Abstract
Objective
To describe 2 cases from a single academic institution of improvement in stiff-person syndrome (SPS) symptoms during pregnancy and to review the clinical outcomes of SPS in 6 additional pregnancies described in the literature.
Methods
Evaluation of clinical symptoms and treatment changes of disease state during pregnancy.
Results
Seven patients with 9 pregnancies are described in women with a diagnosis of SPS. Six of 7 (86%) women were positive for glutamic acid decarboxylase (GAD65) antibody. In 5 of 9 (56%) pregnancies, symptomatic medications (antispasmodics) were significantly reduced with stabilization or improvement in symptoms through pregnancy. Nine live, healthy pregnancies resulted. All 7 (100%) women experienced worsening of symptoms after the birth of their children, and symptomatic therapies were resumed and/or increased.
Conclusions
The immune pathogenesis of SPS continues to be explored. Immunomodulatory shifts during pregnancy may influence changes of clinical SPS symptoms and provide insight into the unique pathogenesis of SPS. Some women with SPS may be able to reduce symptomatic medications related to clinical improvement during pregnancy. Women with SPS may safely carry pregnancies to term, delivering healthy and unaffected babies.
Stiff-person syndrome (SPS) is a rare neuroimmunologic condition characterized by severe, debilitating spasms of axial and limb muscles.1 The symptoms may be triggered or exacerbated by environmental factors, including unexpected loud noises or tactile stimulation (startle response), exposure to cold weather, and anxiety-provoking situations (agoraphobia). The prevalence of SPS is approximately 1–2 in a million persons with women being affected more commonly at rates of up to 2:1 compared with men.1 SPS is speculated to be a humorally mediated immune process and/or cell-mediated immune process. Antibodies against glutamic acid decarboxylase (anti-GAD65 Ab) are found in the serum in up to 80% of patients with classic SPS.1–3 However, it is unclear whether these antibodies are truly pathogenic in all cases of SPS because the antibody titer does not seem to correlate with severity of disease or treatment response, and these antibodies can be seen in other conditions (diabetes mellitus and thyroid diseases, which may demonstrate lower positive antibody titers than in SPS).3 Hence, it has been suggested that SPS may also involve nonantibody-dependent cellular immune functions; the presence of intrathecal GAD65-specific CD4+ T cells in patients with SPS supports this,4,5 although the pathogenic role of T cells in SPS is yet to be elucidated.
Pregnancy presents a unique state in which to study autoimmune conditions. The immunomodulatory effects of pregnancy hormones, particularly estradiol, have been described in several autoimmune diseases including rheumatoid arthritis and MS.6,7 Estradiol induces a shift away from Th1-inflammatory cytokines toward anti-inflammatory Th2-cytokines, and this Th1/Th2 shift appears to peak in the 2nd and 3rd trimesters.6 Conditions that are Th1-mediated tend to demonstrate improvement in the 2nd and 3rd trimesters of pregnancy, with worsening of disease after delivery. Despite the known female gender prevalence, little is known regarding the effects of pregnancy in SPS.
Methods
Studies and case reports were systematically identified in PubMed, Google Scholar, and EMBASE using the key terms “Stiff Person Syndrome,” “Stiff Man Syndrome,” and “Pregnancy”; 18 articles were identified, and 5 relevant articles were included for review. Two patients within our Stiff Person Syndrome Center at the Johns Hopkins Hospital with a diagnosis of SPS before pregnancy were also identified and included after chart review.
Data availability
Anonymized data are available to qualified investigators on request for the purposes of replicating procedures or results by contacting the corresponding author.
Johns Hopkins case reports
Case 1
A 23-year-old woman presented for evaluation of severe, intermittent spasms, and stiff gait that was triggered by startle, bright lights, and temperature extremes. Before pregnancy, she had a prolonged hospitalization for encephalopathy, intractable spasms, and severe torso rigidity; she received IV immunoglobulin (IVIG) during the hospitalization with improvement. Serum anti-GAD65 Ab obtained during her hospitalization was elevated at 1017 U/mL (normal <5 U/mL, Quest Diagnostics). One year later, her severe spasms were controlled with diazepam (30 mg 3 times a day) and baclofen (20 mg 4 times a day) although her torso rigidity and mobility were still problematic. Subsequently, she became pregnant and noted marked improvements in her ongoing SPS symptoms during the second and third trimester. Repeat serum anti-GAD65 Ab during pregnancy was >256,000 U/mL. Given her improvement during pregnancy, she was able to wean her antispasmodic therapies to baclofen 20 mg and diazepam 10 mg daily. Within a couple of weeks after vaginal delivery of a healthy preterm baby boy at 35 weeks gestational age (GA), the patient reported an increase in her SPS symptoms and triggers (startle and agoraphobia) which required increasing baclofen to 20 mg 3 times a day and diazepam 10 mg twice a day. She also started gabapentin 600 mg twice a day for neuropathic pain. At her 4-month postpartum neurology follow-up, she reported a decrease in the frequency of her startle spasms, rigidity, and agoraphobia.
Case 2
A 27-year-old woman was diagnosed with SPS after developing persistent severe, painful limb, and girdle spasms along with torso rigidity and back pain. Initial anti-GAD65Ab was positive with levels beyond the limit of detection but without endpoint titers obtained. She was treated with multiple antispasmodics, including baclofen, diazepam, and lorazepam (dosing regimens unknown), with little improvement. She subsequently received several months of IV immunoglobulin (IVIG) therapy, which provided only transient symptomatic improvement; therefore, IVIG was eventually discontinued. Over the next few years, she had 2 pregnancies. She delivered one healthy singleton baby girl and subsequently had one twin pregnancy with intrauterine fetal demise of one twin at 32 weeks, requiring caesarean delivery of the second, healthy twin. During both pregnancies, the patient reported subjective improvement in her pain and spasms, which required a fewer number of daily medications for breakthrough symptoms (clonazepam 0.5 mg twice daily and as-needed lorazepam at 1 mg). Within few days of delivery, she had significant worsening of her spasms, rigidity, and diffuse body pain. These symptoms persisted in a constant fashion for several weeks, which required increasing doses of clonazepam (up to 2 mg 3 times daily). At the time, she presented for re-evaluation; after the birth of her children, anti-GAD65 Ab endpoints were >3,500 U/mL on 2 occasions (normal <5 U/mL, Quest Diagnostics). Her symptoms were moderately well controlled several months later with clonazepam 1.5 mg twice daily, pregabalin 100 mg twice daily, and the use of transcutaneous electrical nerve stimulation unit.
Review of literature
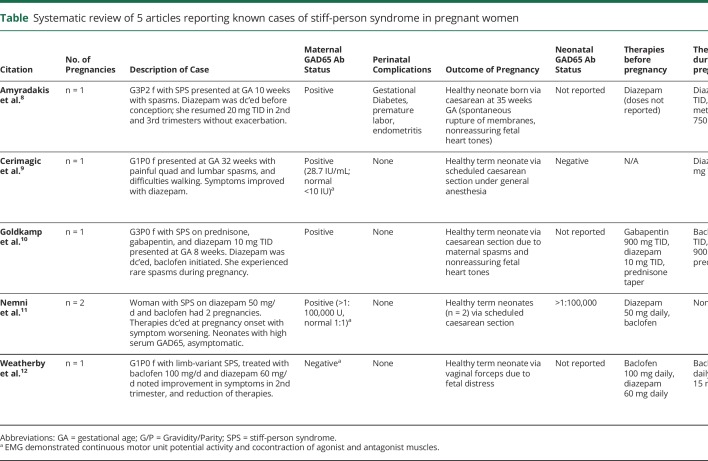
Five publications report 6 pregnancies in 5 women with SPS or limb-variant SPS (SLS) (table). Four of these patients were diagnosed before conception, and one patient was diagnosed with SLS during the third trimester of pregnancy. Four of 5 patients (80%) were noted to have positive anti-GAD65 Ab. One woman with negative anti-GAD65 Ab demonstrated EMG findings consistent with SPS: continuous motor unit fiber activity and cocontraction of agonist and antagonist muscles. None of the women received immunomodulatory treatments during pregnancy, and only one had received immunomodulatory therapy (IVIG) before pregnancy. In 2 of 6 pregnancies (33%), preconception symptomatic therapies were reduced or discontinued with ultimate stabilization of symptoms; in 2 pregnancies (33%), prepregnancy therapies were discontinued completely with some worsening of symptoms; in 2 pregnancies (33%), diazepam was initiated in the 2nd or 3rd trimester with improvement of symptoms. All 6 pregnancies (100%) resulted in live births; 5 of 6 pregnancies (83%) resulted in full-term babies. Five pregnancies were delivered through caesarean section: 2 of these were because of nonreassuring fetal heart tones and increased maternal spasms despite an epidural and 3 were scheduled caesarean sections. One infant delivered through vaginal delivery experienced nonreassuring fetal heart tones and was delivered with forceps assistance. The mother of this infant experienced increased spasms related to pain from episiotomy. In all 6 pregnancies (100%), the postpartum course required reinitiation or continuation of benzodiazepines, with 4 of 6 (67%) requiring additional therapies. Two infants born to a mother with high positive anti-GAD65 Ab titer (>1:100,000) experienced asymptomatic positive anti-GAD65 Ab titers (>1:100,000), with subsequent normalization of antibodies by 24 months of age. Clinical information and antibody titers are not available in the remaining 4 infants.
Table.
Systematic review of 5 articles reporting known cases of stiff-person syndrome in pregnant women
Discussion
Here, we present 2 cases of anti-GAD65-associated SPS in women who experienced symptomatic improvement during 3 pregnancies. Our single institution experience and review of the literature suggest that pregnancy may be associated with an improvement in clinical symptoms of SPS after the first trimester. This may be of special interest to patients and providers desiring to decrease benzodiazepines toward the end of pregnancy to avoid neonatal dependence at delivery.
Our experience of improvement of SPS symptoms during pregnancy is also supported by the case reported by Weatherby et al.,12 who noted improvement in symptoms in a woman whose diazepam and baclofen were significantly reduced during the second trimester of pregnancy. The experiences of Amyradakis and Goldkamp suggest that symptoms of SPS may worsen in the first trimester of pregnancy, requiring reinitiation of therapy with stabilization of disease throughout the second and third trimester.8,10 However, these cases warrant questioning whether improved symptoms later in pregnancy is related to immune pathogenesis of the disease vs maintenance symptomatic treatment. The experience of Nemni et al.11 describing worsening of symptoms with complete discontinuation of therapies suggests that the disease remains at least somewhat active during pregnancy and highlights that some patients may benefit from continued symptomatic therapy throughout pregnancy.
Serum elevations in estradiol during pregnancy shift cytokine production by decreasing inflammatory Th1 cells and increasing anti-inflammatory Th2 and Th17 cells. This shift away from Th1-mediated immunity toward Th2 reduces proinflammatory cytokines (IFNγ, IL2, TNFα) and increases anti-inflammatory cytokines (IL-4,5,10,13).13 In healthy patients, this shift confers protection to the developing fetus because pregnancy is a state of constant bidirectional flow between maternal immunity and a nonself-antigen (the fetus). For patients with autoimmune diseases mediated by predominately Th1 cellular immunity (rheumatoid arthritis, MS), this shift may also confer relative protection to the mother from their autoimmune disease. In particular, protection against these diseases is seen during the 2nd and 3rd trimesters, when estradiol peaks.6,7
Although SPS has been speculated to be a humorally mediated autoimmune disease associated with anti-GAD65 Abs, the pathogenicity of this antibody, directed at a cytoplasmic antigen, has been questioned. A detailed discussion of anti-GAD65 Abs is beyond the scope of this review, yet the realization that these may not be pathogenic in all conditions (diabetes mellitus I), has led to rising interest in the role of antibody-independent functions of B-cells in SPS, and further, the role of cellular immunity in this disorder. The putative pathogenic role of T cells in SPS has been supported by the association of SPS with HLA DQB1*020114 and further by the presence of specific GAD65-epitope binding CD4+ T cells in blood samples of patients with SPS.5 It has subsequently been demonstrated that the phenotype of intrathecally produced GAD65-specific T cells favored a mixed Th1 and Th1/Th2 profiles.4 This latter point is particularly important when considering clinical changes of SPS in pregnant women.
When offering guidance to patients and their practitioners, we note most reported pregnancies in women with SPS were carried safely to term, with few or no complications to the mother or child. Our experience suggests that vaginal delivery may be safe in this population, although fetal distress during labor may lead to a caesarean delivery. Mothers may experience transient increase in spasms during labor and immediately after delivery, perhaps related to the increase of stress of labor and pain related to uterine contractions. Worsening of symptoms immediately postpartum may require increase in antispasmodics in this period. Whether the latter is related to immune shifts intrapartumand postpartum or simply the stress surrounding labor remains unknown. A major limitation of this review is the lack of serum immunologic data regarding potential shifts in Th1 and Th2 subsets; measuring Th1 and Th2 subsets in serum and CSF during the latter 2 trimesters of pregnancy may corroborate the current hypothesis, which is primarily focused on the clinical outcomes of mothers with SPS.
Although 2 of the infants in this review demonstrated asymptomatic high titers of serum anti-GAD65 Ab after delivery, further studies regarding passive transfer of this antibody in infants may provide insight into the factors that induce a pathogenic antibody. Collection of neonatal serologic data and tracking clinical outcomes may contribute to the understanding of passive transfer of anti-GAD65 Ab, as reported in 2 of the infants.11 The observation of SPS symptoms temporally improving with fluctuations in pregnancy hormones may support the role of Th1-mediated cellular immunity in these patients. Monitoring larger cohorts of women with SPS who may desire pregnancy would provide valuable information regarding appropriate disease management during pregnancy.
Glossary
- GA
gestational age
- SPS
stiff-person syndrome
Appendix. Authors
Study Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
This manuscript has not been published and is not under consideration for publication elsewhere. M. E. Esch has no conflicts of interest to disclose. S. D. Newsome has received funding from scientific advisory board meetings with Biogen, Genentech, Celgene, and EMD Serono; he has served as an advisor for Gerson Lehrman Group and BioIncept; a clinical adjudication committee member for a MedDay Pharmaceuticals clinical trial; he has received grant/research funding (paid directly to institution) from Biogen, Genentech, Department of Defense, National MS Society, and the Patient-Centered Outcomes Research Institute. Go to Neurology.org/NN for full disclosures.
References
- 1.Dalakas MC, Fujii M, Li M, McElroy B. The clinical spectrum of anti-GAD antibody-positive patients with stiff-person syndrome. Neurology 2000;55:1531–1535. [DOI] [PubMed] [Google Scholar]
- 2.Holmoy T, Geis C. The immunological basis for treatment of stiff person syndrome. J Neuroimmunology 2011;231:55–60. [DOI] [PubMed] [Google Scholar]
- 3.Newsome SD. Other Proven and Putative Autoimmune Disorders of the CNS: Anti-GAD Associated Neurological Disorders, Stiff-Person Syndrome and Progressive Encephalopathy With Rigidity and Myoclonus (PERM). Neurobiology of Disease. In: Johnston MV, Adams HP, Fatemi A, editors. 2nd ed New York, NY: Oxford Universtiy Press; 2016:675–682. [Google Scholar]
- 4.Skorstad G, Hestvik A, Vartdal F, Holmoy T. Cerebrospinal fluid T cell responses against glutamic acid decarboxylase 65 in patients with stiff person syndrome. J Autoimunity 2009;32:1. [DOI] [PubMed] [Google Scholar]
- 5.Lohmann T, Londei M, Hawa M, Leslie RD. Humoral and cellular autoimmune responses in stiff person syndrome. Ann N Y Acad Sci 2003;998:215–222. [DOI] [PubMed] [Google Scholar]
- 6.Doria A, Iaccarino L, Arienti S, et al. . Th2 immune deviation induced by pregnancy: the two faces of autoimmune rheumatic diseases. Reprod Toxicol 2006;22:234–241. [DOI] [PubMed] [Google Scholar]
- 7.Confavreaux C, Hutchinson M, Hours M, et al. . The pregnancy in multiple sclerosis Group. Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med 1998;339:285–291. [DOI] [PubMed] [Google Scholar]
- 8.Amyradakis G, Carlan SJ, Bhullar A, Eastwood J. Pregnancy and stiff person syndrome. Am J Med 2012;125:3. [DOI] [PubMed] [Google Scholar]
- 9.Cerimagic D, Bilic E. Stiff-person syndrome first manifesting in pregnancy. Gynecol Obstet Invest 2009;67:134–136. [DOI] [PubMed] [Google Scholar]
- 10.Goldkamp J, Blaskiewicz R, Myles T. Stiff person syndrome and pregnancy. Obstet Gynecol 2011;118:454–457. [DOI] [PubMed] [Google Scholar]
- 11.Nemni R, Caniatti LM, Gironi M, Bazzigaluppi E, De Grandis D. Stiff person syndrome does not always occur with maternal passive transfer of GAD65 antibodies. Neurology 2004;62:2101–2102. [DOI] [PubMed] [Google Scholar]
- 12.Weatherby SJM, Wolner P, Clarke CE. Pregnancy in stiff-limb syndrome. Mov Disord 2004;19:852–854. [DOI] [PubMed] [Google Scholar]
- 13.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 1993;14:353–356. [DOI] [PubMed] [Google Scholar]
- 14.Pugliese A, Solimena M, Awdeh ZL, et al. . Association of HLA-DQB1*0201 with stiff-man syndrome. J Clin Endocrinol Metab 1993;77:1550–1553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data are available to qualified investigators on request for the purposes of replicating procedures or results by contacting the corresponding author.