Abstract
Acute respiratory distress syndrome is characterized by dyspnea at presentation, tachypnea on physical examination, findings of bilateral infiltration in chest radiography, refractory hypoxia, and high mortality. Although the main treatment approach is to address the underlying disease, there are also pharmacological and nonpharmacological options for supportive treatment. There is currently no pharmacological agent with proven efficacy in this syndrome, and many drugs are being studied for this purpose. One of these is the endothelin receptor antagonist bosentan.
Keywords: ARDS, bosentan, pharmacological treatment
Introduction
Acute respiratory distress syndrome (ARDS) is a sudden-onset condition characterized by dyspnea, refractory hypoxia, pathological signs of diffuse alveolar destruction, and high mortality. Although sepsis and pneumonia frequently play a major role in its etiology, it is caused by many pulmonary and nonpulmonary factors [1]. ARDS is an inflammatory condition involving the disruption of the alveolar–capillary barrier, flooding of protein-rich edema fluid into the alveolar space, and cell recruitment due to immune system stimulation [1, 2]. The main treatment approach for ARDS is to treat the underlying disease. However, there are also pharmacological and nonpharmacological options for supportive treatment. Nonpharmacological options are lung-protective mechanical ventilation, conservative fluid therapy, and prone position. Pharmacological options include drugs, such as inhaled nitric oxide (NO) and corticosteroid [3–6].
Many experimental drugs that reduce inflammation are also used in the treatment of ARDS, one of which is the endothelin-1 (ET-1) receptor antagonist bosentan [7, 8]. Of the four known ET receptors (ETA, ETB1, ETB2, and ETC), bosentan acts on both endothelin A and B receptors [9, 10]. ET-1 is a peptide produced by endothelial cells and plays an important role in lung inflammation. Several studies have demonstrated the proinflammatory effects of ET-1 in the airways [11, 12]. In contrast, ET receptor antagonists attenuate the proinflammatory effects of ET-1 in animal models of airway inflammation [10, 13]. Studies on the anti-inflammatory effect of ET-1 receptor antagonists are ongoing.
Unfortunately, a pharmacological agent that effectively reduces short- and long-term mortality in ARDS has yet to be identified. Various new agents are being investigated, including bosentan, which has both anti-inflammatory and pulmonary arterial pressure-lowering effects.
Pharmacological Therapy in ARDS
Pathophysiology of ARDS
ARDS is a clinical condition that remains poorly understood due to its complex pathogenesis. It involves a disequilibrium between pro- and anti-inflammatory pathways, complement activation, endothelial cell activation, polymorphonuclear neutrophil and macrophage activation (Figures 1, 2), oxidative stress, and transcriptional factor activation [14]. The clinical manifestation of ARDS is a result of diffuse alveolar destruction caused by intense inflammation. Tumor necrosis factor and interleukin (IL) 1 produced in the early phase and the proinflammatory cytokines IL-6 and IL-8 that appear in the later phases of disease induce leukocyte migration to the area. Accumulated leukocytes in the lungs become activated and secrete reactive oxygen species and proteases that damage the capillary endothelium and alveolar epithelium. This disrupts the normal barrier that protects against alveolar edema. Protein leaks from the vascular space into the interstitium, eliminating the osmotic gradient that allows reabsorption. Extravasation to the interstitium exceeds lymphatic capacity, and the alveolar spaces become flooded with a fluid rich in protein and debris. This fluid disrupts surfactant structure and function, leading to alveolar collapse. Intrapulmonary shunting and ventilation–perfusion mismatch cause hypoxemia, and physiological dead space results in increased ventilation (hypercapnia). Interstitial and alveolar edema and atelectasis lead to reduced compliance. In the early phase, decreased lung compliance is associated with interstitial edema and exudation, whereas in the late phase, it is due to widespread interstitial fibrosis. Increased airway resistance is also common in patients with ARDS. Pulmonary arterial pressure is increased by the pathological changes discussed above, as well as hypoxemia and mechanical ventilation. The average pulmonary arterial pressure in patients with ARDS is usually >30 mmHg [15–17].
Figure 1.
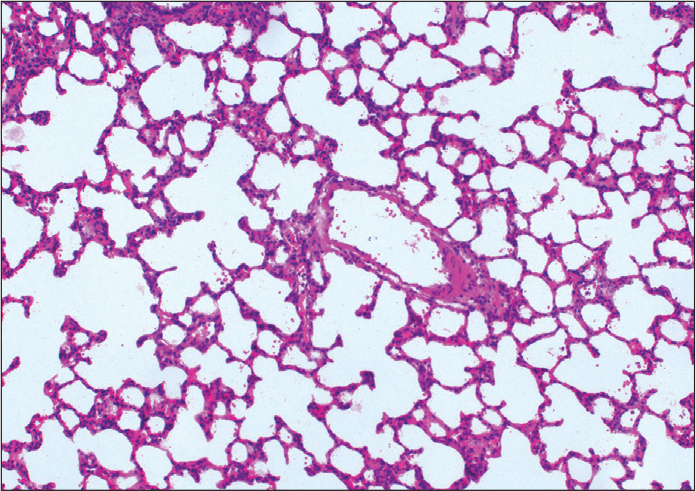
Appearance of normal lung (H&E, ×100).
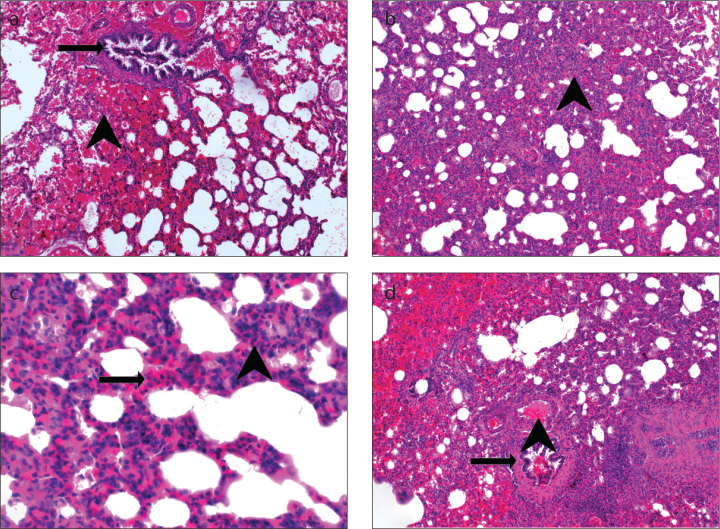
Figure 2. a–d.
Appearance of a lung with ARDS: (a) hemorrhage (arrowhead), terminal bronchiole (arrow) (H&E, ×100); (b) thickening of the interalveolar septa (arrowhead), alveolar filling defects (H&E, ×100); (c) PNL infiltration (arrowhead), MNL infiltration (arrow) (H&E, ×200); (d) vasodilation–congestion (arrowhead), terminal bronchiole (arrow) (H&E, ×100).
The pathological features observed in ARDS are referred to as diffuse alveolar damage. This process includes exudative, proliferative, and fibrotic phases that are generally interrelated and overlapping. The exudative phase usually begins in the first week. This phase is characterized by interstitial–intra-alveolar edema, hemorrhage, and the formation of hyaline membranes in the alveolar ducts. The alveoli are atelectatic and edematous, and the alveolar ducts are dilated. There is widespread destruction of type I cells. Extensive endothelial damage and intravascular fibrin accumulation are common. Days 4–10 are the proliferative phase. In this phase, the accumulated exudate becomes organized and type II cells proliferate, whereas in the alveolar wall, fibroblasts and myofibroblasts proliferate and infiltrate the adjacent fibrinous exudate. Fibroblasts transform the exudate into granulation tissue, which later becomes fibrous tissue with the accumulation of collagen. In the final phase, epithelial cells cover the organized granulation tissue and transform the intra-alveolar exudate into the interstitial tissue. The fibrotic phase manifests with collagenous fibrosis, microcystic honeycombing in some cases, and rarely, bronchopulmonary dysplasia. These changes result in the regeneration of the alveolar basement membrane. In addition to the primary damage described previously, secondary damage occurs in ARDS as a result of mechanical ventilation using high pressure or volume and high oxygen concentration, as well as ventilator-associated pneumonia. This secondary damage is pathologically indistinguishable from the primary damage. Vascular changes also occur during all of these phases, manifesting with thrombotic, fibroproliferative, and obliterative changes from intimal edema to the development of pulmonary hypertension in the terminal phase [1, 15, 16].
Current treatment approach to ARDS
ARDS is primarily managed by identifying the underlying disease and administering appropriate, disease-specific treatment. For instance, good outcomes can be achieved in patients with sepsis-associated ARDS with the use of appropriate antibiotics and source control [18].
Secondary treatment approaches involve supportive therapies. The main supportive method is lung-protective mechanical ventilation. Ventilator-induced lung injury can be an important cause of poor clinical outcomes in patients with ARDS. Therefore, studies are being conducted to determine the appropriate mechanical ventilation strategies to reduce the incidence and severity of ventilator-induced lung injury. Some of these mechanical ventilation strategies include low tidal volume ventilation, open lung ventilation, and high positive end-expiratory pressure (PEEP). Moreover, recruitment maneuvers and PEEP titration are other important mechanical ventilation practices in ARDS [19].
A large randomized trial demonstrated shorter duration of assisted ventilation with a conservative fluid management strategy [20]. This effect may be attributable to avoiding fluid administration after shock reversal [21]. Diuretics and albumin given after shock reversal improved oxygenation and tended to shorten the duration of mechanical ventilation in small randomized trials [22, 23]. However, albumin was not associated with reduced mortality in a larger trial including the general intensive care unit population [24]. In addition, there is evidence that albumin may be harmful in patients with traumatic brain injury [25]. With respect to nutritional support, similar mortality rates were observed with trophic and early full-calorie enteral nutrition [26], whereas potentially unfavorable outcomes were reported with aggressive early caloric supplementation with parenteral nutrition [27].
Less-than-effective drugs used in ARDS
Despite the many medical advances in recent years, there is no pharmacological treatment for ARDS that reduces mortality in the long or short term. Many drugs have been used in the treatment of ARDS to date, one of which is inhaled NO. This treatment temporarily improves oxygenation and may improve long-term pulmonary functions in survivors, but does not reduce mortality and may be associated with acute kidney injury [28].
One of the most frequently used drugs today is glucocorticoids. The use of glucocorticoids may improve oxygenation and airway pressures. Moreover, it may speed up radiographic recovery in patients with ARDS associated with pneumonia. However, these agents have not been proven to offer a consistent survival benefit; while some studies suggest that they may be useful [29], other authors have reported no advantage [30]. In addition, glucocorticoid therapy is harmful in patients with pneumonia and if initiated ≥14 days after ARDS diagnosis [31].
Ineffective or harmful drugs used in ARDS
Many potential treatments for ARDS were considered to be promising but were shown in studies to be either ineffective or harmful. Surfactant replacement therapy, neutrophil elastase inhibition, anticoagulation, and drugs, such as nonsteroidal anti-inflammatory drugs (ketoconazole and lisofylline) and statins, failed in clinical trials [32].
Other agents demonstrated to be ineffective or harmful include antioxidant preparations (N-acetylcysteine, procysteine (l-2-oxothiazolidine-4-carboxylate), glutamine, omega-3 fatty acids, selenium, beta carotene, zinc, vitamins E and C, and lisofylline), intravenous prostaglandin E1, ibuprofen, activated protein C, short-acting beta-2 agonists, and keratinocyte growth factor [33].
Drugs in ongoing clinical studies for ARDS
There are many drugs for the treatment of ARDS currently in the experimental or clinical stages of development. One of these drugs is aspirin, an antiplatelet and anti-inflammatory agent that showed potential in preclinical and observational clinical studies for the prevention and treatment of ARDS [34, 35]. However, aspirin was reported to have no benefit in preventing ARDS in a randomized study of 390 at-risk patients [36].
Although there are studies on granulocyte–monocyte colony stimulating factor (GM-CSF), it has not been adopted as routine therapy for adults with ARDS due to insufficient evidence. GM-CSF plays an important role in the repair of injured lung and in the enhancement of alveolar macrophage function [37, 38]. Preclinical studies have suggested that bronchoalveolar lavage with GM-CSF is associated with improved survival in patients with ARDS [39].
Experimental studies have been conducted in an effort to determine whether certain therapies are beneficial in ARDS. One of these is stem cell therapy. Preclinical studies have demonstrated that exogenous mesenchymal stem cell (MSC) therapy may mitigate lung injury and support repair. In animal studies, MSCs were found to secrete growth factors and cytokines that can modulate local inflammation and support tissue repair, improve bacterial clearance, and potentially differentiate into mature cells to replace the injured cells [40, 41].
Another candidate drug is macrolide. Macrolide antibiotics have both antimicrobial and anti-inflammatory effects. Animal models have indicated that these agents may be beneficial in the treatment of ARDS [42, 43].
Several anti-inflammatory agents that are in early human trials but do not yet have published data include SB-681323, carbon monoxide, tissue factor antibody, interferon-beta, and sevoflurane [33].
Bosentan
Mechanism of action and anti-inflammatory effect
ARDS has many underlying causes and is not a homogeneous clinical condition. For this reason, it is difficult to find an appropriate pharmacological agent for this heterogeneous disease. There are many ongoing clinical and experimental studies in this area [44–47]. One of the experimental drugs used in ARDS is bosentan [7, 8].
Bosentan acts on the two ET receptors, ETA and ETB [9, 10]. ET has been of great interest as a long-acting, powerful vasoconstrictor and mitogen. It is also known to play a role in the regulation of apoptosis [48]. In addition to their roles in embryonic development and physiological stasis, ETs are also involved in the development and persistence of many pathophysiological conditions, such as atherosclerosis, hypertension, congestive heart failure, renal failure, and pulmonary hypertension. Elevated ET levels have been reported in certain pathological conditions, including subarachnoid hemorrhage, myocardial infarction, cardiogenic and septic shock, and Raynaud’s disease, and in chronic hemodialysis [49]. ET receptor antagonists have been used in these diseases since the early 1990s. The first ETA receptor antagonist to be used for this purpose was BQ-123. Its use advanced to phase II pharmacological trials. ETA/B receptor antagonist bosentan, which can be administered orally due to its nonpeptide structure, is currently in phase III clinical trials for congestive heart failure and hypertension [50]. All ET receptor antagonists, primarily selective ETA and nonselective ETA/B receptor antagonists, have demonstrated utility in many diseases [51].
Bosentan is used in the treatment of diseases, such as pulmonary hypertension due to its effect on vascular structures, and also exerts an anti-inflammatory effect via ET-1. Under normal physiological conditions, ET-1 binds to the ETB receptor in endothelial cells, allowing the production of NO and prostacyclin. Moreover, it induces cytokine, growth factor, collagen, and aldosterone production, thus leading to proinflammatory effects [52]. It also affects inflammation through leukocyte–endothelium interactions mediated by the upregulation of P selectin by ET-1 [53]. In addition, it is believed that ET-1 has important proinflammatory activity in the airways via GM-CSF through chemoattractant agents, such as IL-6 or IL-8 [54]. Transforming growth factor-β induces the secretion of ET-1, which has many proinflammatory effects, including fibroblast migration [55].
Experimental studies demonstrating the relationship between bosentan and ARDS
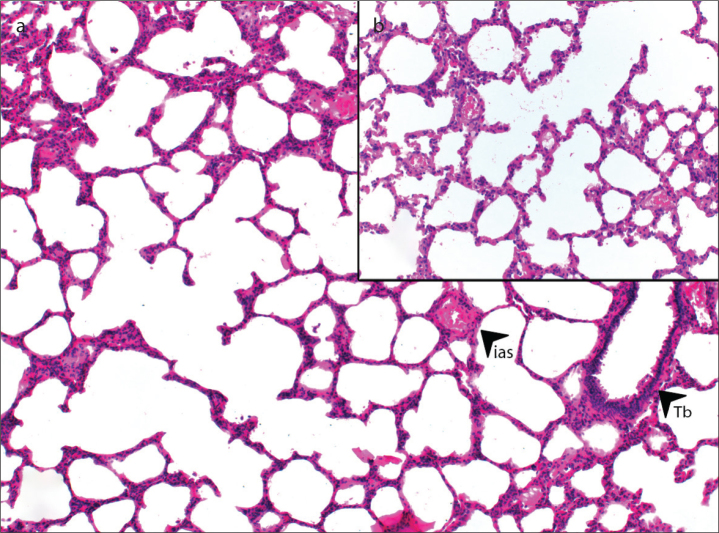
Many experimental studies have demonstrated the relationship between bosentan and ARDS or the anti-inflammatory effect of bosentan. One of these is an experimental study conducted in our center comparing corticosteroid therapy and bosentan in ARDS treatment [7]. Our study showed that bosentan was as effective as dexamethasone in the treatment of lung injury in ARDS. It was determined that bosentan at a dose of 100 mg/kg substantially reduced lung injury and could be among the first-choice treatments due to its regenerative effects (Figure 3).
Figure 3. a, b.
Appearance after treatment with 100 mg/kg bosentan: (a) ias, interalveolar septum; Tb, terminal bronchiole (H&E, ×100); (b) (H&E, ×200).
In a study demonstrating that bosentan is effective in ARDS therapy by reducing the amount of free oxygen radicals produced by polymorphonuclear leukocytes, the effective dose was reported as 90 mg/kg [8]. In another study, bosentan was shown to be effective in reducing infiltration of circulating neutrophils and plasma extravasation from the pulmonary microvascular bed via ETA receptor-related mechanisms in animals with experimentally induced ARDS [56]. Another study in an induced sepsis model showed that ETA and ETB receptor antagonists prevented leakage from the microvascular bed more efficiently than ETA receptor antagonists [57]. ARDS does not only affect the alveolocapillary bed but also affect the vascular bed, causing increased pulmonary arterial pressure. This exacerbates the patient’s clinical condition. In an experimental lipopolysaccharide-induced ARDS model, ET receptor antagonists were shown to reduce pulmonary arterial pressure [58]. Although earlier experimental studies suggested that bosentan is effective in the treatment, another study showed that it was ineffective during treatment but effective during pretreatment [59].
ARDS is characterized by consecutive exudative, proliferative, and fibrotic phases. One of the major problems in ARDS is fibroblast recruitment in the proliferative phase and the fibrotic phase that follows. ET receptor antagonists are effective not only against cell migration or fluid leakage during the exudative phase but also against fibrosis [60].
In addition to experimental animal studies, the clinical use of bosentan as an adjunct to ongoing therapy was shown to improve the clinical condition of a patient with ARDS secondary to viral infection [61].
With the variety of etiological factors and pathogenetic pathways and the absence of a pharmacological treatment approach proven to be completely effective, pharmacological and nonpharmacological studies performed on ARDS continue to be relevant [62–64].
Future directions
Owing to the complexity of inflammation in ARDS, drugs that block only one of the inflammatory mediators have not yielded satisfactory outcomes in humans. Therefore, there is a clear need for new drug(s) with different mechanisms of action.
Conclusion
Bosentan has a strong anti-inflammatory effect in the medical treatment of ARDS. This anti-inflammatory effect and its significant impact on recovery make bosentan a potential first-line drug. More extensive clinical studies are required.
Footnotes
Peer-review: Externally peer-reviewed.
Conflict of Interest: The author has no conflicts of interest to declare.
Financial Disclosure: The author declared that this study has received no financial support.
References
- 1.Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med. 2017;377:562–72. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal NR, King LS, D’Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol. 2014;306:L709–25. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 4.The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–84. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 5.Pham T, Serpa Neto A, Pelosi P, et al. Outcomes of Patients Presenting with Mild Acute Respiratory Distress Syndrome: Insights from the LUNG SAFE Study. Anesthesiology. 2019;130:263–83. doi: 10.1097/ALN.0000000000002508. [DOI] [PubMed] [Google Scholar]
- 6.Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Araz O, Demirci E, Yilmazel Ucar E, et al. Comparison of reducing effect on lung injury of dexamethasone and bosentan in acute lung injury: an experimental study. Multidiscip Respir Med. 2013;8:74. doi: 10.1186/2049-6958-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trabold B, Pawlik M, Nietsch R, et al. Bosentan reduces oxidative burst in acid aspiration-induced lung injury in rats. Injury. 2009;40:946–9. doi: 10.1016/j.injury.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Fagan KA, McMurtry IF, Rodman DM. Role of endothelin-1 in lung disease. Respir Res. 2001;2:90–101. doi: 10.1186/rr44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujitani Y, Trifilieff A, Tsuyuki S, Coyle AJ, Bertrand C. Endothelin receptor antagonists inhibit antigen-induced lung inflammation in mice. Am J Respir Crit Care Med. 1997;155:1890–4. doi: 10.1164/ajrccm.155.6.9196091. [DOI] [PubMed] [Google Scholar]
- 11.Filep JG, Fournier A, Földes-Filep E. Acute pro-inflammatory actions of endothelin-1 in the guinea-pig lung: involvement of ETA and ETB receptors. Br J Pharmacol. 1995;115:227–36. doi: 10.1111/j.1476-5381.1995.tb15868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langleben D, DeMarchie M, Laporta D, Spanier AH, Schlesinger RD, Stewart DJ. Endothelin-1 in acute lung injury and the adult respiratory distress syndrome. Am Rev Respir Dis. 1993;148:1646–50. doi: 10.1164/ajrccm/148.6_Pt_1.1646. [DOI] [PubMed] [Google Scholar]
- 13.Finsnes F, Skjønsberg OH, Tønnessen T, Naess O, Lyberg T, Christensen G. Endothelin production and effects of endothelin antagonism during experimental airway inflammation. Am J Respir Crit Care Med. 1997;155:1404–12. doi: 10.1164/ajrccm.155.4.9105086. [DOI] [PubMed] [Google Scholar]
- 14.Guo R, Ward PA. Role of oxidants in lung injury during sepsis. Antioxid Redox Signal. 2007;9:1991–2002. doi: 10.1089/ars.2007.1785. [DOI] [PubMed] [Google Scholar]
- 15.Petitjeans F, Pichot C, Ghignone M, Quintin L. Early severe acute respiratory distress syndrome: What’s going on? Part I: pathophysiology. Anaesthesiol Intensive Ther. 2016;48:314–38. doi: 10.5603/AIT.2016.0057. [DOI] [PubMed] [Google Scholar]
- 16.Lee WL, Downey GP. Leucocyte elastase. Physiological functions and role in acute lung injury. Am J Respir Crit Care Med. 2001;164:896–904. doi: 10.1164/ajrccm.164.5.2103040. [DOI] [PubMed] [Google Scholar]
- 17.Confalonieri M, Salton F, Fabiano F. Acute respiratory distress syndrome. Eur Respir Rev. 2017;26:144. doi: 10.1183/16000617.0116-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 19.Patel BK, Wolfe KS, Pohlman AS, et al. Effect of Noninvasive Ventilation Delivered by Helmet vs Face Mask on the Rate of Endotracheal Intubation in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA. 2016;315:2435–41. doi: 10.1001/jama.2016.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 21.Semler MW, Wheeler AP, Thompson BT, Bernard GR, Wiedemann HP, Rice TW. Impact of initial central venous pressure on outcomes of conservative versus liberal fluid management in acute respiratory distress syndrome. Crit Care Med. 2016;44:782–9. doi: 10.1097/CCM.0000000000001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin GS, Moss M, Wheeler AP, Mealer M, Morris JA, Bernard GR. A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit Care Med. 2005;33:1681–7. doi: 10.1097/01.CCM.0000171539.47006.02. [DOI] [PubMed] [Google Scholar]
- 23.Martin GS, Mangialardi RJ, Wheeler AP, Dupont WD, Morris JA, Bernard GR. Albumin and furosemide therapy in hypoproteinemic patients with acute lung injury. Crit Care Med. 2002;30:2175–82. doi: 10.1097/00003246-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 24.The SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–56. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 25.The SAFE Study Investigators. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357:874–84. doi: 10.1056/NEJMoa067514. [DOI] [PubMed] [Google Scholar]
- 26.The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–17. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 28.Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005;353:2683–95. doi: 10.1056/NEJMra051884. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal R, Nath A, Aggarwal AN, Gupta D. Do glucocorticoids decrease mortality in acute respiratory distress syndrome? A metaanalysis. Respirology. 2007;12:585–90. doi: 10.1111/j.1440-1843.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 30.Meduri GU, Marik PE, Chrousos GP, et al. Steroid treatment in ARDS: a critical appraisal of the ARDS network trial and the recent literature. Intensive Care Med. 2008;34:61–9. doi: 10.1007/s00134-007-0933-3. [DOI] [PubMed] [Google Scholar]
- 31.The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–84. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 32.Boyle AJ, Mac Sweeney R, McAuley DF. Pharmacological treatments in ARDS; a state-of-the-art update. BMC Med. 2013;11:166. doi: 10.1186/1741-7015-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duggal A, Ganapathy A, Ratnapalan M, Adhikari NK. Pharmacological treatments for acute respiratory distress syndrome: systematic review. Minerva Anestesiol. 2015;81:567–88. [PubMed] [Google Scholar]
- 34.Boyle AJ, Di Gangi S, Hamid UI, et al. Aspirin therapy in patients with acute respiratory distress syndrome (ARDS) is associated with reduced intensive care unit mortality: a prospective analysis. Crit Care. 2015;19:109. doi: 10.1186/s13054-015-0846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Janz DR, Bastarache JA, et al. Prehospital aspirin use is associated with reduced risk of acute respiratory distress syndrome in critically ill patients: a propensity-adjusted analysis. Crit Care Med. 2015;43:801–7. doi: 10.1097/CCM.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kor DJ, Carter RE, Park PK, et al. Effect of Aspirin on Development of ARDS in At-Risk Patients Presenting to the Emergency Department: The LIPS-A Randomized Clinical Trial. JAMA. 2016;315:2406–14. doi: 10.1001/jama.2016.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paine R, Wilcoxen SE, Morris SB, et al. Transgenic overexpression of granulocyte macrophage-colony stimulating factor in the lung prevents hyperoxic lung injury. Am J Pathol. 2003;163:2397–406. doi: 10.1016/S0002-9440(10)63594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baleeiro CE, Christensen PJ, Morris SB, et al. GM-CSF and the impaired pulmonary innate immune response following hyperoxic stress. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1246–55. doi: 10.1152/ajplung.00016.2006. [DOI] [PubMed] [Google Scholar]
- 39.Matute-Bello G, Liles WC, Radella F, 2nd, et al. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Crit Care Med. 2000;28:1–7. doi: 10.1097/00003246-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Mac Sweeney R, McAuley DF. Mesenchymal stem cell therapy in acute lung injury: is it time for a clinical trial? Thorax. 2012;67:475–6. doi: 10.1136/thoraxjnl-2011-201309. [DOI] [PubMed] [Google Scholar]
- 41.McIntyre LA, Moher D, Fergusson DA, et al. Efficacy of Mesenchymal Stromal Cell Therapy for Acute Lung Injury in Preclinical Animal Models: A Systematic Review. PLoS One. 2016;11:e0147170. doi: 10.1371/journal.pone.0147170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawashima M, yatsunami J, Fukuno Y, et al. Inhibitory effects of 14-membered ring macrolide antibiotics on bleomycin-induced acute lung injury. Lung. 2002;180:73–89. doi: 10.1007/PL00021246. [DOI] [PubMed] [Google Scholar]
- 43.Leiva M, Ruiz-Bravo A, Jimenez-Valera M. Effects of telithromycin in in vitro and in vivo models of lipopolysaccharide-induced airway inflammation. Chest. 2008;134:20–9. doi: 10.1378/chest.07-3056. [DOI] [PubMed] [Google Scholar]
- 44.Ucar EY, Araz O, Yilmaz N, Akgun M. Recurrent postpartum eosinophilic pneumonia presenting as acute respiratory distress syndrome. Eurasian J Med. 2011;43:200–2. doi: 10.5152/eajm.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karaman A, Omeroglu M, Emet M, Kerget B, Subasi ID, Alper F. Lenalidomide Induced Late-Onset Acute Respiratory Distress Syndrome. Eurasian J Med. 2016;48:228–9. doi: 10.5152/eurasianjmed.2015.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viswanathan S, Kumar S, Kandan B. Indoxacarb-Related ARDS, Neurotoxicity and Orange Urine. Eurasian J Med. 2013;45:135–7. doi: 10.5152/eajm.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Celik MG, Saracoglu A, Saracoglu T, et al. Effects of Propofol and Midazolam on the Inflammation of Lungs after Intravenous Endotoxin Administration in Rats. Eurasian J Med. 2015;47:109–14. doi: 10.5152/eajm.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eberl LP, Egidy G, Pinet F, Juillerat-Jeanneret L. Endothelin receptor blockade potentiates fasl-induced apoptosis in colon carcinoma cells via the protein kinase c-pathway. J Cardiovasc Pharmacol. 2000;36:S354–6. doi: 10.1097/00005344-200036001-00103. [DOI] [PubMed] [Google Scholar]
- 49.Gulati A, Srimal RC. Endothelin mechanisms in the central nervous system: A target for drug development. Drug Dev Res. 2004;26:361–87. doi: 10.1002/ddr.430260402. [DOI] [Google Scholar]
- 50.Ray A, Hegde LG, Chugh A, Gupta JB. Endothelinreceptor antagonists: Current and future perspectives. Drug Discov today. 2000;5:455–64. doi: 10.1016/S1359-6446(00)01557-9. [DOI] [PubMed] [Google Scholar]
- 51.Wu C. Recent discovery and development of endothelin receptor antagonists. Exp Opin Ther Patents. 2000;10:1653–68. doi: 10.1517/13543776.10.11.1653. [DOI] [Google Scholar]
- 52.Rubin LJ, Roux S. Bosentan: a dual endothelin receptor antagonist. Exp Opin Invest Drugs. 2002;11:991–1002. doi: 10.1517/13543784.11.7.991. [DOI] [PubMed] [Google Scholar]
- 53.Murohara T, Lefer AM. Autocrine effects of endothelin-1 on leukocyte-endothelial interaction: stimulation of endothelin B receptor subtype reduces endothelial adhesiveness via a nitric oxide-dependent mechanism. Blood. 1996;88:3894–900. doi: 10.1182/blood.V88.10.3894.bloodjournal88103894. [DOI] [PubMed] [Google Scholar]
- 54.Mullol J, Baraniuk JN, Logun C, Benfield T, Picado C, Shelhamer JH. Endothelin-1 induces GM-CSF, IL-6 and IL-8 but not G-CSF release from a human bronchial epithelial cell line (BEAS-2B) Neuropeptides. 1996;30:551–6. doi: 10.1016/S0143-4179(96)90038-4. [DOI] [PubMed] [Google Scholar]
- 55.Ahmedat AS, Warnken M, Seemann WK, et al. Pro-fibrotic processes in human lung fibroblasts are driven by an autocrine/paracrine endothelinergic system. Br J Pharmacol. 2013;168:471–87. doi: 10.1111/j.1476-5381.2012.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guimarães CL, Trentin PG, Rae GA. Endothelin ET(B) receptor-mediated mechanisms involved in oleic acid-induced acute lung injury in mice. Clin Sci (Lond) 2002;103(Suppl 48):340S–344S. doi: 10.1042/CS103S340S. [DOI] [PubMed] [Google Scholar]
- 57.Hele DJ, Birrell MA, Webber SE, Foster ML, Belvisi MG. Effect of endothelin antagonists, including the novel ET(A) receptor antagonist LBL 031, on endothelin-1 and lipopolysaccharide-induced microvascular leakage in rat airways. Br J Pharmacol. 2000;131:1129–34. doi: 10.1038/sj.bjp.0703691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frank DU, Lowson SM, Roos CM, Rich GF. Endotoxin alters hypoxic pulmonary vasoconstriction in isolated rat lungs. J Appl Physiol. 1996;81:1316–22. doi: 10.1152/jappl.1996.81.3.1316. [DOI] [PubMed] [Google Scholar]
- 59.Hubloue I, Biarent D, Abdel Kafi S, et al. Endothelin receptor blockade in canine oleic acid-induced lung injury. Intensive Care Med. 2003;29:1003–6. doi: 10.1007/s00134-003-1683-5. [DOI] [PubMed] [Google Scholar]
- 60.Zuo WL, Zhao JM, Huang JX, et al. Effect of bosentan is correlated with MMP-9/TIMP-1 ratio in bleomycin-induced pulmonary fibrosis. Biomed Rep. 2017;6:201–5. doi: 10.3892/br.2016.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo Q, Huang JA, Fraidenburg DR. Bosentan as rescue treatment in refractory hypoxemia and pulmonary hypertension in a patient with ARDS and H7N9 influenza virus infection. Lung. 2014;192:635–6. doi: 10.1007/s00408-014-9602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akgun M, Mirici A, Meral M, et al. hypersensitivity pneumonitis case complicated with acute respiratory distress syndrome after bronchoscopy. Respir Med. 2005;99:1195–7. doi: 10.1016/j.rmed.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 63.Bos LD, Martin-Loeches I, Schultz MJ. ARDS: challenges in patient care and frontiers in research. Eur Respir Rev. 2018;27 doi: 10.1183/16000617.0107-2017. pii: 170107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Slutsky AS, Villar J. Early Paralytic Agents for ARDS? Yes, No, and Sometimes. N Engl J Med. 2019;380:2061–3. doi: 10.1056/NEJMe1905627. [DOI] [PubMed] [Google Scholar]