformer images of the oscillatory neural responses at each frequency. The respective color legend for each image is displayed to the right. Responses in the theta (4–8 Hz), alpha (8–14 Hz), and gamma (64–72 Hz) ranges were found to originate from bilateral occipital sources, whereas activity in the beta (16–24 Hz) band originated from the primary motor cortex contralateral to movement. Note that the beta band was excluded from further analysis because of its motor-related origin.
Neural dynamics: age and cognitive status interact in HIV-infected adults
Next, we investigated the potential for interacting effects of age and group on these neural responses, by computing whole-brain Fisher Z comparisons separately for the theta, alpha, and gamma band responses. In the alpha band, a significant interaction between age and group was observed in the left parieto-occipital cortices between unimpaired HIV-infected participants and those with HAND (zpeak = −3.31, p < 0.01, corrected). In contrast, significant interaction effects in the gamma band were found in the right parieto-occipital cortices (controls vs HAND; zpeak = 2.99, p < 0.01, corrected), as well as the right and left prefrontal cortices (HIV vs HAND; right: zpeak = −3.31, p < 0.01, corrected; left: zpeak = −3.16, p < 0.01, corrected). Finally, a significant interaction effect was found in the theta range within the premotor cortex (HIV vs HAND; zpeak = −2.91, p < 0.01, corrected).
Because the goal of this study was to investigate the visuospatial processing circuitry involved in HAND-related cognitive declines, we have focused interpretation on these effects (i.e., posterior and prefrontal clusters) and not those in sensorimotor regions. As stated above, for the alpha left parieto-occipital peak (figure 4), the interaction was driven by differences between unimpaired HIV-infected adults and those with HAND. In contrast, the interaction in the gamma right parieto-occipital peak (figure 5) was driven by differences between uninfected adults and HIV-infected adults with HAND. Specifically, in the alpha band, both the control (rpeak = −0.21, p = 0.048) and unimpaired HIV-infected (rpeak = −0.52, p < 0.001) participants exhibited a negative relationship between neural activity and age in the left parieto-occipital cortex such that as age increased, neural activity became more negative. Although the signs of these relationships were negative, it is important to note that the response of interest was a desynchronization (i.e., a decrease in activity from baseline), meaning that more negative values indicate a stronger neural response to the task at this frequency.22 Thus, the relationships indicate that control and unimpaired HIV-infected participants exhibited a more robust alpha desynchronization response in left parieto-occipital regions as age progressed. In contrast to the other 2 groups, participants with HAND did not exhibit this negative relationship between alpha activity and age, resulting in a significant interaction at this peak. As per the gamma responses, there was an interaction in the right parieto-occipital cortex such that participants with HAND exhibited a robust negative relationship between neural activity and age (i.e., as age increased, neural activity decreased; rpeak = −0.49, p = 0.011), whereas controls and unimpaired HIV-infected participants exhibited no such relationship. Finally, the interactions in the prefrontal cortices reflected a significant increase in gamma with age in those with HAND (right: rpeak = 0.51, p = 0.008; left: rpeak = 0.48, p = 0.013), whereas unimpaired HIV-infected and control participants again showed no such effect. Of note, the prefrontal gamma responses across all 3 groups were largely centered on zero, reducing our faith in the signal-to-noise ratio of this response.
Figure 4. Interaction between age and cognitive status on the posterior alpha response in HIV-infected participants.
The brain slice (top left) represents the voxel-wise interaction between age and cognitive status in HIV-infected participants (i.e., HIV vs HAND). Peak voxel amplitude values were extracted from the cluster shown in this map and plotted as a function of age by group to visualize the differing relationships between age and neural activity. *p < 0.05. HAND = HIV-associated neurocognitive disorder.
Figure 5. Interaction between age and cognitive status on the posterior gamma response.
The brain slice (top left) represents the voxel-wise interaction between age and cognitive status on gamma oscillations (i.e., controls vs HAND). Peak voxel amplitude values were extracted from the cluster shown in this map and plotted as a function of age by group to visualize the differing relationships between age and neural activity. *p < 0.05. HAND = HIV-associated neurocognitive disorder.
Although these findings were groundbreaking and illuminated distinct brain aging trajectories, a major question remained regarding these effects. Basically, are any of the relationships observed here between age and neural activity in HIV-infected adults largely a reflection of the duration of infection? As would be expected, disease duration and age exhibit a high degree of covariance, and so we examined the potential for a statistical mediation of our interaction effects (i.e., the alpha- and gamma-band interactions) by disease duration. Our results indicated that disease duration did not mediate the effect of age on alpha range neural activity in either of the HIV-infected groups, but did mediate gamma-range neural activity in those with HAND (figure 6). Specifically, disease duration fully mediated the effect of age on gamma activity in these participants (Δr = 0.18, b = −0.03, p = 0.022, 95% CI = −0.07, 0.00), and predicted gamma response amplitude above and beyond age (rpartial = −0.55, p = 0.005), signifying that the length of HIV infection was actually responsible for this relationship. Intriguingly, this was not the case in unimpaired HIV-infected adults (Δr = 0.04).
Figure 6. Mediation of the age/gamma activity relationship by disease duration.
The brain slice (left) represents the voxel-wise interaction between age and cognitive status on gamma oscillations (i.e., controls vs HAND). Peak voxel amplitude values were extracted from the cluster shown in this map and plotted as a function of time since diagnosis (in years) separately by cognitive status in HIV-infected participants to visualize the differing relationships. Graphical representations of the mediation models computed in unimpaired HIV-infected participants and those with HAND are displayed below, with the regression coefficients (R) above each respective relationship. The mediated relationship between age and gamma activity is indicated in bold text. *p < 0.05. HAND = HIV-associated neurocognitive disorder.
Discussion
Although earlier work has found aberrant neural dynamics during visuospatial processing2 and visual attention4 in participants with HAND, the interaction between cognitive status and age on these patterns of brain activity has not been investigated. Using advanced neuroimaging and statistical analyses, we found that age and cognitive impairment interact in unique ways in HIV-infected adults during performance of visuospatial tasks. More specifically, alpha- and gamma-range neural oscillatory patterns in occipito-parietal regions were found to covary with age differently, depending on the cognitive status of the participant. Broadly, visual alpha oscillations represent the gross disinhibition of visual processing circuits,23–25 whereas visual gamma activity has been implicated in more fine-tuned stimulus encoding.26–28 The general pattern of these results was such that unimpaired HIV-infected adults exhibited neural dynamics more similar to the uninfected participants, whereas those with HAND exhibited markedly irregular patterns. Furthermore, visual dynamics in the gamma-band were significantly predicted by the time since HIV-diagnosis in those with HAND, and this measure fully mediated the relationship between gamma oscillations and age in these participants. These results are discussed below, with a focus on implications for the field of neuroHIV.
The neural responses observed in this study have all been reported and studied extensively in the past. Theta activity in primary visual regions is widely accepted as being representative of early stimulus recognition and processing in the cortical visual stream,23,29,30 whereas the more lateralized alpha decreases (from baseline levels) are known to represent the engagement of later visual regions and scale as a function of attentional load.23 Finally, the gamma increases in more posterior visual regions are known to be modulated by a number of fine-tuned stimulus features and also by attention,26–28,31,32 and thus such responses are thought to represent object representation in visual cortices. Hence, broadly, the theta increases in the primary visual cortex can be interpreted as an earlier component of visual processing, whereas higher-order functions appear to be represented in the alpha and gamma ranges.
Visuospatial dysfunction in HIV-infected participants has been well documented; however, the underlying neurophysiology of these impairments has remained elusive until fairly recently.2–4,10,33 In addition to these functional imaging studies, the visual cortices have been found to be preferentially vulnerable to HIV-related degeneration,34 an effect which may be due to latent viral reservoirs instigating immune system decline in these regions.34,35 Despite these advances, the role of aging in these visuospatial deficits has not been examined, which is surprising because these same cognitive faculties degrade substantially as a function of age in uninfected populations.36–41 Furthermore, although numerous studies have reported little or no evidence for an interaction between age and HIV infection on neurophysiology and neuropsychological assessments,42–46 none of these investigations included the cognitive status of the HIV-infected participants as a factor. Thus, the findings reported herein are the first such investigation of the intersection between age, HIV infection, and cognitive status on brain function.
In the alpha band, increases in age were linked to stronger neural responses in the parieto-occipital cortices in both cognitively normal groups. Because this response indexes the usage of visuospatial resources and is modulated by attention,23,24 this relationship should be interpreted as a compensatory recruitment of visual processing circuits as cognitively intact individuals age. In contrast, no such relationship existed in adults with HAND. This indicates that impaired HIV-infected participants are unable to fully recruit the circuitry underlying parieto-occipital alpha responses, which is essential for efficient visuospatial processing. Similarly, the relationship between visual neural dynamics in the gamma range and age in those with HAND also differed from that of cognitively intact adults. However, in contrast to the alpha findings, neither the uninfected controls nor the unimpaired HIV-infected participants exhibited a significant relationship between age and gamma neural responses, whereas those with HAND had significantly reduced visual gamma activity as age progressed. Gamma activity in parieto-occipital cortices is important for the indexing of saliency in visual space26–28 and the integration of these saliency maps with goal-oriented motor planning,47,48 which is known to covary with healthy aging.12 Thus, in this context, reduced gamma indicates deficient mapping of the visual stimulus in participants with HAND. Importantly, although the relationship with alpha activity appeared to be truly resultant of aging effects, the similar relationship between occipital gamma and age was found to actually be due to the length of HIV infection. This implies that although parieto-occipital gamma activity is almost certainly degraded in a subset of infected individuals (i.e., those who develop HAND), this degradation scales with the amount of time that the brain is exposed to the virus and not necessarily chronological age. An important consideration here, however, is that disease duration and time on cART are highly covariable (r = 0.93 in participants with HAND; r = 0.90 in all HIV-infected participants). This collinearity between these variables precludes us from investigating their independent contributions to the mediated age-gamma effect, and thus it is possible that time spent on cART plays a role in this decline.
A number of significant interactions between age and cognitive status on oscillatory neural activity were also observed in HIV-infected participants in extravisual cortices. Specifically, we found such interactions on theta-frequency activity in premotor cortex and gamma-frequency activity in bilateral prefrontal cortices. Tentatively, these findings might reflect prefrontal neural sources involved in the so-called top-down modulation of visual processing, which scale as a function of age in cognitively impaired participants. Indeed, previous research from our laboratory has suggested that similar spectrally, temporally, and spatially defined neural responses differ as a function of cognitive status in HIV-infected participants.4 However, the overall response amplitude in each of these regions was qualitatively small in this study, reducing our confidence in these results. Further research is certainly necessary, perhaps using more cognitively demanding tasks specifically targeting frontal attention systems, to more effectively study the complex interactions between aging, cognitive status, and HIV infection on neural oscillations in these regions. Of particular interest would be studies investigating the impact of these factors on fronto-visual functional connections that are known to be formed during visuospatial attention processing.13,14,24,49–52
This study provides the first evidence of an interaction between age and cognitive status on neural activity in adults infected with HIV. More specifically, we found that the age-related trajectory of occipito-parietal alpha activity differs in those with HAND compared with both unimpaired HIV-infected adults and uninfected controls. This is significant, as parieto-occipital alpha oscillations are perhaps the most widely studied and behaviorally relevant of the visual population–level neural responses. Furthermore, occipital gamma responses initially appeared to exhibit a similar age-by-cognitive status interaction in those with HAND, but this relationship was ultimately found to result from the length of HIV infection.
Intriguingly, the same occipito-parietal responses found to interact with age and cognitive status in this study were also aberrant in our previous study of occipital neural dynamics in HAND.2 These spectrally specific responses are often implicated in higher-order cognitive processing, such as selective attention and working memory, and future studies should investigate the age-by-cognitive status interaction on the oscillatory neural responses serving these higher-order processes. Indeed, recent evidence suggests that attention- and working memory–related alpha oscillations are aberrant in HAND,4,53 making the investigation of related aging effects all the more imperative. In addition, although this study marks one of the largest MEG studies of HIV infection and HAND to date, an even larger group of cognitively impaired HIV-infected participants would have facilitated the examination of potentially linear effects of HAND severity. Future studies might focus specifically on larger cohorts of participants with HAND to tease apart this kind of relationship. Regardless, these findings provide compelling new evidence that visual neural responses are differentially affected by aging in HIV-infected adults with and without cognitive impairments and open up key new questions for the neuroHIV community.
Acknowledgment
The authors thank the participants for volunteering to participate in the study, as well as our staff and local collaborators for their contributions to the work. They also specifically thank Nichole Knott for extensive help with the MEG recordings and the MRI scanning. Finally, they acknowledge the enormous contribution of Kevin R. Robertson, PhD, Professor of Neurology and Director of the AIDS Neurological Center at the University of North Carolina at Chapel Hill. Dr. Robertson designed and analyzed all the neuropsychological testing and sadly died during the conduct of the study.
Glossary
- HAND
HIV-associated neurocognitive disorder
- cART
combined antiretroviral treatment
- ANI
asymptomatic neurocognitive impairment
- MND
mild neurocognitive disorder
- HAD
HIV-associated dementia
- RT
reaction time
Appendix. Authors

Study funding
Supported by the NIH (R01-MH103220, R01-MH116782, R01-MH118013, P30-MH062261, and F31-AG055332) and the NSF (#1539067).
Disclosure
This research was supported by grants R01-MH103220 (TWW), R01-MH116782 (TWW), R01-MH118013 (TWW), P30-MH062261 (HSF), and from the NIH F31-AG055332 (AIW), and grant 1539067 from the National Science Foundation (TWW). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript. B.R. Groff reports no disclosures. A.I. Wiesman is funded by NIH grant F31-AG055332. M.T. Rezich and J. O'Neill report no disclosures. K.R. Robertson is deceased; disclosures are not included for this author. H.S. Fox is funded by NIH grant P30-MH062261. S. Swindells receives research grants to her institution from ViiV Healthcare. T.W. Wilson is funded by NIH grants R01-MH103220, R01-MH116782, and R01-MH118013 and by NSF grant 1539067. Go to Neurology.org/NN for full disclosures.
References
- 1.Tozzi V, Balestra P, Murri R, et al. Neurocognitive impairment influences quality of life in HIV-infected patients receiving HAART. Int J STD AIDS 2004;15:254–259. [DOI] [PubMed] [Google Scholar]
- 2.Wiesman AI, O'Neill J, Mills MS, et al. Aberrant occipital dynamics differentiate HIV-infected patients with and without cognitive impairment. Brain 2018;141:1678–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson TW, Fox HS, Robertson KR, et al. Abnormal MEG oscillatory activity during visual processing in the prefrontal cortices and frontal eye-fields of the aging HIV brain. PLoS One 2013;8:e66241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lew BJ, McDermott TJ, Wiesman AI, et al. Neural dynamics of selective attention deficits in HIV-associated neurocognitive disorder. Neurology 2018;91:e1860–e1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aylward EH, Brettschneider PD, McArthur JC, et al. Magnetic resonance imaging measurement of gray matter volume reductions in HIV dementia. Am J Psychiatry 1995;152:987–994. [DOI] [PubMed] [Google Scholar]
- 6.Stout JC, Ellis RJ, Jernigan TL, et al. Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. HIV Neurobehavioral Research Center Group. Arch Neurol 1998;55:161–168. [DOI] [PubMed] [Google Scholar]
- 7.Ances BM, Hammoud DA. Neuroimaging of HIV-associated neurocognitive disorders (HAND). Curr Opin HIV AIDS 2014;9:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heindel WC, Jernigan TL, Archibald SL, Achim CL, Masliah E, Wiley CA. The relationship of quantitative brain magnetic resonance imaging measures to neuropathologic indexes of human immunodeficiency virus infection. Arch Neurol 1994;51:1129–1135. [DOI] [PubMed] [Google Scholar]
- 9.Chang L, Speck O, Miller EN, et al. Neural correlates of attention and working memory deficits in HIV patients. Neurology 2001;57:1001–1007. [DOI] [PubMed] [Google Scholar]
- 10.Chang L, Tomasi D, Yakupov R, et al. Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol 2004;56:259–272. [DOI] [PubMed] [Google Scholar]
- 11.Masters MC, Ances BM. Role of neuroimaging in HIV-associated neurocognitive disorders. Semin Neurol 2014;34:89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiesman AI, Wilson TW. The impact of age and sex on the oscillatory dynamics of visuospatial processing. Neuroimage 2019;185:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiesman AI, Heinrichs-Graham E, Proskovec AL, McDermott TJ, Wilson TW. Oscillations during observations: dynamic oscillatory networks serving visuospatial attention. Hum Brain Mapp 2017;38:5128–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiesman AI, Mills MS, McDermott TJ, Spooner RK, Coolidge NM, Wilson TW. Polarity-dependent modulation of multi-spectral neuronal activity by transcranial direct current stimulation. Cortex 2018;108:222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007;69:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan battery: demographically adjusted neuropsychological norms for African American and Caucasian adults. Psychol Assess Resour 2004. [Google Scholar]
- 17.Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 2006;51:1759–1768. [DOI] [PubMed] [Google Scholar]
- 18.Ernst MD. Permutation methods: a basis for exact inference. Stat Sci 2004;19:676–685. [Google Scholar]
- 19.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 2007;164:177–190. [DOI] [PubMed] [Google Scholar]
- 20.Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci USA 2001;98:694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Embury CM, Wiesman AI, Proskovec AL, et al. Neural dynamics of verbal working memory processing in children and adolescents. Neuroimage 2019;185:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheeringa R, Fries P, Petersson KM, et al. Neuronal dynamics underlying high- and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron 2011;69:572–583. [DOI] [PubMed] [Google Scholar]
- 23.Klimesch W. α-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci 2012;16:606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiesman AI, Groff BR, Wilson TW. Frontoparietal networks mediate the behavioral impact of alpha inhibition in visual cortex. Cereb Cortex 2019;29:3505–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiesman AI, Wilson TW. Alpha frequency entrainment reduces the effect of visual distractors. J Cogn Neurosci 2019:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertrand O, Tallon-Baudry C. Oscillatory gamma activity in humans: a possible role for object representation. Int J Psychophysiol 2000;38:211–223. [DOI] [PubMed] [Google Scholar]
- 27.Vidal JR, Chaumon M, O'Regan JK, Tallon-Baudry C. Visual grouping and the focusing of attention induce gamma-band oscillations at different frequencies in human magnetoencephalogram signals. J Cogn Neurosci 2006;18:1850–1862. [DOI] [PubMed] [Google Scholar]
- 28.Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci 2009;29:15721–15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Başar E, Basar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol 2001;39:241–248. [DOI] [PubMed] [Google Scholar]
- 30.Başar-Eroglu C, Başar E, Demiralp T, Schürmann M. P300-response: possible psychophysiological correlates in delta and theta frequency channels. A review. Int J Psychophysiol 1992;13:161–179. [DOI] [PubMed] [Google Scholar]
- 31.Womelsdorf T, Fries P, Mitra PP, Desimone R. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature 2006;439:733–736. [DOI] [PubMed] [Google Scholar]
- 32.Muthukumaraswamy SD, Singh KD. Visual gamma oscillations: the effects of stimulus type, visual field coverage and stimulus motion on MEG and EEG recordings. Neuroimage 2013;69:223–230. [DOI] [PubMed] [Google Scholar]
- 33.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev 2009;19:152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson PM, Dutton RA, Hayashi KM, et al. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci 2005;102:15647–15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson KA, Cherry CL, Bell JE, McLean CA. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am J Pathol 2011;179:1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plude DJ, Doussard-Roosevelt JA. Aging, selective attention, and feature integration. Psychol Aging 1989;4:98–105. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins L, Myerson J, Joerding JA, Hale S. Converging evidence that visuospatial cognition is more age-sensitive than verbal cognition. Psychol Aging 2000;15:157–175. [DOI] [PubMed] [Google Scholar]
- 38.Curran T, Hills A, Patterson MB, Strauss ME. Effects of aging on visuospatial attention: an ERP study. Neuropsychologia 2001;39:288–301. [DOI] [PubMed] [Google Scholar]
- 39.Moffat SD, Elkins W, Resnick SM. Age differences in the neural systems supporting human allocentric spatial navigation. Neurobiol Aging 2006;27:965–972. [DOI] [PubMed] [Google Scholar]
- 40.Baccini M, Paci M, Del Colletto M, Ravenni M, Baldassi S. The assessment of subjective visual vertical: comparison of two psychophysical paradigms and age-related performance. Atten Percept Psychophys 2014;76:112–122. [DOI] [PubMed] [Google Scholar]
- 41.Bigelow RT, Semenov YR, Trevino C, et al. Association between visuospatial ability and vestibular function in the baltimore longitudinal study of aging. J Am Geriatr Soc 2015;63:1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ances BM, Vaida F, Yeh MJ, et al. HIV infection and aging independently affect brain function as measured by functional magnetic resonance imaging. J Infect Dis 2010;201:336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr 2012;59:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valcour V, Paul R, Neuhaus J, Shikuma C. The effects of age and HIV on neuropsychological performance. J Int Neuropsychol Soc 2011;17:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wendelken LA, Valcour V. Impact of HIV and aging on neuropsychological function. J Neurovirol 2012;18:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology 2013;80:1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Womelsdorf T, Fries P. Neuronal coherence during selective attentional processing and sensory-motor integration. J Physiol Paris 2006;100:182–193. [DOI] [PubMed] [Google Scholar]
- 48.Van Der Werf J, Jensen O, Fries P, Medendorp WP. Gamma-band activity in human posterior parietal cortex encodes the motor goal during delayed prosaccades and antisaccades. J Neurosci 2008;28:8397–8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szczepanski SM, Crone NE, Kuperman RA, Auguste KI, Parvizi J, Knight RT. Dynamic changes in phase-amplitude coupling facilitate spatial attention control in fronto-parietal cortex. Plos Biol 2014;12:e1001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hipp JF, Engel AK, Siegel M. Oscillatory synchronization in large-scale cortical networks predicts perception. Neuron 2011;69:387–396. [DOI] [PubMed] [Google Scholar]
- 51.Fellrath J, Mottaz A, Schnider A, Guggisberg AG, Ptak R. Theta-band functional connectivity in the dorsal fronto-parietal network predicts goal-directed attention. Neuropsychologia 2016;92:20–30. [DOI] [PubMed] [Google Scholar]
- 52.Kwon S, Watanabe M, Fischer E, Bartels A. Attention reorganizes connectivity across networks in a frequency specific manner. Neuroimage 2017;144:217–226. [DOI] [PubMed] [Google Scholar]
- 53.Wilson TW, Proskovec AL, Heinrichs-Graham E, et al. Aberrant neuronal dynamics during working memory operations in the aging HIV-infected brain. Sci Rep 2017;7:41568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available via the corresponding author on reasonable request and are being made publicly available via the National NeuroAIDS Tissue Consortium database on completion of the study.









