Abstract
Objective
To explore the repertoire of glycan-specific immunoglobulin G (IgG) antibodies in treatment-naive patients with relapsing-remitting multiple sclerosis (RRMS).
Methods
A systems-level approach combined with glycan array technologies was used to determine specificities and binding reactivities of glycan-specific IgGs in treatment-naive patients with RRMS compared with patients with noninflammatory and other inflammatory neurologic diseases.
Results
We identified a unique signature of glycan-binding IgG in MS with high reactivities to the dietary xenoglycan N-glycolylneuraminic acid (Neu5Gc) and the self-glycan N-acetylneuraminic acid (Neu5Ac). Increased reactivities of serum IgG toward Neu5Gc and Neu5Ac were additionally observed in an independent, treatment-naive cohort of patients with RRMS.
Conclusion
Patients with MS show increased IgG reactivities to structurally related xenogeneic and human neuraminic acids. The discovery of these glycan-specific epitopes as immune targets and potential biomarkers in MS merits further investigation.
CNS tissue damage in patients with multiple sclerosis (MS) is mediated by both cellular and humoral immune factors, and clonal T- and B-cell expansions within MS lesions and the CSF suggest that the pathogenic immune responses in MS are driven by distinct, yet incompletely defined antigens.1 A pathogenic role for antibodies is further supported by the marked deposition of immunoglobulin G (IgG) at least in a subset of demyelinating MS lesions.2
Glycans, polymers of glycosidically linked sugars, represent one of the most basic cellular components of mammals and other organisms and exist as free glycan entities as well as being covalently attached to proteins or lipids. During the last decade, glycans have become increasingly recognized as participants in neural cell interactions as well as in myelin formation and maintenance. Some glycan structures, attached to proteins and expressed on the surface of neuronal and glial cells, are specifically enriched in the mammalian brain and have pivotal functions in nervous system development and regeneration following CNS tissue injury.3
Despite the paradigm that glycans are T cell–independent antigens and the observation that antibodies recognizing carbohydrate epitopes in chronic immune-mediated neuropathies such as multifocal motor neuropathy are frequently immunoglobulin M isotypes, there is evidence that CD4+ T cells are involved in the generation of carbohydrate-specific IgG antibodies following glycovaccination,4 and switched carbohydrate-specific IgG antibodies are universally found in humans.5,6 Furthermore, carbohydrate epitopes in conjunction with carrier protein-derived peptides can bind major histocompatibility class II molecules and stimulate glycan-specific CD4+ T cells to produce interleukins 2 and 4—cytokines essential for providing T-cell help to antibody-producing B cells.7. Here, we used a systems-level approach combined with glycan microarray technologies to evaluate the repertoire of carbohydrate-specific IgG antibodies in treatment-naive patients with relapsing-remitting MS (RRMS).
Methods
Standard protocol approvals, registrations, and patient consents
All patients included in this study were enrolled at the Department of Neurology, University Hospital Basel, Switzerland. Institutional review board approval was granted by the local ethics committee, and participants provided written informed consent for participation. All patients with MS were treatment naive and had relapsing-remitting disease. Serum and CSF samples were collected and stored at −80°C following standardized procedures.
Glycan microarray
IgG derived from serum and CSF samples were purified using Protein G Sepharose 4 Fast Flow (GE Healthcare, Opfikon, Switzerland) according to the manufacturer's instruction, dialyzed in phosphate-buffered saline (PBS) (Sigma-Aldrich Chemie GmbH, Buchs, Switzerland), and sterilized by 0.2 μM filtration. Acrylamide gel electrophoresis, Coomassie stainings, and immunoblots were performed to test IgG integrity and purity.8 Purified IgGs derived from patients with MS, noninflammatory neurologic diseases (NIND), and other inflammatory neurological diseases (OIND) were pooled. Pooled samples were adjusted to similar concentrations of IgG molecules as determined by photometry (NanoDrop1000; Thermo Scientific, Basel, Switzerland), subsequently screened for carbohydrate recognition on the Consortium for Functional Glycomics (CFG) array version 5.3, and detected at 50 μg/mL using the anti-human IgG mAb clone HP-6043-Biot (5 μg/mL) coupled to streptavidin-Alexa633 (Invitrogen, Basel, Switzerland). Antibody binding was quantified as relative fluorescence unit (RFU), and the obtained data were evaluated using a systems biology approach, as described in reference 5.
Bio-Plex assay
The Bio-Plex glycan suspension assay was performed as previously described.6 Briefly, end-biotinylated glycopolymers (Laboratory of Carbohydrate Chemistry, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Moscow, Russian Federation) were coupled to fluorescent carboxylated beads with a distinct ratio of red and infrared fluorescent dyes (Bio-Rad Laboratories Inc., Hercules, CA). Antibody diluent (PBS-1% bovine serum albumin; Sigma-Aldrich Chemie GmbH) incorporating 2,000 beads of each region/well (50 μL/well) was added to a 96-well multiscreen HTS filter plate (Millipore Corp., Billerica, MA) previously soaked with 100 μL of antibody diluent for 5 minutes. The plate was washed twice with 100 μL washing buffer (PBS-0.02% Tween 20) using a vacuum manifold (Bio-Rad Laboratories Inc.). Human serum samples were added to wells (in antibody diluent 1:20 [50 μL/well]) and incubated on a shaker for 1 hour at room temperature (RT) in the dark. After incubation, the plate was washed 3 times with washing buffer. Secondary antibodies (R-PE-conjugated goat anti-human IgG H + L; Southern Biotechnology Associates Inc., Birmingham, AL, 25 ng/well) were added and incubated on a shaker for 1 hour at RT in the dark. The plate was washed 3 times with washing buffer, and beads were resuspended and shaken vigorously for 30 seconds in 100 μL of antibody diluent. The plate was analyzed on the Bio-Plex array reader, with which data were acquired in real time, analyzing 50 beads by their median fluorescence intensity using Bio-Plex Manager 6.1 software (Bio-Rad Laboratories Inc.).
Statistical analysis
Pearson correlation matrix, heatmap, and hierarchical clustering were performed using the package gplots from “R” (The R Foundation for Statistical Computing, version 3.0.2 [rdrr.io/cran/gplots/]). For correlation analysis and heatmap construction, negative values were set to zero. To exclude the observations that markedly deviate from the rest of the data, outlier exclusion was performed via the robust regression and outlier removal method (Q = 1%). Statistical significance of the results was reported using 1-way analysis of variance, followed by the Tukey multiple comparison test. A p value <0.05 was considered statistically significant. The asterisks depicted in the figures translate into the following grouping: *p < 0.05, **p < 0.01,***p < 0.001. All quantitative analyses were performed with GraphPad Prism v5.0a or 6.0c for Mac OSX (GraphPad Software, Inc., San Diego, CA).
Data availability
Any data not published within the article will be shared by request from any qualified investigator.
Results
Glycan array analysis indicates unique anti-carbohydrate IgG repertoire in MS
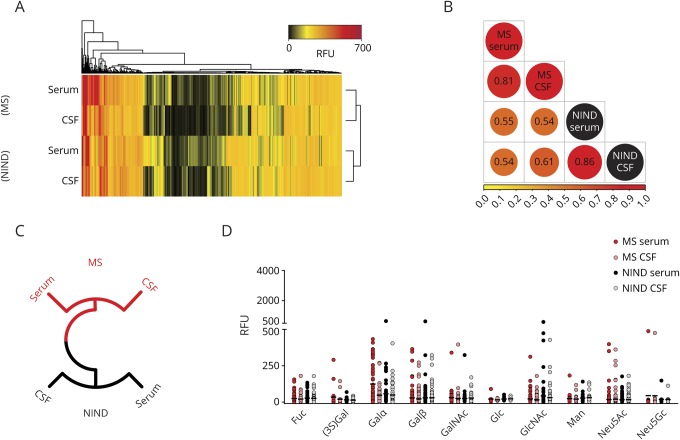
Glycan array technology, a powerful tool to study protein-carbohydrate interactions on a large scale, has recently been used to examine glycan-binding profiles of IgG from healthy donors.5 To interrogate the anti-carbohydrate IgG repertoire in patients with MS, we first purified and pooled IgG from serum and CSF samples derived from 25 treatment-naive patients with RRMS and 30 patients with NIND matched by age and sex (table). Pooled IgG samples were normalized to concentrations of 50 μg/mL5 and screened for binding to 600 distinct glycans using the CFG glycan array version 5.3. Hierarchical dendrogrammed clustering analysis was performed to compute a reactivity matrix of IgG preparations based on sample RFU values,5 as shown in figure 1A. The reactivity matrix indicated that antibody repertoires between patients with MS and NIND were distinct for at least certain glycans (figure 1A). A relatively close relationship between glycan recognition patterns of serum and CSF IgG was observed for patients with MS (r = 0.81) and NIND (r = 0.86), respectively. The repertoire differences between both cohorts were confirmed by Pearson correlation (figure 1B) and radial dendrogram computation (figure 1C). Since the chemical structure of the terminal carbohydrate moiety has been linked to immunogenicity and hence the IgG immune profile in healthy individuals,5 we next determined IgG reactivities to glycans with specific terminal carbohydrates. Although most terminal glycan structures were recognized by both patients with MS and NIND, high reactivities to individual terminal N-acetylneuraminic acid (Neu5Ac) epitopes and the structurally related N-glycolylneuraminic acid (Neu5Gc) were exclusively observed in patients with MS (figure 1D).
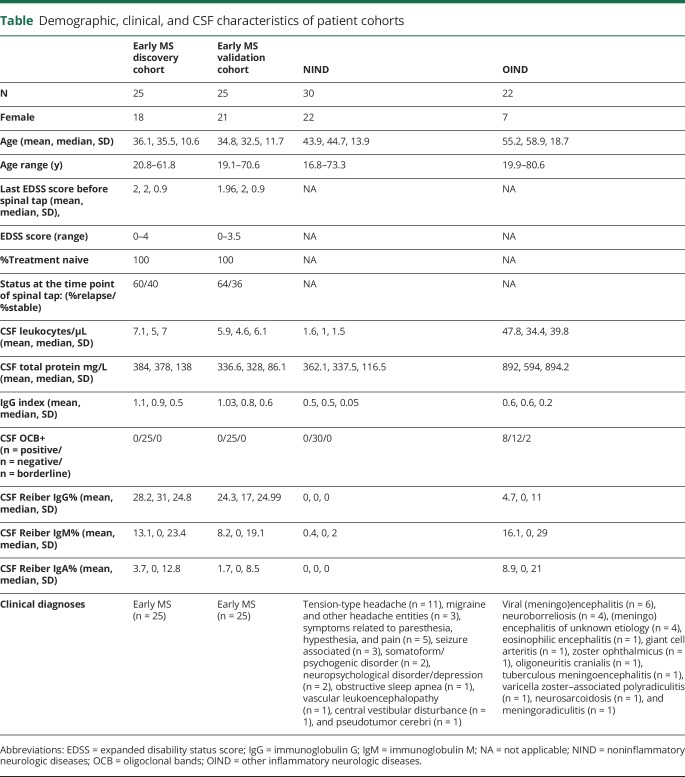
Table.
Demographic, clinical, and CSF characteristics of patient cohorts
Figure 1. Recognition of carbohydrate structures in glycan array version 5.3 by sera and CSF of patients with MS and NIND.
(A) Heatmap of glycan-binding reactivity, clustered by dendrogram algorithm. Columns represent the reactivity of each specific glycan (n = 600) at an immunoglobulin G concentration of 50 μg/mL, and rows represent the immune profile for each patient subgroup (MS, n = 25; NIND, n = 30). (B) Pearson correlation comparison of glycan recognition in sera and CSF among patients. (C) Circlized dendrogram for recognition of carbohydrate antigens based on RFU. Clusters based on recognition similarity are shown in the same color. (D) Recognition of specific terminal carbohydrate moieties by sera or CSF from patients with MS and NIND. Neu5Ac = N-acetylneuraminic acid; Neu5Gc = N-glycolylneuraminic acid; NIND = noninflammatory neurologic disease; RFU = relative fluorescence unit.
Increased IgG reactivity to xenogeneic and human neuraminic acids in MS
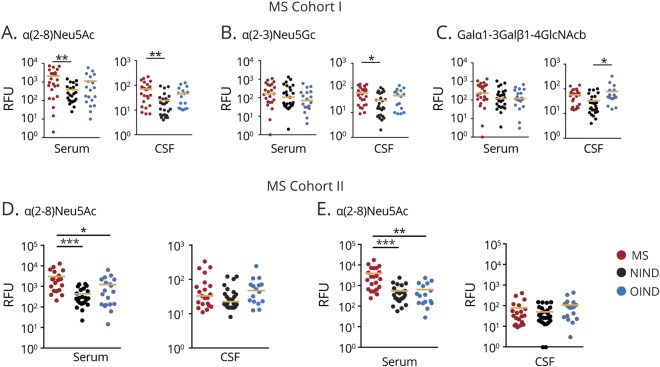
Terminal glycan recognition was further evaluated in individual serum and CSF samples derived from our MS discovery and NIND cohorts using an independent bead-based multiplex glycan suspension assay. To assess whether IgG reactivities are largely determined by inflammation or more specifically related to MS, we additionally recruited a cohort of 22 patients with OIND (table). Based on the results obtained with the CFG glycan array with exclusively increased reactivities to distinct Neu5Ac and Neu5Gc epitopes in MS, we specifically determined IgG responses to (Neu5Ac2-8)3, an α(2–8)-linked homooligomer of Neu5Ac, hereafter termed α(2–8)Neu5Ac, and to the Neu5Gc epitope Neu5Gcα2-3Galβ1-3GlcNAcβ, hereafter termed α(2–3)Neu5Gc. As a reference, we additionally assessed IgG responses to the α(1–3)-linked galactose epitope (Galα1-3Galβ1-4GlcNAcβ) because anti-Gal-α antibodies are frequently detectable in immunocompetent individuals and produced throughout life as a result of continuous antigenic stimulation by carbohydrate antigens on gastrointestinal bacteria of the normal flora.9
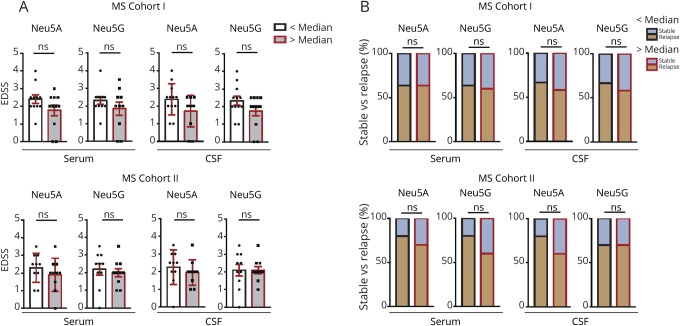
Antibody reactivities toward neuraminic acid epitopes were significantly increased in patients with MS compared with patients with NIND and OIND. Both serum and CSF IgG reactivities toward α(2–8)Neu5Ac and CSF IgG responses toward α(2–3)Neu5Gc were significantly elevated in patients with MS (figure 2, A and B). In contrast, IgG reactivities to the Gal-α epitope were unchanged in patients with MS (figure 2C). To validate increased immunoreactivity to neuraminic acid epitopes in MS, we additionally recruited an independent second cohort of treatment-naive patients with RRMS (table) and reperformed the Bio-Plex assay. In these patients (MS cohort II), serum IgG reactivities to both α(2–8)Neu5Ac and α(2–3)Neu5Gc were increased compared with NIND and OIND, whereas CSF IgG reactivities did not differ significantly (figure 2, D and E), indicating a systemically increased immune response to both epitopes. We observed a strong overlap of IgG reactivities to Neu5Ac and Neu5Gc in individual patients. In cohort I, 73% and 92% of patients with MS with above-median anti-Neu5Ac levels also show above-median anti-Neu5Gc levels in serum and CSF samples, respectively. In cohort II, 90% and 90% of patients with MS with above-median anti-Neu5Ac levels also show above-median anti-Neu5Gc levels in serum and CSF samples, respectively. Patients with high IgG reactivities (above median) did not differ from patients with low (below median) IgG reactivities to Neu5Ac and Neu5Gc epitopes in clinical disease severity as determined by the expanded disability status score and disease activity as defined by having a relapse or being in remission during the time of blood and CSF sampling (figure 3). Altogether, these data demonstrate that the anti-carbohydrate IgG repertoire in patients with MS contains elevated reactivities toward both α(2–8)Neu5Ac and α(2–3)Neu5Gc neuraminic acids.
Figure 2. Increased reactivity to sialic acid–terminated glycan epitopes Neu5Ac and Neu5Gc in patients with MS.
Recognition of α(2–8)Neu5Ac, α(2–3)Neu5Gc, and Galα1-3Galβ1-4GlcNAcβ carbohydrate structures by sera and CSF. patients with MS from discovery cohort I (A–C) and independent cohort II (D and E) compared with patients with other neurologic diseases NIND and OIND as screened by suspension array. Each dot represents an individual patient. Bar represents median. Significant values are reported using 1-way ANOVA, followed by Tukey multiple comparison test. *p < 0.05; **p < 0.01, ***p < 0.001. ANOVA = analysis of variance; Neu5Ac = N-acetylneuraminic acid; Neu5Gc = N-glycolylneuraminic acid; NIND = noninflammatory neurologic disease; OIND = other inflammatory neurologic disease; RFU = relative fluorescence unit.
Figure 3. High vs low IgG reactivities to Neu5Ac and Neu5Gc epitopes in relation to MS clinical disease severity and activity.
Patients with MS were stratified by means of their high IgG reactivities to Neu5Ac and Neu5Gc epitopes (above median vs below median). (A) Above- and below-median groups were compared with regard to the EDSS score and (B) clinical disease activity as defined by having a relapse or being in remission during the time of blood and CSF sampling. Each dot represents an individual patient. SEM is depicted. Statistical analysis was performed via the (A) Mann-Whitney test and (B) Fisher exact test. EDSS = expanded disability status score; IgG = immunoglobulin G; Neu5Ac = N-acetylneuraminic acid; Neu5Gc = N-glycolylneuraminic acid; ns = non significant; SEM = standard error of the mean.
Discussion
Our study is the first investigation to systematically interrogate the carbohydrate-specific IgG repertoire in patients with MS. We found that treatment-naive patients with early MS differ from patients with NIND and OIND in their recognition of terminal carbohydrate moieties with increased reactivities toward the neuraminic acid α(2–8)Neu5Ac and the structurally related α(2–3)Neu5Gc.
The α(2–8)Neu5Ac oligomers constitute the building blocks of α(2–8)polysialic acid (PSA), whose major carrier in vertebrates is the neural cell adhesion molecule (NCAM). PSA is widely expressed in the developing brain, absent in the adult brain, but reexpressed upon CNS injury during which it supports repair mechanisms including cell migration, axon guidance, and synaptic plasticity.3,10 Targeted overexpression of PSA or administration of PSA mimetics improves neuronal regeneration and functional recovery upon spinal cord injuries.11,12 In MS lesions, PSA-NCAM is reexpressed on demyelinated axons but absent from myelinated axons of the periplaque or normal-appearing white matter, possibly in an attempt to achieve neuroprotection following demyelinating injury.10 We infer from these data that IgG specific for α(2–8)Neu5Ac oligomers could also bind to NCAM decorated with α(2–8)Neu5Ac within MS plaques.
The structurally almost identical and Neu5Ac derivative Neu5Gc cannot be synthesized by humans due to a loss-of-function mutations in the gene encoding the sialic acid–modifying enzyme cytidine monophosphate-N-acetylneuraminic acid hydroxylase but is found in most nonhuman mammals and is enriched in red meat from livestock (beef, pork, and lamb).13 Despite the absence of endogenously produced Neu5Gc, experimental studies in humans and in Cmah−/− mice revealed that dietary Neu5Gc can become incorporated into tissues in trace amounts, essentially making Neu5Gc a dietary “xeno-autoantigen”, and transfer of Neu5Gc-specific polyclonal IgG antibodies to Cmah−/− mice fed with Neu5Gc was shown to promote systemic inflammation.13–16 Based on the aforementioned studies, it has been proposed that anti-Neu5Gc antibodies may contribute to the development of various human chronic inflammatory diseases that are not seen in great apes, such as MS.15,17,18 Although healthy diets have been associated with a reduced risk to develop MS,19 studies investigating red meat consumption and risk of MS have been inconclusive so far,20–22 and it is unknown whether and to which extend dietary Neu5Gc can be incorporated into tissues including the CNS. However, given the structural similarity between Neu5Gc and Neu5Ac and the large overlap of reactivities toward both carbohydrate epitopes in individual patients, systemically increased IgG responses to both terminal glycan structures in patients with MS might partly result from cross-reactive antibody species23: exposure to a dietary xenoantigen expressed in peripheral tissues could elicit immune reactivity to a self-antigen, reexpressed upon myelin injury in MS lesions. This scenario does not necessarily depend on intrathecal production or enhanced reactivities of glycan-specific CSF IgG.
Limitations of our study include the small size of the cohorts investigated. Although increased IgG reactivities to α(2–8)Neu5Ac and α(2–3)Neu5Gc were confirmed in an independent cohort of patients with MS, our findings clearly require validation in larger independent studies. Patients with OIND did not differ from patients with NIND in their serum and CSF immunoreactivity profile toward Neu5Ac and Neu5Gc. However, we cannot exclude the possibility that the increased response to the aforementioned epitopes might result from polyclonal B lineage cell stimulation driven by chronic inflammation without being specific for MS. Second, the functional relevance of our findings is hypothetical, and longitudinal studies are required to assess whether immune reactivities to Neu5Ac and Neu5Gc are associated with MS disease activity and progression. Whether IgG specific for neuraminic acid–containing epitopes are germline configured or selected for high affinity during germinal center reactions in patients with MS and healthy individuals requires further investigation. Our findings, therefore, should provide incentive to conduct larger prospective and experimental investigations to examine the functional role of terminal glycan structures as immune targets and potential biomarkers in MS.
Acknowledgment
The authors thank the patients for their continuous cooperation. They thank Alain Despont, DBMR, University of Bern, for performing the Bio-Plex analyses and Alexander Chinarev (Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Science, Moscow, Russia) for synthesizing end-biotinylated glycopolymers.
Glossary
- CFG
Consortium for Functional Glycomics
- IgG
immunoglobulin G
- NCAM
neural cell adhesion molecule
- Neu5Ac
N-acetylneuraminic acid
- Neu5Gc
N-glycolylneuraminic acid
- NIND
noninflammatory neurologic diseases
- OIND
other inflammatory neurologic diseases
- PSA
α(2–8)polysialic acid
- RFU
relative fluorescence unit
- RT
room temperature
- RRMS
relapsing-remitting MS
Appendix. Authors
Study funding
No targeted funding reported.
Disclosure
K.F. Boligan has nothing to disclose. J. Oechtering has received in the last 3 years travel grants from Bayer, Biogen, and Novartis and served on a scientific advisory board for Roche, not related to this work. C.W. Keller, B. Peschke, R. Rieben, and N. Bovin have nothing to disclose. L. Kappos reports grants from Actelion, grants from Almirall, grants from Bayer HealthCare, grants from Biogen, grants from Excemed, grants from Genzyme, grants from df-mp, grants from Merck, grants from Mitsubishi, grants from Novartis, grants from Roche, grants from Pfizer, grants from Receptos, grants from Sanofi, grants from Santhera, grants from Teva, grants from Vianex, grants from Allergan, grants from CSL Behring, other from Neurostatus products, grants from European Union, grants from the Roche Research Foundation, grants from the Swiss Multiple Sclerosis Society, grants from the Swiss National Research Foundation, grants from INNO-Swiss, grants from Baxalta, and grants from Desitin, outside the submitted work during the last 3 years. R.D. Cummings is supported by grant GM62116 to the CFG and grants P41GM103694 and GM098791. J. Kuhle receives speaker fees, research support, and/or served on advisory boards during the last 3 years for ECTRIMS, Swiss Multiple Sclerosis Society, Swiss National Research Foundation, University of Basel, Bayer, Biogen, Genzyme, Merck, Novartis, Roche, and Teva. S. von Gunten's laboratory research is supported by the Swiss National Science Foundation (SNSF) grants 310030_162552 and 310030_184757 and Swiss Cancer League/Swiss Cancer Research grant KFS-3941-08-2016. J.D. Lünemann receives research support from the Swiss National Science Foundation (31003A-169664), the Sassella Foundation, the Hartmann Müller Foundation, the Olga Mayenfisch Foundation, and the Swiss Multiple Sclerosis Society. Go to Neurology.org/NN for full disclosures.
References
- 1.Hohlfeld R, Dornmair K, Meinl E, Wekerle H. The search for the target antigens of multiple sclerosis, part 1: autoreactive CD4+ T lymphocytes as pathogenic effectors and therapeutic targets. Lancet Neurol 2016;15:198–209. [DOI] [PubMed] [Google Scholar]
- 2.Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 2000;47:707–717. [DOI] [PubMed] [Google Scholar]
- 3.Kleene R, Schachner M. Glycans and neural cell interactions. Nat Rev Neurosci 2004;5:195–208. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Rouphael N, Duraisingham S, et al. . Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol 2014;15:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider C, Smith DF, Cummings RD, et al. . The human IgG anti-carbohydrate repertoire exhibits a universal architecture and contains specificity for microbial attachment sites. Sci Transl Med 2015;7:269ra1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jandus P, Frias Boligan K, Smith DF, et al. . The architecture of the IgG anti-carbohydrate repertoire in primary antibody deficiencies (PADs). Blood 2019;134:1941–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med 2011;17:1602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quast I, Keller CW, Maurer MA, et al. . Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J Clin Invest 2015;125:4160–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med 1984;160:1519–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charles P, Reynolds R, Seilhean D, et al. . Re-expression of PSA-NCAM by demyelinated axons: an inhibitor of remyelination in multiple sclerosis? Brain 2002;125:1972–1979. [DOI] [PubMed] [Google Scholar]
- 11.Papastefanaki F, Chen J, Lavdas AA, Thomaidou D, Schachner M, Matsas R. Grafts of Schwann cells engineered to express PSA-NCAM promote functional recovery after spinal cord injury. Brain 2007;130:2159–2174. [DOI] [PubMed] [Google Scholar]
- 12.Saini V, Lutz D, Kataria H, Kaur G, Schachner M, Loers G. The polysialic acid mimetics 5-nonyloxytryptamine and vinorelbine facilitate nervous system repair. Sci Rep 2016;6:26927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tangvoranuntakul P, Gagneux P, Diaz S, et al. . Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA 2003;100:12045–12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedlund M, Padler-Karavani V, Varki NM, Varki A. Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci USA 2008;105:18936–18941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samraj AN, Pearce OM, Läubli H, et al. . A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci USA 2015;112:542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorvignit D, Boligan KF, Relova-Hernández E, et al. . Antitumor effects of the GM3(Neu5Gc) ganglioside-specific humanized antibody 14F7hT against Cmah-transfected cancer cells. Sci Rep 2019;9:9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varki A. Are humans prone to autoimmunity? Implications from evolutionary changes in hominin sialic acid biology. J Autoimmun 2017;83:134–142. [DOI] [PubMed] [Google Scholar]
- 18.'t Hart BA. Why does multiple sclerosis only affect human primates? Mult Scler 2016;22:559–563. [DOI] [PubMed] [Google Scholar]
- 19.Black LJ, Rowley C, Sherriff J, et al. . A healthy dietary pattern associates with a lower risk of a first clinical diagnosis of central nervous system demyelination. Mult Scler 2019;25:1514–1525. [DOI] [PubMed] [Google Scholar]
- 20.Gusev E, Boiko A, Lauer K, Riise T, Deomina T. Environmental risk factors in MS: a case-control study in Moscow. Acta Neurol Scand 1996;94:386–394. [DOI] [PubMed] [Google Scholar]
- 21.Zhang SM, Willett WC, Hernán MA, Olek MJ, Ascherio A. Dietary fat in relation to risk of multiple sclerosis among two large cohorts of women. Am J Epidemiol 2000;152:1056–1064. [DOI] [PubMed] [Google Scholar]
- 22.Black LJ, Bowe GS, Pereira G, et al. . Higher non-processed red meat consumption is associated with a reduced risk of central nervous system demyelination. Front Neurol 2019;10:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leviatan Ben-Arye S, Schneider C, Yu H, et al. . Differential recognition of diet-derived Neu5Gc-neoantigens on glycan microarrays by carbohydrate-specific pooled human IgG and IgA antibodies. Bioconjug Chem 2019;30:1565–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any data not published within the article will be shared by request from any qualified investigator.