Abstract
Objective
We aimed to investigate the relationship between the use of fluoro-2-deoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT), maximized standardized uptake value (SUVmax) values of tumors, and tumor differentiation and tumor markers during the initial staging of patients with gastric cancer.
Materials and Methods
The study included 50 patients (14 women and 36 men; mean age: 63±11 years; age range: 31–80 years) who had undergone initial staging with FDG-PET/CT after the diagnosis of gastric cancer with endoscopic biopsy between January and June 2013. Serum alpha fetoprotein (AFP), carcinogenic antigen 19-9 (CA 19.9), carcinoembryonic antigen (CEA), and C-reactive protein (CRP) levels were measured in patients prior to imaging. PET/CT images were evaluated for primary tumors, locoregional spread, and distant organ metastases, and classified by tumor-node-metastasis staging. Semiquantitative data were collected by SUVmax measurements in pathological regions of involvement. Data were analyzed statistically.
Results
FDG-PET/CT showed primary gastric cancer with a sensitivity of 87%. Imaging findings were normal in 3 patients (1/3; mucinous adenocarcinoma, 2/3; signet-ring cell adenocarcinoma). With FDG-PET/CT, 3/50 patients were classified into Stage 1B, 3/50 patients into Stage 2, 5/50 patients into Stage 3A, 5/50 patients into Stage 3B, 5/50 patients into 3C and 29/50 patients into Stage 4. The mean SUVmax was calculated as 11.35±4.3 (poorly differentiated adenocarcinoma: 5.4±1.7; moderately differentiated adenocarcinoma: 10.3±4.8) for the primary tumor and 14.9±6.3 for tumor metastasis. A positive correlation was evident between the measured SUVmax and stage and the grade of primary tumor (p<0.05). While the relationship between SUVmax and levels of serum AFP and CRP was statistically significant (p<0.05), the relationship between SUVmax and levels of serum CA 19-9 and CEA was not statistically significant (p>0.05).
Conclusion
The SUVmax of primary tumors was associated with the degree of differentiation of primary tumors and the biochemical tumor markers CRP and AFP. The fact that SUVmax of primary tumors is high supplies clues about the presence of the factors affecting prognosis of the disease.
Keywords: Gastric cancer, FDG-PET/CT, initial staging, SUVmax, tumor markers
Introduction
Gastric cancer is the world’s third-leading cause of cancer-related deaths in men after lung cancer, and the second-most common cause in women after breast cancer [1]. It is difficult to make an early diagnosis of gastric cancer because it frequently manifests in the form of weight loss and anemia [2]. Early diagnosis and accurate staging are therefore important.
The gold standard method for the diagnosis of gastric cancer is still endoscopy and biopsy. However, computed tomography (CT), endoscopic ultrasonography, and diagnostic laparoscopy are becoming increasingly useful. The most important drawback of these methods is that there is insufficient evidence supporting diagnoses of metastatic lymph nodes due to relatively low detection rates [3]. Positron emission tomography (PET) is a molecular imaging method that provides physiological information required for clinical diagnosis based on changes in tissue metabolism. Many malignant tumors can be noninvasively visualized through PET using fluoro-2-deoxyglucose (FDG) labeled with fluorine-18 due to increased glucose metabolism [4]. FDG-PET/CT is an imaging modality in which CT can compensate for the limited spatial resolution of PET, and anatomical and morphological information is completed with metabolic and molecular information and has a leading role in staging, treatment-response evaluation, prognosis determination and restaging stages in various types of malignancies [5, 6].
Alpha fetoprotein (AFP), carcinogenic antigen 19-9 (CA 19-9), carcinoembryonic antigen (CEA) and C-reactive protein (CRP) are tumor markers used for the early diagnosis, prognosis prediction, and post-treatment recurrence investigation of gastric cancer [7]. The prognostic significance of CRP in esophageal, gastric, liver, pancreatic, colorectal, and prostate cancers is mentioned frequently [8].
In this study, we investigated the initial staging performance of FDG-PET/CT in patients with gastric cancer, as well as the relationship between PET/CT findings and the degree of tumor differentiation and tumor markers.
Materials and Methods
The study group consisted of 50 patients (14 women and 36 men with a mean age of 63±11 years; age range: 31–80 years) diagnosed with gastric cancer by endoscopic biopsy who underwent initial staging with FDG-PET/CT before treatment between January and June 2013. To avoid false-positive indications for tumor markers, patients diagnosed with chronic inflammatory/infectious disease, collagen-tissue disease, and concurrent second primary cancer were excluded from the study prior to imaging. Informed consent forms were obtained from the patients before the PET/CT study. In cancer staging, the revised American Joint Committee on Cancer’s Cancer Staging Manual, 7th edition, was used [9].
FDG-PET/CT imaging protocol
Fasting blood glucose levels of patients were measured before the PET/CT study. PET/CT scans were not performed in patients with fasting blood glucose levels higher than 150 mg/dL. The patients received an intravenous injection of 8–12 mCi (296–444 MBq) of FDG 60 min after they drank 25 mL of an oral contrast agent (Omnipaque 300 mg/50 mL; GE Healthcare) in 1 L of water. One hour after the injection, non-diagnostic CT imaging was performed for anatomic localization and attenuation correction. CT imaging was performed using the 70 mAs and 120 keV values from the thigh to the cranium, with the patient in supine position. Following CT imaging, PET imaging was performed with the patient in the same position from the proximal thigh to the base of the skull, with 9 bed positions for 2 min in each position. A PET/CT device (Biograph 16 TruePoint ; Siemens, Germany) was used in all patients for FDG-PET/CT.
Evaluation of images
PET/CT images were evaluated by two experienced nuclear medicine specialists. The evaluators were informed about each patient’s medical history, the results of the patient’s previous anatomical imaging studies, complaints of the patients, tumor markers, and histopathological examination results. Final decisions were made by consensus if the two evaluators reached conflicting evaluations.
In FDG-PET/CT images, all focal hypermetabolic activity involvements that were higher than ground activity, with the exception of FDG’s physiological involvement areas were accepted as pathological involvement. Focal hypermetabolic areas in the gastric tissue were evaluated as local lesions and those in the liver with higher concentrations than adjacent parenchymal tissues were considered liver metastasis. Focal linear activities detected with the gastric mucosa were accepted as changes related to inflammation. Increased focal or diffuse activity involvements in the mesenteric area that did not correspond to the bowel wall were accepted as peritoneal carcinomatosis. Moderately diffuse or segmental hypermetabolic areas were considered physiological involvements and increased focal hypermetabolic areas were accepted as metastases in areas that corresponded to the bowel wall. Metastases other than liver metastases were accepted as distant metastases, and liver metastases were evaluated separately. SUVmax values of each pathological lesion read were measured and recorded.
Tumor markers
Biochemical measurements of tumor markers including AFP, CA 19-9, CEA, and CRP were made in each patient after histological diagnosis and before imaging. An immunochemical method and a Beckman Coulter Immunoassay device (DXI 800, CA, USA) were used for serum AFP, CA 19-9, and CEA measurements. Serum CRP levels were measured by a nephelometric method using a Siemens BN Behring analyzer (Siemens Healthcare Diagnostics, Germany).
Statistical analysis
Statistical analysis and descriptive statistics were performed using The Statistical Package for the Social Sciences (SPSS) for Windows 15.0 software (SPSS Inc., Chicago, IL, USA). Data were presented as number, percentage, mean, and standard deviation. The Kolmogorov–Smirnov test was used to determine whether the variables were in compliance with normal distribution. The Mann–Whitney U test was used for analyses of two groups of numerical variables such as serum CEA or CRP levels, and the Kruskal–Wallis test was used for group analyses with more than two groups. Chi-square tests and Fisher’s exact test were used to analyze categorical variables. As in the investigation of the relationship between serum AFP and SUVmax levels of the primary tumor, Spearman correlation analysis was used to determine whether two numerical variables were correlated with each other. Statistical significance level was accepted as p<0.05.
Ethical dimensions of the research
The compatibility of the study with ethical principles was evaluated by the Ethics Committee of Ataturk University Faculty of Medicine. This article was approved by the Ethics Committee of the Non-Drug Clinical Trials of Ataturk University Faculty of Medicine, with a 28.02.2013 date and with number 39 and decision number 13. Written permission was received from the directors of the Ataturk University Training and Research Hospital for the study to be carried out. Patients provided verbal and written consent about their willingness to participate.
Results
The study group consisted of 50 patients (36 men [72%], 14 women [28%]) diagnosed with gastric cancer after endoscopic biopsy. The mean age of the patients was 62.9±11.0 and the age range was 31–80 years. As a result of histopathological examination of biopsy materials; 11/50 patients (22%) were reported to have moderately differentiated adenocarcinoma, 7/50 patients (14%) had poorly differentiated adenocarcinoma, 2/50 patients (4%) had neuroendocrine differentiated adenocarcinoma, 3/50 patients (6%) had signet-ring cell carcinoma, 3/50 patients (6%) had mucinous adenocarcinoma, and 24/50 patients (48%) were reported as untyped/other adenocarcinoma. Demographic characteristics and histopathological examination findings of the patients are summarized in Table 1. After initial staging by PET/CT, surgical treatment decisions were taken for 24/50 (48%) of the patients and 26/50 (52%) were deemed inoperable. Of the 24 patients for whom surgical intervention was determined, 14 had a total gastrectomy and 10 had a distal subtotal gastrectomy. D2 lymph-node dissection was applied in all total gastrectomies, whereas lymph-node dissection was D1 in distal gastrectomies.
Table 1.
Demographic, histopathological and clinical stage related features of patients
| Features | Number of patients (%) |
|---|---|
| Gender | |
| Male | 36 (72%) |
| Female | 14 (28%) |
| Type of operation | |
| Distal subtotal gastrectomy | 10 (20%) |
| Total gastrectomy | 14 (28%) |
| Inoperable | 26 (52%) |
| Primary tumor pathology | |
| Slightly differentiated adenocarcinoma | 7 (14%) |
| Moderately differentiated adenocarcinoma | 11 (22%) |
| Signet ring cell adenocarcinoma | 3 (6%) |
| Mucinous adenocarcinoma | 3 (6%) |
| Neuroendocrine differentiated adenocarcinoma | 2 (4%) |
| Untyped/other adenocarcinoma | 24 (48%) |
| Postoperative lymphovascular invasion | |
| Yes | 20 (83%) |
| No | 4 (17%) |
| Postoperative perineural invasion | |
| Yes | 18 (75%) |
| No | 6 (25%) |
| TNM stage | |
| IB | 3 (6%) |
| II | 3 (6%) |
| IIIA | 5 (10%) |
| IIIB | 5 (10%) |
| IIIC | 5 (10%) |
| IV | 29 (58%) |
PET/CT findings
Prior to the treatment, the presence of a primary tumor (T), nodal invasion (N), and distant metastasis (M) were evaluated according to FDG-PET/CT findings. The tumor stage was found to be Tx in 26/50 patients (52%), T1b in 3/50 patients (6%), T2 in 1/50 patients (2%) and T4a in 20/50 patients (40%). When evaluated in terms of lymph-node spread; 26/50 patients (52%) were Nx, 4/50 patients (8%) were N0, 7/50 patients (14%) were N1, 6/50 patients (12%) were N2, 4/50 patients (8%) were N3a, and 3/50 patients (6%) were N3b. While the presence of distant metastases (liver in 23 patients and extrahepatic metastases in 6 patients) was detected in 29/50 patients (58%), there was no finding detected through imaging in 21/50 patients (42%) that was compatible with distant metastasis. In conclusion, according to clinical tumor-node-metastasis staging; 3/50 patients (6%) were classified as Stage 1B, 3/50 patients (6%) as Stage 2, 5/50 patients (10%) as Stage 3A, 5/50 patients (10%) as Stage 3B, 5/50 patients (10%) as Stage 3C, and 29/50 patients (58%) as Stage 4 (Table 1). The sensitivity of FDG-PET/CT in demonstrating a primary tumor was 87%. Of the three patients whose primary gastric tumor could be demonstrated by PET/CT, one was reported as mucinous-type adenocarcinoma and two as signet-ring cell adenocarcinoma. (Figure 1).
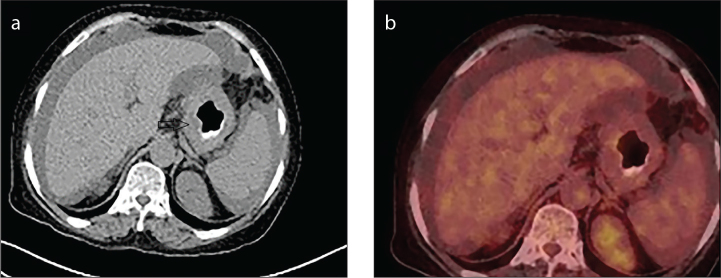
Figure 1. a, b.
(a) Wall thickening and an appearance of the mass are observed in the primary tumor area (arrow head) of the case with a signet-ring cell gastric adenocarcinoma in axial section CT image. (b) In the PET/CT fusion image of the same patient, metabolic F-18-FDG involvement in the primary tumor and other areas in the image is within normal limits.
Mean SUVmax as a semiquantitative index was calculated as 11.35 (3.20–26.91) for all patients. Differences between the mean SUVmax (14.9±6.3) of the patients with distant metastasis (29/50 patients) and the mean SUVmax (7.4±4.4) of patients without distant metastasis were statistically significant (p<0.05). Similarly, the difference between the mean SUVmax (13.5±6.7) (Figure 2) measured in the patients with liver metastasis (23/50 patients) and the mean SUVmax (9.8±6.2) measured in patients without liver metastasis was also found to be statistically significant (p<0.05). The mean SUVmax value increased as the degree of differentiation of the tumor increased. The mean SUVmax was 5.4±1.7 in poorly differentiated adenocarcinomas, while the mean SUVmax measurement was 10.3±4.8 in moderately differentiated adenocarcinomas. The difference between these two groups was statistically significant (p<0.01). A statistically significant and increasing relationship was evident between the disease stage and the SUVmax measurement result of the primary tumor (p<0.01).
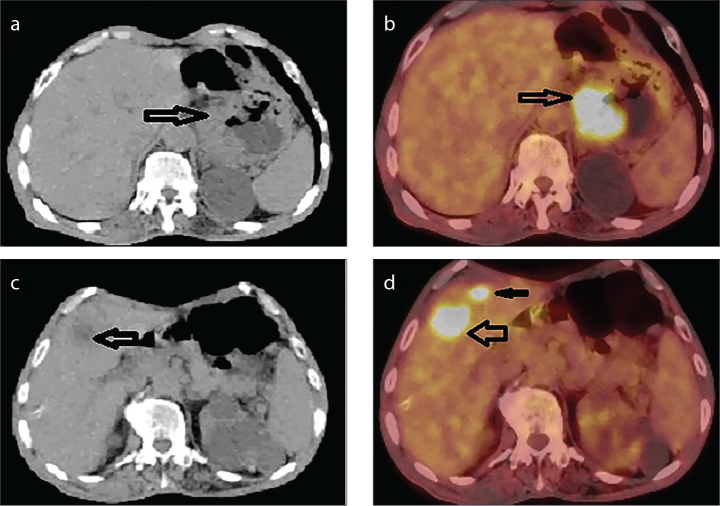
Figure 2. a–d.
A case of adenocarcinoma located in gastric cardia region. (a) Wall thickening (arrow) is observed in axial section CT image. (b) Hypermetabolic FDG involvement (arrow) is observed in primary tumor (SUVmax: 12.29) in an axial section PET/CT fusion image. (c) Tumor metastasis in liver is observed as hypodense area (arrow) in an axial section CT image. (d) Axial PET/CT fusion image shows focal hypermetabolic FDG involvement (SUVmax: 15.9) in liver metastases (arrows).
Compliance with tumor markers
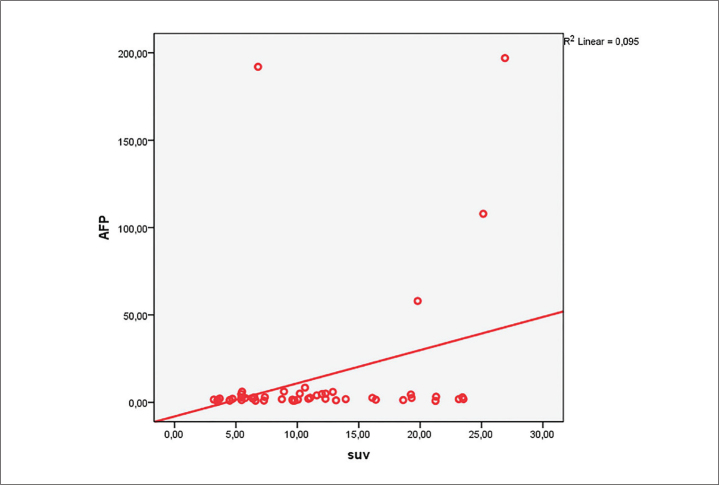
Serum AFP positivity rate was 30% (15/50 patients) in the study patients. While the mean SUVmax measurement of patients with serum AFP levels within the normal range was 10.26±6.3, this value was 13.9±6.9 in patients with elevated AFP levels. We detected a weak positive correlation between the primary tumor SUVmax and AFP reading of patients (r=0.238, p<0.05) with liver metastasis (Figure 3).
Figure 3.
Linear correlation of the relationship between serum AFP levels in 50 patients with gastric cancer forming the study group and SUVmax readings of the primary tumor.
Serum CA 19-9 measurement results were positive in 44% of the patients (22/50). However, no statistically significant relation was detected in a correlation analysis between serum CA 19-9 levels and disease stage, presence of distant metastasis, and SUVmax measured in primary tumors.
Serum CEA measurement results were positive in 56% (28/50) of the patients. A significant weak correlation was found between serum CEA level and disease stage (r=0.203, p<0.05). No statistically significant relationship was found between serum CEA measurement results and SUVmax readings of the primary tumor.
Serum CRP levels exceeded the upper limit of the normal reference range in 52% of patients (26/50 patients). More than half of the patients with high serum CRP (16/26 patients) had a late-stage disease. SUVmax values of the primary tumor and serum CRP level were positively correlated, but the relationship was not statistically significant.
Discussion
SUVmax values obtained from a PET-CT scans of patients with gastric cancer was correlated with tumor differentiation, presence of liver metastasis, presence of distant metastasis, and biochemical tumor markers.
Surgical treatment is currently the treatment of choice for middle-stage and even some advanced-stage gastric cancers, making early diagnosis, correct clinical staging and selection of appropriate surgical procedure of prognostic importance [10].
The criterion by which CT is used in metastatic lymph nodes is size increase. However, in infective and inflammatory processes, lymph nodes may increase in size, leading to false evaluations. Several studies comparing CT and PET in terms of preoperative lymph-node staging in gastric cancers have shown that PET is not superior to CT in terms of sensitivity. This is mainly due to the low resolution of PET scans, which makes it difficult to distinguish pathologic lymph nodes from adjacent primary tumors. However, it has been reported that pathological lymph nodes can be detected more accurately when PET and CT scans are combined [11].
PET/CT is reportedly not used in T staging and can show the primary tumor with a sensitivity of 58%–94% [12]. FDG involvement has been shown to be significantly lower in signet-ring cell adenocarcinoma, mucinous adenocarcinoma, and poorly differentiated adenocarcinoma subtypes of gastric cancer [13]. Some investigators have reported that, although PET/CT has a lower diagnostic efficacy in signet-ring cell and mucinous adenocarcinomas, it can distinguish between other histological types [14]. Signet-ring cell and mucinous adenocarcinoma associated with false-negative results on PET/CT were found to have insufficient GLUT-1 expression in tumor cell membranes [11, 15]. Similarly, the histopathology of study patients whose primary tumor could not be visualized was reported as mucinous adenocarcinoma in one patient and signet-ring cell adenocarcinoma in two patients.
SUVmax is a semiquantitative parameter calculated by taking into account the amount of FDG administered to the patient, the patient’s weight, and the physical decay of FDG. The value of SUVmax is reportedly higher in fast-growing tumors with high glucose use compared with slow-growing tumors [16].
Previous studies have reported a correlation between the degree of differentiation of the tumor and the level of FDG involvement in gastric cancer, that the SUVmax value was significantly lower in well-differentiated types compared with poorly differentiated types, and that there could be a significant differences in SUVmax values in cases with lymph-node metastasis. [17]. Diverse results were also present in the literature. In one previous study, the authors found that moderately differentiated (grade 2) gastric adenocarcinomas had higher SUVmax values compared with poorly differentiated ones (grade 3) [13]. Our results confirmed those findings as we encountered a significant difference between poorly differentiated and moderately differentiated adenocarcinomas in terms of in SUVmax (5.4±1.7 vs 10.3±4.8, p<0.05). In addition, a strong and significant relationship was evident between the SUVmax of the primary tumor and the stage of the disease and distant metastasis. These findings partially support the previous reports.
Serum AFP levels are frequently a marker for germ cell tumors, hepatocellular cancer, and AFP producing gastric cancer [18]. Several studies have reported that high serum AFP in gastric cancer is associated with liver metastasis [18, 19]. High serum AFP is also known as a poor prognosis indicator in patients with liver metastasis [19]. In our efforts, a relationship was found between high serum AFP and histopathological tumor type. In addition, a statistically significant relationship between serum AFP values and SUVmax values in patients with liver metastasis is shown.
CA 19-9 is a tumor marker with an adhesion molecule. It has been reported that this marker is 16%–44% positive in gastric cancer [20]. This rate was found to be 44% in our patient group. High serum CA 19-9 in gastric cancer was associated with lymph-node metastasis and peritoneal metastasis [21]. A statistically significant relation has been found between high serum CA 19-9 and disease stage, tumor size, serosal invasion, peritoneal metastasis, and acid [22]. Another study reported a correlation between high serum CA 19-9 and lymph-node involvement, disease stage, vascular invasion, and tumor size [23]. A relationship has also been found between high serum CA 19-9 and liver metastasis, depth of tumor invasion, primary tumor size, and resectability of primary tumors [20]. We found no statistically significant correlation between serum CA 19-9 level and tumor stage, distant metastasis, and SUVmax.
CEA is the most studied tumor marker in terms of correlation with gastrointestinal malignancies. Serum CA 19-9 and CEA measurements are now routinely used in the management of gastric cancer. Preoperative CEA levels can also be used to predict tumor prognosis [20]. According to the literature, high serum CEA levels are detected in 15.9%–57.6% of patients with gastric cancer. [20]. High levels of serum CEA were found in 56% of our patients, which is within the limits reported in previous studies. Some authors have reported that serum CEA positivity is associated with liver metastasis, but not with histopathological type or tumor stage [24]. One study reported a significant relationship between serum CEA positivity and the patient’s gender (males exhibiting a higher rate of positivity), disease stage, tumor size, lymph-node metastasis, liver metastasis, serosal invasion, as well as peritoneal metastasis [25]. Another study found a relationship between high serum CEA level and advanced-stage disease, large tumor size, serosa invasion, liver metastasis, and resectability of the primary tumor [22]. In a study of 663 patients with gastric cancer, a significant relationship was found between high serum CEA and liver metastasis, peritoneal involvement, and advanced-stage disease [20]. Another large study (549 gastric cancer cases) showed a significant correlation between high serum CEA and liver metastasis, primary tumor resectability, and tumor depth [26]. In this study, a strong correlation was found between disease stage and the presence of liver metastases and high serum CEA. However, we found no significant relationship between the histopathological subtype and SUVmax value of the primary tumor and high serum CEA. In this study, no statistically significant relationship was found between serum CEA measurement results and SUVmax readings of the primary tumor.
The production of CRP is regulated by interleukin (IL)-1 and IL-6 tumor necrosis factor from proinflammatory cytokines. Circulating products of these cytokines increase the synthesis of CRP in hepatocytes [27]. CRP is also produced by some tumor cells [28]. High serum CRP has been reported in patients with gastric cancer [18]. Multiple studies have shown that high serum CRP levels can be used as an indicator of poor prognosis in lung, prostate, ovarian, and gastrointestinal malignancies [29]. Serum CRP level is an inexpensive technique that can be easily measured in routines [30]. High serum CRP detected in malignant cases is likely a secondary response to tumor necrosis, regional tissue damage, and associated inflammation. [31]. In this study group, serum CRP level readings were high in 52% of the patients. In addition, a positive but statistically non-significant correlation was found between the SUVmax of the primary tumor and the serum CRP level.
There are some limitations of our study. First, the sample size was relatively small and our findings need to be supported by studies with a larger sample size. Second, although our findings are valid for gastric cancer, no significant correlation was found for mucinous and signet-ring cell subtypes.
In our study, the SUVmax value of primary tumor was connected with the degree of differentiation of primary tumors and the biochemical tumor markers CRP and AFP. The fact that the SUVmax value of the primary tumor is high supplies clues about the presence of the factors affecting the prognosis of the disease.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of the Non-Drug Clinical Trials of Ataturk University Faculty of Medicine, with a 28.02.2013 date and with number 39 and decision number 13.
Informed Consent: Informed consent is not necessary due to the retrospective nature of this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.M., A.K.A; Design - A.M., A.K.A.; Supervision - A.M., A.K.A Resources - A.M., A.K.A.; Materials - A.M., A.K.A, A.S.; Data Collection and/or Processing - A.M., A.K.A, A.S.; Analysis and/or Interpretation - A.M., A.K.A, A.S.; Literature Search - A.M., A.K.A, A.S.; Writing Manuscript – A.M.; Critical Review - A.M., A.S.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1. [Accessed on 27 Feb 2019]. https://www.who.int/news-room/fact-sheets/detail/cancer.
- 2.Polat FR, Duran Y. Gastric Cancer And The İmportance Of Early Diagnosis. Namık Kemal Dergisi. 2018;6:32–5. [Google Scholar]
- 3.Kim AY, Han JK, Seong CK, Kim TK, Choi BI. MRI in staging advanced gastric cancer: is it useful compared with spiral CT? J Comput Assist Tomogr. 2000;24:389–94. doi: 10.1097/00004728-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Karapolat İ. Akciğer Kanserinde Tedavi Yanıtını Değerlendirmede FDG PET/BT Görüntüleme. Nucl Med Semin. 2018;4:43–51. doi: 10.4274/nts.2018.007. [DOI] [Google Scholar]
- 5.Aydos U, Akdemir ÜÖ, Atay LÖ. Positron Emission Tomography/Magnetic Resonance Imaging Practices in Oncological Imaging. Nuclear Medicine Seminars. 2017;1:22–51. doi: 10.4274/nts.004. [DOI] [Google Scholar]
- 6.Duman K, Simsek A, Gorgulu S, Yagci G, Peker Y. The role of 2-[f-18] fluoro 2-deoxy d-glucose positron emission tomography in the preoperative staging of gastric cancer. Eurasian J Med. 2013;45:149–54. doi: 10.5152/eajm.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heikkilä K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health. 2007;61:824–33. doi: 10.1136/jech.2006.051292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gockel I, Dirksen K, Messow CM, Junginger T. Significance of preoperative C-reactive protein as a parameter of the perioperative course and long term prognosis in squamous cell carcinoma and adenocarcinoma of the oesophagus. World J Gastroenterol. 2006;12:3746–50. doi: 10.3748/wjg.v12.i23.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Guo W, Hu Y, et al. Superiority of the 8th edition of the TNM staging system for predicting overall survival in gastric cancer: Comparative analysis of the 7th and 8th editions in a monoinstitutional cohort. Mol Clin Oncol. 2018;9:423–31. doi: 10.3892/mco.2018.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu L, Chen M, Guo D, et al. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS One. 2017;12:e0182692. doi: 10.1371/journal.pone.0182692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SK, Kang W, Lee JS, et al. Assesment of Lymph node metastasis using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006;33:148–55. doi: 10.1007/s00259-005-1887-8. [DOI] [PubMed] [Google Scholar]
- 12.Lynch TB. PET/CT in Clinical Practice. Springer-Verlag; London: 2007. Introduction; pp. 1–15. [Google Scholar]
- 13.Stahl A, Ott K, Weber WA, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003;30:288–95. doi: 10.1007/s00259-002-1029-5. [DOI] [PubMed] [Google Scholar]
- 14.Kim DW, Park SA, Kim GC. Detecting the recurrence of gastric cancer after curative resection: comparison of FDG PET/CT and contrast-enhanced abdominal CT. J Korean Med Sci. 2011;26:875–80. doi: 10.3346/jkms.2011.26.7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mochiki E, Kuwano H, Katoh H, et al. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg. 2004;28:247–53. doi: 10.1007/s00268-003-7191-5. [DOI] [PubMed] [Google Scholar]
- 16.Balcı AE, Balcı TA, Çerçi SS, editors. Mediyastenin Kitle Lezyonlarının Değerlendirilmesinde Nükleer Tıbbın Katkısı. TÜSAD; 2015. Mediyasten Hastalıkları ve Cerrahisi; pp. 77–112. [Google Scholar]
- 17.Yoshioka T, Yamaguchi K, Kubota K, et al. Evaluation of 18F-FDG PET in patients with advanced, metastatic, or recurrent gastric cancer. J Nucl Med. 2003;44:690–9. [PubMed] [Google Scholar]
- 18.Göksel S. Mide Kanserinde etyoloji, patogenez ve patoloji. İstanbul: İstanbul Onkoloji Enstitüsü Yayınları; 1998. pp. 181–216. [Google Scholar]
- 19.Chang YC, Nagasue N, Kohno H, et al. Clinicopathologic features and long-term results of alpha fetoprotein producing gastric cancer. Am J Gastroenterol. 1990;85:1480–5. [PubMed] [Google Scholar]
- 20.Ychou M, Duffour J, Kramar A, Gourgou S, Grenier J. Clinical significance and prognostic value of CA 72-4 compared with CEA and CA 19-9 in patients with gastric cancer. Dis Markırs. 2000;16:105–10. doi: 10.1155/2000/595492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kodera Y, Yamanura Y, Torii A. The prognostic value of preoperative serum levels of CEA and CA 19-9 in patients with gastric cancer. Am J Gastroenterol. 1996;91:49–56. [PubMed] [Google Scholar]
- 22.Bayrak M, Ölmez ÖF, Kurt E, et al. Lokal İleri ve Metastatik Mide Kanserli Hastalarda Tedavi Öncesi AFP, CEA ve CA 19-9 Serum Seviyeleri ile Klinikopatolojik Faktörlerin Arasındaki İlişki. Uludağ Üniversitesi Tıp Fakültesi Derg. 2011;37:139–43. [Google Scholar]
- 23.Dilege E, Mihmanli M, Demir U, et al. Prognostic value of preoperative CEA and CA 19-9 levels in resectable gastric cancer. Hepatogastroenterology. 2010;57:674–7. [PubMed] [Google Scholar]
- 24.Ucar E, Semerci E, Ustun H, Yetim T, Huzmeli C, Gullu M. Prognostic value of preoperative CEA, CA 19-9, CA 72-4, and AFP levels in gastric cancer. Adv Ther. 2008;25:1075–84. doi: 10.1007/s12325-008-0100-4. [DOI] [PubMed] [Google Scholar]
- 25.Dirican A, Ünal B, Işık B, et al. Corelation between Preoperative Serum CEA, CA19-9, and AFP Levels and Clinicopathologic Factors. Journal of Inonu University Medical Faculty. 2008;15:233–7. [Google Scholar]
- 26.Ishigami S, Natsugoe S, Hokita S, et al. Clinical importance of preoperative carcinoembryonic antigen and carbohydrate antigen 19-9 levels in gastric cancer. J Clin Gastroenterol. 2001;32:41–4. doi: 10.1097/00004836-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32:274–8. doi: 10.3109/07853890009011772. [DOI] [PubMed] [Google Scholar]
- 28.Nozoe T, Korenaga D, Futatsugi M, Saeki H, Maehara Y, Sugimachi K. Immunohistochemical expression of C-reactive protein in squamous cell carcinoma of the esophagus significance as a tumor markır. Cancer Lett. 2003;192:89–95. doi: 10.1016/S0304-3835(02)00630-4. [DOI] [PubMed] [Google Scholar]
- 29.Hefler LA, Concin N, Hofstetter G, et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res. 2008;14:710–4. doi: 10.1158/1078-0432.CCR-07-1044. [DOI] [PubMed] [Google Scholar]
- 30.Ledue TB, Weiner DL, Sipe J, Poulin SE, Collins MF, Rifai N. Analytical evaluation of particle-enhanced immunonephelometric assays for C-reactive protein, serum amyloid A, and mannose binding protein in human serum. Ann Clin Biochem. 1998;35:745–53. doi: 10.1177/000456329803500607. [DOI] [PubMed] [Google Scholar]
- 31.Slaviero KA, Clarke SJ, Rivory LP. Inflammatory response: an unrecognised source of variability in the pharmacokinetics and pharmacodynamics of cancer chemotherapy. Lancet Oncol. 2003;4:224–32. doi: 10.1016/S1470-2045(03)01034-9. [DOI] [PubMed] [Google Scholar]