Abstract
Background
Patients with severe mental disorders have a high risk of metabolic-related complications like metabolic syndrome (MetS), diabetes mellitus (DM), hypertension and lipid derangements, and these factors may predispose them to a high mortality rate. Data is very scarce regarding MetS among patients with severe mental illness in Ethiopia. Therefore, this study aimed to assess the prevalence of MetS and its associated factors among patients with severe mental illness.
Methods
A cross-sectional study was conducted in Hawassa University Comprehensive Specialized Hospital from January to June 2019 among adult patients attending a psychiatric outpatient department, Southern Ethiopia. A systematic random sampling technique was used to select 245 study subjects. Socio-demographic and other data were collected using a structured questionnaire. Both the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) and International Diabetes Federation (IDF) guidelines were used to define MetS.
Results
The prevalence of MetS was 24.5% and 26.9% by NCEP-ATP and IDF criteria respectively. In both definitions, females had significantly higher MetS when compared to males (31.4% vs 19.6%; p=0.03 by NCEP), and (34.3% vs 21.7%; p =0.03 by IDF), respectively. Duration >5 years with mental illness indicated higher MetS when compared to duration ≤ 5 years (42.9% vs 19.9%, p=0.001; and 46.9% vs 21.9%, p<0.0001) in NCEP and IDF, respectively. In addition, marital status [AOR (95% CI): 2.4 (1.1–5.3)], and BMI [AOR (95% CI): 8.4(4.0–17.6)], duration > 5 years with mental illness [AOR (95% CI): 2.8(1.2–6.5)], and age >40 years [AOR (95% CI): 2.7(1.2–6.1)] were significantly associated factors of MetS by NCEP. While BMI, age >40 years and duration > 5 years with mental illness were associated with MetS by IDF.
Conclusion
Long-time experience with severe mental illness and antipsychotic therapy might predispose patients to metabolic complications with significant risks of cardiovascular events. Therefore, intensive screening of patients for MetS/components is required during follow-up based on national non-communicable diseases guideline. Besides, the proper intervention of patients concerning lifestyle changes and averting risk full behaviors is mandatory.
Keywords: severe mental illness, antipsychotic agents, metabolic syndrome, Hawassa, Southern-Ethiopia
Introduction
Psychotic problems and mental sickness consequences reduced the quality of life in patients.1,2 In addition, patients with schizophrenia, depression or bipolar illnesses have poor physical health and low life expectancy in comparison to the general population. Patients with severe mental disorders are more likely to be overweight and to have diabetes mellitus (DM), hypertension and lipid derangements, and these factors may predispose them to a high mortality rate.3,4 In addition, patients with severe mental illnesses (SMI) have a high risk for developing metabolic-related complications like metabolic syndrome (MetS) and abnormality of its components that include overweight to obesity, type-2 DM, dyslipidemia, and hypertension.4 Besides patients with SMI have also a high prevalence of MetS and obesity when compared to the general population.5 Moreover, MetS and obesity are commodities that linked with psychiatric disorders and more than 40% of the cases were affected by such complications,6 and almost 60% mortality related to physical commodities, largely with cardiovascular diseases (CVD).7 Schizophrenic patients had a high prevalence of overweight or obesity when compared to individuals without schizophrenic disorders.8 In addition, schizophrenia also is associated with a high risk of DM when compared with the general population.4 Factors like inactive/sedentary lifestyle, abnormal eating behaviors, stressful characteristics and or psychotropic medication may increase the rate MetS in schizophrenic, schizoaffective or bipolar disordered patients.9–12 Other factors like using second-generation antipsychotic drugs (olanzapine and clozapine) can be associated with obesity and mainly causes metabolic disorders, with a significant gaining of weight, lipid derangements, and blood glucose level alterations.13,14 The increased rate of depression and its symptoms were associated with MetS, whereas anxiety-related problems were not associated with MetS.15 Furthermore, most studies have shown that patients with SMI have a high risk of death, due to cardiovascular events, with a minimum of three times risks full when compared to the general population.16,17
To the best of our knowledge, data are very scarce regarding MetS among patients with severe mental problems in Ethiopia, mainly in the study area. Therefore, the current study aimed to assess the prevalence and its associated factors of MetS among patients with severe mental illness.
Methods
Study Setting and Study Population
This hospital-based cross-sectional study was conducted in Hawassa University Comprehensive Specialized Hospital at the psychiatric outpatient department from January to June 2019. Hawassa is the capital city of the Southern nation’s nationalities and Peoples Region (SNNPR) and located 275 km to the South direction from Addis Ababa (the capital city of Ethiopia). The hospital established in November 2005 and expected to serve for more than twenty million peoples of the region including neighboring regions. This hospital is one of the health facilities found in the region, which provides public health services for patients. All participants aged ≥18 years who had a regular follow up in the psychiatric outpatient department and receiving treatment for a minimum of one year were eligible for the study. However, patients receiving lipid levels affecting drugs and pregnant women were not included in the study.
Sample Size and Sampling Technique
The sample size estimation was based on a 29% prevalence rate of MetS among patients with SMI that was conducted in Jimma, Southwest Ethiopia, and the required sample size was computed using a single population proportion formula at 95% confidence interval (CI).18
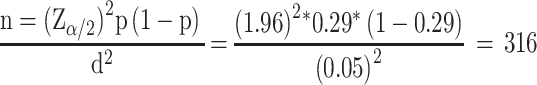 |
Where, P= proportion of MetS, Z/2= Critical value at 95% level of confidence (Z =1.96) d= Margin of error (5%), n=the required sample size that is 316. However, sample size correction was done using Cochran’s sample size correction formula based on the number of psychiatric population and a 12% non-response rate was also considered. Following this, the final sample size calculated to be 250. To select the study subjects from the study population, the patients flow in the psychiatric outpatient was checked for one week. Hence, the trend indicated the average weekly patients’ flow was around 60–70 cases for psychiatric patients. Lastly, every fourth patient was selected using a systematic random sampling technique.
Definition of MetS
According to the modified National Cholesterol Education Adult Treatment Panel (NCEP-ATP) III guideline, patients should have a minimum of three of the following abnormal components to be classified in the group of MetS. (1) triglycerides (TGs) ≥150 mg/d; (2) the high density lipoprotein-cholesterol (HDL-c) <40mg/dl in males and <50mg/dl in females (3) systolic/diastolic blood pressure (BP) ≥130/85mmHg (previously diagnosed hypertension/on treatment); (4) Fasting blood sugar (FBS) ≥100mg/dl (previously diagnosed type 2 DM and or treatment); and (5) waist circumference (WC) >102 cm in males and >88cm in females.19 Whereas according to IDF definition, MetS described as the presence of abdominal obesity (WC ≥94 cm in males and ≥80 cm in females for Sub-Sahara Africans) plus any two of the other four criteria which are basically alike to those mentioned in NCEP-ATP III above.20
Data Collection and Assessments
Data on socio-demographic, anthropometric, clinical information and recent treatment information was collected using pre-tested and structured questionnaires. Next to this, trained psychiatric nurses who were working in the psychiatric outpatient department did the measurements of blood pressure, height, weight, and WC of the study subjects. In addition, BP was measured using a digital electronic sphygmomanometer (Omron, Healthcare, Japan) after patients rested and fully stabilized for a minimum of 5–10 min in the department. Regarding measurement precision, two readings were taken within a three-minute difference and lastly, the average was taken to evaluate the BP level. Both weight and height of patients were measured according to the WHO stepwise approach,21 using a digital electronic Adult scale (ASTO) that contain both weight scale as well as height scales. The weight and height measured when an individual has stood on a weight scale wearing light clothes without shoes. Moreover, body mass index (BMI) was calculated as the weight in kilogram divided by height squared (m2) and then the result was classified based on the international conventions: BMI <18.5 Kg/m2 (underweight), 18.5–24.9 Kg/m2(normal weight), 25–29.9 kg/m2(overweight), and ≥30 kg/m2 (obesity).22 Furthermore, WC was measured according to the WHO stepwise technique,23 with the erect stand-up position following normal out breathing by non- stretching tape to the nearest 0.1cm.
Blood Collection and Biochemical Analysis
About 4–5 milliliters of venous blood was collected from each study subject after overnight fasting and then serum was obtained for lipid profiles and FBS investigations. Serum biochemicals determination was done using the A25TM BioSystem Random Access chemistry instrument (BioSystems S.A. Costa Brava 30, Barcelona (Spain) (BioSystemsTM, Spain)). Fasting serum TGs and blood sugar were analyzed using an enzymatic colorimetric method (Linear chemicals, Montgat, Spain). While serum HDL-c was analyzed by enzymatic colorimetric method using total cholesterol reagent after a selective precipitation technique of lipoproteins that contain apo-lipoproteins (very-low-density lipoprotein cholesterol, low-density lipoprotein cholesterol and apolipoprotein a) through phosphotungstic acid or magnesium chloride method (Linear chemicals, Montgat, Spain).
Statistical Analysis
The questionnaires were double-checked and entered into Statistical Package for Social Sciences (SPSS), Version 20 for statistical analysis. Descriptive statistics (frequency, means, standard deviation, and percentages) were used to describe study subjects with different variables. Chi-square and or fisher’s exact test were used to evaluate the significance of categorical variables with the study outcome. Besides, the Student’s t-test was used to assess the differences between quantitative continuous data and the study groups. Both bivariate and multivariate binary logistic regression models were used to evaluate distributional differences of the categorical variables in relation to the study groups. Moreover, only a variable with p-value < 0.25 was considered for multivariable analysis and finally p-value <5% was accepted as statistical significance.
Data Quality
The quality of data was maintained by pre-testing of 10% questionnaires in Adare General Hospital, which is around 5 Km distant from the study hospital. Next to this, all required improvement was done in the questionnaire following pretest feedback. Only trained psychiatric nurses and laboratory technologists have participated in data collection, blood sample collection, and laboratory diagnosis.
Moreover, commercially prepared quality control samples run was done daily prior to running the study samples in order to assess the function of the instrument’s, laboratory chemicals, and procedural performances with the strict following of standard operating procedures (SOPs).
Ethical Considerations
Ethical clearance was obtained on August 02, 2017 from the institutional review board (IRB) of Hawassa University College of Medicine and health sciences (Ref No: IRB 100 09) and adhered to the codes of the declaration of Helsinki for Ethical Principles for Medical Research Involving Human Subjects (World Medical Association Declaration of Helsinki: Nov 27, 2013). Permission request letter together with ethical clearance also was submitted to the Hawassa University Comprehensive specialized Hospital Clinical and Academic Director office to get go-ahead permission. In addition, the participants were well informed about the protocol of the study and written informed consent obtained from every study subject before data collection. Individuals were well informed about the full right to refuse participation/withdraw at any point in the study. Further, the privacy of the data was well-kept.
Results
Socio-Demographic and Other Features of the Study Participants
A total of 250 psychiatric patients approached, of them, 5 were refused to take part in the study. Of 245 SMI participants, 59.2% were males and 40.8% were females with a mean (±SD) age of 32 (±11.4) years old. Regarding ethnicity: 28.6%, 20.8%, and 16.7%, 12.7%, and 21.2% were Sidama, Amhara, Oromo, Wolayita and other ethnic groups, respectively. About 58.4% and 55.1% of the study participants were non-married, and Protestants in religion, respectively. The majority (86.1%) have a sedentary lifestyle, 1.6% were previously diagnosed diabetes cases, and 1.6% were known hypertensives. The mean (SD) since the diagnosis of mental illness was 3.8(2.3) years (Table 1).
Table 1.
Socio-Demographic and Behavioral Characteristics of Patients with Severe Mental Problems
| Variables | Total n=245 | Male n=143 | Female n=102 | p-value | |
|---|---|---|---|---|---|
| Mean Age, years (±SD) | 32(11.2) | 31.5(11.3) | 32.7(11.1) | 0.43 | |
| <20 | 24(9.8) | 14(9.8) | 10(9.8) | ||
| 20–34 | 138(56.3) | 85(59.4) | 53(52) | ||
| 35–49 | 64(26.1) | 32(22.4) | 32(31.4) | ||
| ≥50 | 19(7.8) | 12(8.4) | 7(6.9) | 0.45 | |
| Marital status= | Single | 143(58.4) | 89(62.2) | 54(52.9) | |
| Married | 99(40.4) | 52(36.4) | 47(46.1) | 0.31 | |
| Divorced/Widow/er | 3(1.2) | 2(1.4) | 1(1.0) | ||
| Education = Unable to read and write | 12(4.9) | 3(2.1) | 9(8.8) | ||
| Primary | 93(38) | 50(35) | 43(42.2) | ||
| Secondary | 55(22.4) | 34(23.8) | 21(20.6) | ||
| Tertiary | 85(34.7) | 56(39.2) | 29(28.4) | <0.036 | |
| Occupation = | Farmers | 16(6.5) | 14(9.8) | 2(2.0) | |
| Employed | 58(23.7) | 35(24.5) | 23(22.5) | ||
| Merchants | 17(6.9) | 12(8.4) | 5(4.9) | ||
| Housewife | 28(11.4) | 0(0.0) | 28(27.5) | ||
| Students | 55(22.4) | 33(23.1) | 22(21.6) | ||
| Jobless | 71(29.0) | 49(34.3) | 22(21.6) | <0.0001 | |
| Religion= | Orthodox | 78(31.8) | 46(32.2) | 32(31.4) | |
| Protestant | 135(55.1) | 75(52.4) | 60(58.8) | ||
| Muslim | 26(10.6) | 19(13.3) | 7(6.9) | 0.40 | |
| Others | 6(2.4) | 3(2.1) | 3(2.9) | ||
| Ever drunk alcohol= | No | 231(94.3) | 130(90.9) | 101(90.0) | |
| Yes | 14(5.7) | 13(9.1) | 1(1.0) | 0.009 | |
| Ever smocked cigarette = | No | 227(92.7) | 126(88.1) | 101(99) | 0.001 |
| Yes | 18(7.3) | 17(11.9) | 1(1.0) | ||
| Hypertensives= | No | 241(98.4) | 140(97.9) | 101(99) | 0.64 |
| Yes | 4(1.6) | 3(2.1) | 1(1.0) | ||
| Diabetes = | Yes | 4(1.6) | 2(1.4) | 2(1.6) | 0.99 |
| No | 241(98.4) | 141(98.6) | 100(98) | ||
| Physical exercise= | Sedentary | 211(86.1) | 119(83.2) | 92(90.2) | 0.29 |
| Light | 14(5.7) | 10(7.0) | 4(3.9) | ||
| Moderate | 20(8.2) | 14(9.8) | 6(5.9) | ||
| Khat chewing= | No | 223(91) | 122(85.3) | 101(99) | <0.0001 |
| Yes | 22(9.0) | 21(14.7) | 1(1.0) | ||
| Duration of illness, years, mean (±SD) | 3.8(2.3) | 3.9(2.7) | 3.6(2.5) | 0.63 | |
| 1–4 years | 170(69.4) | 95(66.4) | 75(73.5) | 0.45 | |
| 5–9 years | 65(26.5) | 41(28.7) | 24(23.5) | ||
| ≥10years | 10(4.1) | 7(4.9) | 3(2.9) | ||
Note: Values are in percent unless and otherwise indicated.
Abbreviation: SD, standard deviation.
Types of Mental Disorders, and Other Features of Patients with Severe Mental Problems
About 47.8% of the patients had schizophrenia, 35.5% had a major depressive disorder with psychotic features, 9% had bipolar disorders, 3.7% had delusional disorders, 3.7% had the schizophrenic-form disorder, and the rest 0.4% had schizoaffective disorder types of mental illnesses.
Forty (16.3%) patients were overweight and 6.1% were obese. Patients experienced with SMI for more than five years had significantly higher mean value of BMI, SBP, DBP and TGs when compared to those experienced the problem for less than or equal five years (Table 2).
Table 2.
Characteristics of Patients with the Severe Mental Problem in Relation to Disease Duration
| Variables | Total 245 | Duration ≤5 Years (n=196) | Duration >5 Years (n=49) | p-value |
|---|---|---|---|---|
| Mean BMI, Kg/m2(SD) | 22.9(4.6) | 22.4(4.3) | 24.7(5.5) | <0.007 |
| <18.5 Kg/m2 | 26(10.6) | 24(12.2) | 2(4.1) | |
| 18.5–24.9 Kg/m2 | 164(66.9) | 135(68.9) | 29(59.2) | |
| 25–29.9 Kg/m2 | 40(16.3) | 30(15.3) | 10(20.4) | <0.003 |
| ≥30 Kg/m2 | 15(6.1) | 7(3.6) | 8(16.3) | |
| Mean WC, cm(SD) | 81.8(10.5) | 81.2(10.1) | 84.3(11.4) | 0.06 |
| Mean SBP, mmHg(SD) | 120(9.4) | 119(9.1) | 123.3(10.1) | 0.006 |
| Mean DBP, mmHg(SD) | 78.8(6.8) | 78.3(6.6) | 80.7(7.4) | 0.03 |
| Mean FBS, mg/dl(SD) | 96.9(26.3) | 95.5(24.7) | 102.3(31.6) | 0.10 |
| Mean HDL-c, mg/dl(SD) | 50.2(17.7) | 50.9(17.4) | 47.2(18.4) | 0.18 |
| Mean TGs, mg/dl (SD) | 122.7(58.8) | 118.6(58.2) | 139(59.4) | 0.03 |
Note: Values are in percent unless and otherwise indicated.
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FBS, fasting blood sugar; HDL-c, high-density lipoprotein cholesterol; SD, standard deviation; WC, waist circumference; SBP, systolic blood pressure; TGs, triglycerides.
Treatment Allocations
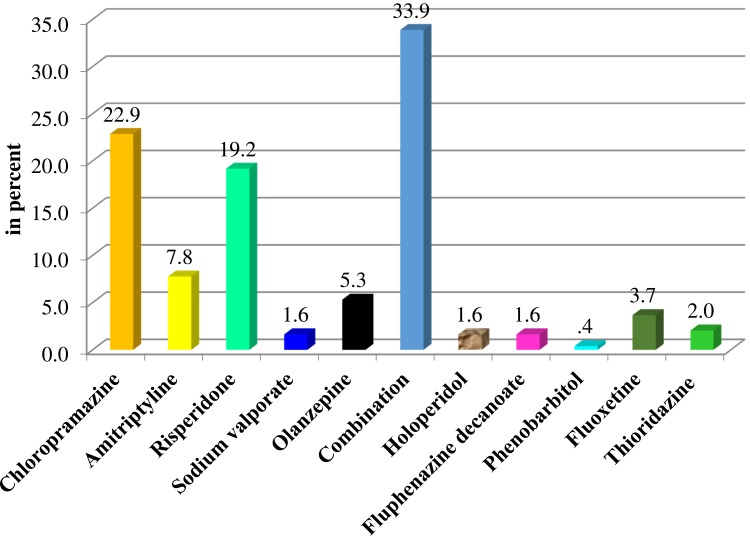
The majority (66.1%) of the study participants were on a single type of drug, whereas 33.9% were taking at least two drugs. Patients on chlorpromazine were 22.9%, while 19.2% were receiving risperidone. About 35.9% were on first-generation antipsychotic drugs and 3.7% were on antidepressants, while rest were receiving second generation and combined treatments (Figure 1).
Figure 1.
Proportion of patients’ allocation to different anti-psychotic agents.
Prevalence of MetS with Gender and the Experiences of Disease Duration
MetS was determined using both IDF and NCEP-ATP III definitions. Based on IDF, the overall prevalence of MetS was 26.9% and patients who experienced mental illness for more than 5 years had a significantly higher proportion of MetS when compared to those experienced less than or equal to 5 years (46.9% vs.21.9%, p<0.0001). Whereas the overall MetS was 24.5% in NCEP-ATP III criteria, and a significant difference was observed with the duration of mental illness. In addition, raised BP (≥130/85mmHg), TG ≥150 mg/dl, BMI ≥25kg/m2, and FBS were significantly higher among patients who experienced the mental problem for more than 5 years when compared to those experienced less than or equal to 5 years (Table 3).
Table 3.
Prevalence of Metabolic Syndrome and Its Components Among Patients with Severe Mental Problems
| Parameters | Total 245(%) | ≤5 Years 196(%) | >5 Years 49(%) | p-value |
|---|---|---|---|---|
| Metabolic syndrome by NCEP-ATP III | 60(24.5) | 39(19.9) | 21(42.9) | 0.001 |
| Metabolic syndrome by IDF | 66(26.9) | 43(21.9) | 23(46.9) | <0.0001 |
| Abdominal obesity by NCEP | 30(12.2) | 21(10.7) | 9(18.4) | 0.14 |
| Abdominal obesity by IDF | 61(24.9) | 45(23.0) | 16(32.7) | 0.16 |
| Triglycerides (≥150mg/dl) | 66(26.9) | 45(23.0) | 21(42.9) | 0.005 |
| Reduced HDL-c | 101(41.2) | 76(38.8) | 25(51.0) | 0.12 |
| Fast blood sugar (≥110mg/dl) | 84(34.3) | 62(31.6) | 24(49.0) | 0.02 |
| Raised SBP (≥130mmHg) | 5(70.6) | 37(18.9) | 17(34.7) | 0.02 |
| Raised DBP (≥85mmHg) | 65(26.5) | 48(24.5) | 17(34.7) | 0.15 |
| BP (≥130/85mmHg) | 54(22.0) | 38(19.4) | 16(32.7) | 0.045 |
| BMI≥25Kg/m2 | 55(22.4) | 37(18.9) | 18(36.7) | 0.007 |
Abbreviations: BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; dl, deciliter; HDL-c, High-Density Lipoprotein-cholesterol; IDF, International Federation of Diabetes; NCEP-ATP, national cholesterol education adult treatment panel; mmHg, millimeter of mercury; SBP, systolic blood pressure.
Females had a significantly higher rate of MetS when compared to males in both criteria (31.4% vs 19.6%, p=0.03 by NCEP-ATP; and 34.3% vs 21.7%, p=0.03 by IDF, respectively). The prevalence of reduced HDL-c was significantly higher in females when compared to males (52% vs 34.3%, p=0.009). In addition, abdominal obesity was significantly higher among females when compared to males both criteria (Figure 2).
Figure 2.
Prevalence of metabolic syndrome and its components among psychiatric patients in relation to sex.
Notes: Red bars represent males; green bars represent females. (P-values: HDL-c, 0.009; BMI, 0.001; abdominal obesity (NCEP) & IDF, <0.0001; SBP, 0.43; DBP, 0.57; TG, 0.89; BP≥130/85mmHg, 0.63; FBS, 0.96; MetS (NCEP) & IDF, 0.03)
Abbreviations: BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; dl, deciliter; HDL-c, High Density Lipoprotein-cholesterol; IDF, international federation of diabetes; NCEP-ATP, national cholesterol education adult treatment panel; mmHg, millimeter of mercury.
Factors Associated with MetS Among Patients with Severe Mental Problems
Both bivariate and multivariable analysis were used to assess the association of independent factors with the prevalence of MetS among patients with SMI in both definitions. Using the NCEP ATP III criteria: being female the crude odds ratio [COR (95% CI): 1.9(1.1–3.4); p=0.04] and age >40years [COR (95% CI): 4.7(2.5–8.8); p<0.0001] were significantly associated with MetS. In addition, marital status [COR (95% CI): 3.3(1.8–6.0); p<0.0001], abnormal BMI [COR (95% CI): 8.9(4.5–17.2); p<0.0001] and disease duration more than 5 years since its diagnosis [COR (95% CI): 3.0(1.5–5.8); p=0.001] were significantly associated with MetS. Moreover, multivariable analysis also was adjusted for the possible confounding factors. Marital status, the adjusted odds ratio [AOR (95% CI): 2.4 (1.1–5.3); p= 0.036], BMI [AOR (95% CI): 8.4(4.0–17.6); p<0.0001], age >40 years [AOR (95% CI): 2.7(1.2–6.1); p<0.016] and more than 5 years experiences with SMI [AOR (95% CI): 2.8(1.2–6.5); p=0.013] were significantly associated factors of MetS (Table 4).
Table 4.
Factors Associated with Metabolic Syndrome Among Psychiatric Patients by NCEP-ATP III
| Variable | MetS= 60(%) | No MetS 185(%) | COR(95% CI) | P-value | AOR(95% CI) | P-value | ||
|---|---|---|---|---|---|---|---|---|
| Gender | Males | 28(46.7) | 115(62.2) | 1.00 | 1.00 | |||
| Females | 32(53.3) | 70(37.8) | 1.9(1.1–3.4) | 0.04 | 1.0(0.51–2.3) | 0.83 | ||
| Age in years: | ≤40 | 28(46.7) | 149(80.5) | 1.00 | 1.00 | |||
| >40 | 32(53.3) | 36(19.5) | 4.7(2.5–8.8) | <0.0001 | 2.7(1.2–6.1) | 0.016 | ||
| Marital status: | Currently single§ | 22(36.7) | 121(65.4) | 1.00 | 1.00 | |||
| Married | 38(63.3) | 64(34.6) | 3.3(1.8–6.0) | <0.0001 | 2.4(1.1–5.3) | 0.036 | ||
| Occupation: | Employed | 18(30) | 40(21.6) | 1.00 | 1.00 | |||
| Merchants | 4(6.7) | 13(7.0) | 1.5(0.78–3.0) | 0.21 | 1.1(0.45–2.6) | 0.84 | ||
| Farmers | 3(5.0) | 13(7.0) | 1.0(0.32–3.4) | 0.94 | 1.2(0.29–4.7) | 0.82 | ||
| Non-employed | 35(58.3) | 119(64.3) | 0.78(0.2–2.9) | 0.72 | 0.34(0.06–1.9) | 0.22 | ||
| BMI, Kg/m2 | Normal | 17(28.3) | 144(77.8) | 1.00 | 1.00 | |||
| Abnormal§ | 43(71.7) | 41(22.2) | 8.9(4.5–17.2) | <0.0001 | 8.4(4.0–17.6) | <0.0001 | ||
| Duration of mental illness | ≤5 years | 39(65.0) | 157(84.9) | 1.00 | 1.00 | |||
| >5 years | 21(35.0) | 28(15.1) | 3(1.5–5.8) | 0.001 | 2.8(1.2–6.5) | 0.013 | ||
| Ever drunk alcohol | No | 59(98.3) | 172(93.0) | 1.00 | ||||
| Yes | 1(1.7) | 13(7.0) | 4.5(0.57–34.8) | 0.15 | 2.6(0.29–23.6) | 0.39 | ||
Notes: §For the statistical analysis, both abnormally high and low body mass index was combined together as abnormal BMI; and both single and divorced cases were merged together as currently single.
Abbreviations: AOR, adjusted odds ratio; BMI, body mass index; COR, crude odds ratio; CI, Confidence Interval; MetS, Metabolic syndrome; NCEP-ATP III, national cholesterol education adult treatment panel III.
Whereas using IDF definition: being female [COR (95% CI): 1.9(1.1–3.3); p=0.03], age >40years [COR (95% CI): 4.5(2.5–8.3); p<0.0001], and the duration more than 5 years with SMI [COR (95% CI): 3.1(1.6–6.1); p=0.001] were significantly associated with MetS. In addition, marital status [COR (95% CI): 3.1(1.7–5.7); p<0.0001], and BMI [COR (95% CI): 8.5(4.5–16.1); p<0.0001] were significantly associated with MetS. Moreover, the multivariable analysis was maintained for possible confounding factors. Age >40 years [AOR (95% CI): 2.5(1.2–5.5); p= 0.02], BMI [AOR (95% CI): 8.2(4.0–16.6); p<0.0001] and the duration more than 5 years with SMI [AOR (95% CI): 2.9(1.3–6.5); p<0.008] were significantly and positively associated factors of MetS (Table 5).
Table 5.
Factors Associated with Metabolic Syndrome Among Psychiatric Patients by IDF
| Variable | MetS= 66(%) | No MetS= 179(%) | COR(95% CI) | P-value | AOR(95% CI) | P-value | ||
|---|---|---|---|---|---|---|---|---|
| Gender | Males | 31(47) | 112(62.6) | 1.00 | 1.00 | |||
| Females | 35(53.0) | 67(37.4) | 1.9(1.1–3.3) | 0.03 | 1.2(0.59–2.4) | 0.61 | ||
| Age in years: | ≤40 | 28(46.7) | 149(80.5) | 1.00 | 1.00 | |||
| >40 | 32(53.3) | 36(19.5) | 4.5(2.5–8.3) | <0.0001 | 2.5(1.2–5.5) | 0.02 | ||
| Marital status: | Currently single§ | 25(37.9) | 118(65.9) | 1.00 | 1.00 | |||
| Married | 41(62.1) | 61(34.1) | 3.1(1.7–5.7) | <0.0001 | 2.1(0.98–4.5) | 0.055 | ||
| BMI, Kg/m2 | Normal | 20(30.3) | 141(78.8) | 1.00 | 1.00 | |||
| Abnormal§ | 46(69.7) | 38(21.2) | 8.5(4.5–16.1) | <0.0001 | 8.2(4.0–16.6) | <0.0001 | ||
| Duration of mental illness | ≤5 years | 43(65.2) | 153(85.5) | 1.00 | 1.00 | |||
| >5 years | 23(34.8) | 26(14.5) | 3.1(1.6–6.1) | 0.001 | 2.9(1.3–6.5) | 0.008 | ||
| Ever drunk alcohol | No | 65(98.5) | 166(92.7) | 1.00 | 1.00 | |||
| Yes | 1(1.5) | 13(7.3) | 5.1(0.65–39.7) | 0.12 | 3.1(0.36–27.2) | 0.30 | ||
Notes: §For the statistical analysis, both abnormally high and low body mass index was combined together as abnormal BMI; and both single and divorced cases were merged together as currently single.
Abbreviations: AOR, adjusted odds ratio; BMI, body mass index; COR, crude odds ratio; CI, Confidence Interval; MetS, Metabolic syndrome; IDF, international diabetes federation.
Discussion
We found the overall prevalence of MetS was 24.5% (95% CI: 19.2–29.8) by NCEP criteria and the finding was almost comparable with the different studies report: 22.2% in Southwest Ethiopia,18 24.3% in Brazil,24,25 23.2% in South Africa,26 and 27.5% in Egypt27 using NCEP. In addition, we found the prevalence of MetS was 26.9% (95% CI: 21.2–32.7) by IDF definition and it was comparable with the study conducted in southwest Ethiopia,18 which was 28.9%. However, the higher rate of MetS was reported by different international studies: 43.6% in Palestine,28 32.3% in Turkey,29 34.7% in Kashmir,30 49% in Australia,31 and 32% in South Africa32 in NCEP criteria. In addition, higher rates also were reported by different studies using IDF like 35% in Hong Kong,9 54% in Australia,31 32.8% in Italy,33 and 38.1% in Egypt.34 The variations may be due to differences in types or generation of antipsychotic agents utilized by the patients, patients’ lifestyle and genetic disparities between populations. In evidence, about 41.6% of our study participants were using the first-generation antipsychotic agents and antidepressants, while the rest 58.4% were on second-generation and combination therapy. Moreover, the result of MetS was higher than the rate reported from the general adult populations of Ethiopia, which was 12.5% by NCEP and 17.9% by IDF.35 This could be suggestive for a metabolic disturbance of patients with severe mental illness receiving antipsychotic agents might develop cardiovascular and other health influences in near future, in spite of lessening the severity of mental illness through treatments when compared to general populations.
The present study indicated that females had significantly higher MetS when compared to males in both definitions. This in line with the reports of several studies.18,25,26,28,30,33 However, the current study indicated that no association between gender and MetS in both definitions. This is not in line with the different studies report25,26,28,33 and this is not in line with the finding of the current study. About 73.5% of our study females SMI experience was less than five years and the trend of our study indicated that the abnormality of MetS components increased with the increased duration of disease and this might be a reason for discordances with different studies finding.
We found that BMI was associated with the presence of MetS in both NCEP and IDF. This report was in line with the study conducted in Palestine,28 Turkey,29 and Brazil.25 Moreover, the study conducted in Palestine,28 also indicated that the duration of SMI was significantly associated with the presence of MetS. This in line with the finding of the current study. However, the study conducted in Southwest Ethiopia revealed insignificant association between MetS and duration of mental illness,18 and this might be due to the classification variability of illness duration for analysis purposes.
We found that a significant association of age with MetS. Similarly, studies indicated a significant association between the age of patients and MetS.18,29,33 Moreover, age greater 40 years and therapy might favor the risks for the development of MetS. 36,37
Furthermore, marital status was significantly associated with MetS by NCEP. This finding in line with the reports of previously conducted several studies.36,38,39 In support Rguibi et al40 reported that weight gaining was significantly higher among the married groups when compared to the non-married group and more satisfaction in marriage like situations might consequence the abnormality of MetS components.
Limitation of the Study
First, we did not include the normal population as the control group, however, we tried to compare findings of this study with previously conducted MetS of Ethiopian normal adult population,25 and this is an evidence for the increment of MetS among patients with SMI when compared to the control group. Second, due to its metrical difficulties, we did not assess, an individual’s consumption type and amount of nutrition. Third, our study was cross-sectional by its nature and signifying that it cannot evidence of MetS and its causative risk factors sufficiently. Fourth, we applied only two criteria to define MetS; a wide-ranged rate might be observed if we used other guidelines like the American Heart Association (AHA) and or world health organization (WHO) criteria. Regardless of the mentioned limitations, this study provides helpful information in the scarce data situation of Ethiopia including the study area.
Conclusion
The present study revealed 26.9% and 24.5% of MetS in IDF and NCEP-ATP III criteria, respectively. In both definitions, females had a higher rate of MetS compared to males with a significant difference. In addition, marital status and BMI were significantly associated with MetS in both definitions. While the duration of patients since the diagnosis of the mental problems was associated with MetS by IDF. Therefore, the duration of SMI experiences and therapeutic conditions might predispose patients to metabolic complications with a significant risk of cardiovascular events. Therefore, intensive screening of patients should be done in each follow-up through the strict adherence of national non-communicable disease guidelines. Moreover, proper intervention and counseling of patients’ are mandatory regarding moderate-intensive physical activities performing, lifestyle modifications and reducing of risk full behaviors. Furthermore, therapeutic options should also be applied for abnormal components of MetS based on appropriate medical decisions.
Acknowledgments
We want to acknowledge psychiatric nurses who were working in the study hospital for their support during socio-demographic, clinical and behavioral related data collection and Mr. Abebe Kifle for his support during blood sample collection. Further, our gratitude is also protracted to psychiatric patients for their voluntary contribution to the study.
Abbreviations
BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; IDF, international diabetic federation; HDL-c, HDL-cholesterol; WC, waist circumference; WHO, World Health Organization; TG, triglycerides; SMI, severe mental illness; SPSS, Statistical package for social sciences; NCEP-ATP, National Cholesterol Education Program, Adult Treatment Panel; MetS, metabolic syndrome.
Data Sharing Statement
The dataset of this article is not openly available, but it can be accessible on reasonable appeal from the corresponding author with the permission of Hawassa University’s comprehensive specialized hospital clinical director office.
Author Contributions
All authors contributed toward data analysis, drafting and revising; gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Funding
This study was financially supported by NORAD project of Hawassa University.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Hansson L. Determinants of quality of life in people with severe mental illness. Acta Psychiatr Scand Suppl. 2006;429:46–50. doi: 10.1111/j.1600-0447.2005.00717.x [DOI] [PubMed] [Google Scholar]
- 2.Talarowska M, Florkowski A, Orzechowska A, Zbolarski K, Wysokiński A. Konsekwencje psychologiczne zespołu metabolicznego. Psychiatria w Praktyce Klinicznej. 2008;1:67–73. [Google Scholar]
- 3.Correll CU, Detraux J, De Lepeleire J, De Hert M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry. 2015;14:119. doi: 10.1002/wps.v14.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Hert M, Dekker JM, Wood D, Kahl KG, Holt RI, Möller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry. 2009;24:412–424. doi: 10.1016/j.eurpsy.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 5.Carpiniello B, Pinna F, Velluzzi F, Loviselli A. Mental disorders in patients with metabolic syndrome. The key role of central obesity. Eat Weight Disord. 2012;17:e259–66. [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome- A new world-wide definition. A Consensus Statement from the international diabetes federation. Diabet Med. 2006;23:469–480. [DOI] [PubMed] [Google Scholar]
- 7.De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10:138–151. doi: 10.1002/wps.2011.10.issue-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gopalaswamy AK, Morgan R. Too many chronic mentally disabled patients are too fat. Acta Psychiatr Scand. 1985;72:254–258. doi: 10.1111/acp.1985.72.issue-3 [DOI] [PubMed] [Google Scholar]
- 9.Bressington DT, Mui J, Cheung EFC, Petch J, Clark AB, Gray R. The prevalence of metabolic syndrome amongst patients with severe mental illness in the community in Hong Kong–a cross sectional study. BMC Psychiatry. 2013;13:87. doi: 10.1186/1471-244X-13-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ventriglio A, Gentile A, Stella E, Bellomo A. Metabolic issues in patients affected by schizophrenia: clinical characteristics and medical management. Front Neurosci. 2015;9:297–303. doi: 10.3389/fnins.2015.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, Nurjono M, Wong A, Salim A. Prevalence of metabolic syndrome among patients with schizophrenia in Singapore. Ann Acad Med. 2012;41:457–462. [PubMed] [Google Scholar]
- 12.De Hert M, Detraux J, Vancampfort D. The intriguing relationship between coronary heart disease and mental disorders. Dialog Clin Neurosci. 2018;20:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123:225–233. doi: 10.1016/j.schres.2010.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonseka TM, Müller DJ, Kennedy SH. Inflammatory cytokines and antipsychotic-induced weight gain: review and clinical implications. Mol Neuropsychiatry. 2016;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi T, Nakao M, Nomura K, et al. Association of the metabolic syndrome with depression and anxiety in Japanese men: a 1-year cohort study. Diabetes Metab Res Rev. 2009;25:762–767. doi: 10.1002/dmrr.1041 [DOI] [PubMed] [Google Scholar]
- 16.Osborn DP. The poor physical health of people with mental illness. West J Med. 2001;175:329–332. doi: 10.1136/ewjm.175.5.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latoo J, Mistry M, Dunne FJ. Physical morbidity and mortality in people with mental illness. BJMP. 2013;6:a621. [Google Scholar]
- 18.Asaye S, Bekele S, Tolessa D, Cheneke W. Metabolic syndrome and associated factors among psychiatric patients in Jimma University Specialized Hospital, South West Ethiopia. Clin Res Rev. 2018;12:753–760. [DOI] [PubMed] [Google Scholar]
- 19.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 20.Zimmet P, Alberti KGM, Serrano Ríos M. A New International Diabetes Federation (IDF) Worldwide definition of the metabolic syndrome: the rationale and the results. Rev Esp Cardiol. 2005;58:1371–1376. [PubMed] [Google Scholar]
- 21.World Health Organization (WHO). Chronic diseases and health promotion: stepwise approach to surveillance (STEPS); 2010. Available from: http://www.who.int/chp/steps/manual/en/. Accessed February19, 2020.
- 22.Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158:1855–1867. doi: 10.1001/archinte.158.17.1855 [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. WHO Stepwise Approach to Surveillance (STEPS). Geneva: World Health Organization (WHO); 2008. [Google Scholar]
- 24.Moreira FP, Jansen K, Cardoso TA, et al. Metabolic syndrome and psychiatric disorders: a population-based study. Braz J Psychiatry. 2019;41:38–43. doi: 10.1590/1516-4446-2017-2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teixeira PJR, Rocha FL. The prevalence of metabolic syndrome among psychiatric inpatients in Brazil. Rev Bras Psiquiatr. 2007;29:330–336. doi: 10.1590/S1516-44462007000400007 [DOI] [PubMed] [Google Scholar]
- 26.Saloojee S, Burns JK, Motala AA. Metabolic syndrome in South African patients with severe mental illness: prevalence and associated risk factors. PLoS One. 2016;11:e0149209. doi: 10.1371/journal.pone.0149209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mousa FA, Dessoki HH, El Kateb SM, Ezzat AA, Soltan MR. Metabolic syndrome in psychiatric patients (comparative study). Egypt J Psychiatry. 2017;38:179–191. doi: 10.4103/ejpsy.ejpsy_24_17 [DOI] [Google Scholar]
- 28.Sweileh WM, Zyoud SH, Dalal SA, Ibwini S, Sawalha AF, Ali I. Prevalence of metabolic syndrome among patients with schizophrenia in Palestine. BMC Psychiatry. 2012;12:235. doi: 10.1186/1471-244X-12-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Çankaya PK, Tiryaki A, Arslan FC, Çankaya S. Comparison of the prevalence of metabolic syndrome in patients with schizophrenia and bipolar disorder: a cross-sectional study from Black Sea region. Am J Psychiatry. 2018;19:346–354. [Google Scholar]
- 30.Hussain T, Margoob MA, Shoib S, Shafat M, Chandel RK. Prevalence of metabolic syndrome among psychiatric inpatients: a hospital based study from Kashmir. J Clin Diagn Res. 2017;11:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.John AP, Koloth R, Dragovic M, Lim SC. Prevalence of metabolic syndrome among Australians with severe mental illness. Med J Aust. 2009;190:176–179. doi: 10.5694/j.1326-5377.2009.tb02342.x [DOI] [PubMed] [Google Scholar]
- 32.Maaroganye K, Mohapi M, Krüger C, Rheeder P. The prevalence of metabolic syndrome and its associated factors in long-term patients in a specialist psychiatric hospital in South Africa. Afr J Psychiatry. 2013;19:16. [DOI] [PubMed] [Google Scholar]
- 33.Ventriglio A, Baldessarini RJ, Vitrani G, et al. Metabolic syndrome in psychotic disorder patients treated with oral and long-acting injected antipsychotics. Front Psychiatry. 2019;9:744. doi: 10.3389/fpsyt.2018.00744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatata H,El-Gohary, G., Abd-Elsalam, M., Elokda, E. Risk of metabolic syndrome among Egyptian patients with schizophrenia. Curr Psychiatr. 2009;16:85–95. [Google Scholar]
- 35.Tran A, Gelaye B, Girma B, et al. Prevalence of metabolic syndrome among working adults in Ethiopia. Int J Hypertens. 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tadewos A, Addis Z, Ambachew H, Banerjee S. Prevalence of dyslipidemia among HIV-infected patients using first-line highly active antiretroviral therapy in Southern Ethiopia: a cross-sectional comparative group study. AIDS Res Ther. 2012;9:31. doi: 10.1186/1742-6405-9-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woyesa SB, Hirigo AT, Wube TB. Hyperuricemia and metabolic syndrome in type II diabetes mellitus patients at Hawassa university comprehensive specialized hospital, South West Ethiopia. BMC Endocr Disord. 2017;17:76. doi: 10.1186/s12902-017-0226-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sirdah MM, Al Laham NA, Abu Ghali AS. Prevalence of metabolic syndrome and associated socioeconomic and demographic factors among Palestinian adults (20–65 years) at the Gaza strip. Diabetes Metab Syndr. 2011;5:93–97. doi: 10.1016/j.dsx.2012.02.024 [DOI] [PubMed] [Google Scholar]
- 39.Al-Daghri NM, Alkharfy KM, Al-Attas OS, et al. Gender dependent associations between socioeconomic status andmetabolic syndrome: a cross-sectional study in the adult Saudi population. BMC Cardiovasc Disord. 2014;14:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rguibi M, Belahsen R. Overweight and obesity among urban Sahraoui women of South Morocco. Ethn Dis. 2004;14:542–547. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- World Health Organization (WHO). Chronic diseases and health promotion: stepwise approach to surveillance (STEPS); 2010. Available from: http://www.who.int/chp/steps/manual/en/. Accessed February19, 2020.