Abstract
PURPOSE
We aimed to evaluate the safety and effectiveness of radiofrequency ablation (RFA) combined with transarterial chemoembolization (TACE) guided by multiple imaging modalities for hepatocellular carcinomas (HCCs) in special (i.e., high-risk or unfavorable) locations compared with those in conventional locations.
METHODS
A total of 122 HCC patients were enrolled, including 85 patients (69.7%) with HCC in conventional locations and 37 (30.3%) with HCC in special locations. The clinical data, overall survival (OS), progression-free survival (PFS), and procedure-related adverse events were analyzed.
RESULTS
RFA combined with TACE was successfully performed in all patients. Three complications (2.5%) occurred, with no significant difference between the conventional (n=1, 1.2%) and special (n=2, 5.4%) locations (P = 0.218). Complete tumor necrosis rate was not significantly different between the conventional (n=73, 85.9%) and special (n=34, 91.9%) locations at one-month imaging (P = 0.353). After a follow-up of 3–48 months, the PFS was 17 months for patients with HCC in conventional locations and 14 months for patients with HCC in special locations; one-year PFS rate was 68.1% in the conventional location group, not significantly (P = 0.741) different from 59.1% in the special location group. The OS was 28 months in the conventional location group while 32 months in the special location group. The cumulative one- and two-year OS rates were 89.9% and 63.3%, respectively, in the conventional location group, not significantly different from 96.3% and 65% in the special location group (P = 0.273). Age (P = 0.043) and tumor size (P < 0.001) were significant prognostic factors for OS, and tumor size (P < 0.001) was the only significant prognostic factor for PFS.
CONCLUSION
RFA guided by multiple imaging modalities combined with TACE may be safe and effective for treating HCCs in special locations.
Being one of the world’s most common malignant tumors, hepatocellular carcinoma (HCC) has an increasing incidence because of widespread hepatitis C virus infection and can cause almost 700 000 deaths in the world (1, 2). Surgical resection of tumor remains the first choice for patients with primary HCCs; however, less than 20% of patients with HCCs undergo potentially curative resection (3). Not all patients with primary HCCs have the right indications for surgical resection. Liver transplantation may offer the best chance for successful treatment but is limited by a shortage of donor organs (4) and advanced age of patients (2, 5). Transarterial chemoembolization (TACE) allows for super-selective delivery of chemotherapeutic agents to hepatic tumor lesions (6), but it is currently suggested only for large and multinodular hepatic cancers (7–10). Radiofrequency ablation (RFA) is effective and safe for small HCCs (≤5 cm) (11); however, for lesions in high-risk locations close to the hepatic hilum, large vessels and extrahepatic organs, RFA is limited in its effect and may cause major complications (2, 12). For lesions at unfavorable sites near the diaphragmatic apex and posterior ribs with poor conspicuity, ultrasonography (US)-guided RFA is often infeasible, and one-fourth of small HCC lesions are invisible when treated with US-guided RFA (13). These high-risk and unfavorable locations are defined as special locations, while other locations are conventional. In order to overcome the limitations of US guidance for RFA, contrast-enhanced US and computed tomography (CT) have been used for guidance of percutaneous RFA. Even so, tumors invisible on US are also invisible on plain CT scan (14).
TACE has been used before RFA for early-stage HCCs (15). The combination of TACE and RFA can increase ablation size since TACE is able to decrease perfusion-mediated heat loss (16). Moreover, when combined with RFA, TACE can increase RFA feasibility (17). Radio-opaque iodized oil used in TACE may accumulate in tumor lesions and present radiographic contrast to a small tumor lesion either of poor conspicuity or in US blind spots like the hepatic dome. Consequently, TACE may allow guidance with fluoroscopy, US, and CT for RFA in the target tumor lesion. The combination of TACE with RFA has been reported in treating small HCCs with good results (18–20). However, few studies have been reported regarding use of multiple imaging modalities in guiding RFA for HCC lesions at high-risk or unfavorable sites. This study aimed to investigate the technical feasibility, safety and effects of percutaneous RFA, guided by multiple imaging modalities for accurate ablation of HCCs in special locations compared with those in conventional locations.
Methods
Patients
This study was approved by our hospital ethics committee (20130088127) and was performed between April 2013 and January 2018, and all patients gave their written informed consent to participate. The inclusion criteria were HCCs with histologic evidence; typical characteristics of HCC on medical imaging; infeasible for surgical resection due to comorbidities; ≤5 cm in diameter; adjacent to the diaphragm or behind ribs on US; <10 mm from intrahepatic large vessels and biliary tract or from the extrahepatic organs including heart, lung, gallbladder, right kidney or gastrointestinal tract; <5 mm from the liver surface, Child-Pugh class A-B, and stage A-B of the Barcelona Clinic Liver Cancer (BCLC) system. The exclusion criteria were HCCs with portal vein tumor thrombosis or extrahepatic metastasis; multiple lesions and contraindication of TACE; range of tumor exceeding 70% of the liver; and high flow intrahepatic arteriovenous shunt. A total of 122 patients met the inclusion criteria and were enrolled in the study to have percutaneous RFA guided by multiple imaging modalities combined with TACE. Eighty-five patients (69.7%) had HCC in conventional locations, while 37 patients (30.3%) in special locations, including 11 HCC lesions (29.7%) adjacent to the stomach or gastrointestinal tract, 10 (27%) close to liver surface, 6 (16.2%) adjacent to the diaphragm, 5 (13.5%) near the large vessels, 2 (5.4%) behind ribs, 2 (5.4%) adjacent to the gallbladder and 1 (2.7%) near large biliary tract (Table 1).
Table 1.
Patient demographics and tumor characteristics
| Conventional (n=85) | Special (n=37) | P | |
|---|---|---|---|
| Sex (M/F) | 62 (73)/23 (28) | 25 (68)/12 (32) | 0.546 |
|
| |||
| Age (years) | 58.36±10.46 | 59.62±9.04 | 0.527 |
|
| |||
| Tumor size (cm) | 3.20±0.89 | 3.03±1.04 | 0.368 |
|
| |||
| Etiology | |||
| Hepatitis B virus | 76 (89) | 33 (89) | |
| Hepatitis C virus | 6 (7) | 2 (5) | |
| Alcoholic | 2 (2) | 1 (3) | |
| Others | 1 (1) | 1 (3) | 0.935 |
|
| |||
| Child-Pugh class | |||
| A | 64 (75) | 29 (78) | |
| B | 21 (25) | 8 (22) | 0.713 |
|
| |||
| BCLC stage | |||
| 0 | 10 (12) | 9 (24) | |
| A | 28 (33) | 10 (27) | |
| B | 47 (55) | 18 (49) | 0.211 |
|
| |||
| Serum AFP level (ng/mL) | |||
| Negative | 18 (21) | 8 (22) | |
| Positive | 67 (79) | 29 (78) | 0.956 |
BCLC, Barcelona Clinic Liver Cancer; AFP, alpha fetal protein.
Data are presented as n (%) or mean ± standard deviation.
TACE and RFA
Before RFA, TACE was used to mark the lesion (adjacent to the diaphragm, behind the ribs, or negative on imaging), to occlude blood flow (in HCC adjacent to large vessels), or prevent possible ischemia of liver (in HCC close to the liver surface) using an emulsion of Amycin (10–20 mg) mixed with super-liquefied lipiodol (5–10 mL). US was used for guidance when the HCC lesion was adjacent to large blood or biliary vessels (for real-time monitoring), close to the liver surface, adjacent to extrahepatic organs, or negative on imaging. Fluoroscopy was applied when the lesion was near the diaphragm and liver surface, behind ribs, or negative on imaging. Cone beam CT (CBCT, DynaCT) was used in all cases (Table 2).
Table 2.
Use of TACE and imaging guidance in special locations
| Location | TACE | US | Fluoroscopy | DynaCT |
|---|---|---|---|---|
| Near diaphragm | ✓ (marking) | × | ✓ (oblique puncture) | ✓ |
| Near big blood vessels | ✓ (flow occlusion) | ✓ (real-time monitoring) | × | ✓ |
| Near gallbladder | Optional | ✓ (real-time monitoring) | × | ✓ |
| Near liver surface | ✓ (bleeding prevention) | ✓ | ✓ | ✓ |
| Behind ribs | ✓ (marking) | × | ✓ (oblique puncture) | ✓ |
| Near gastrointestinal tract | ✓ (marking) | ✓ | × | ✓ |
| Negative on imaging | ✓ (marking) | ✓ | ✓ | ✓ |
TACE, transarterial chemoembolization; US, ultrasonography; CT, computed tomography.
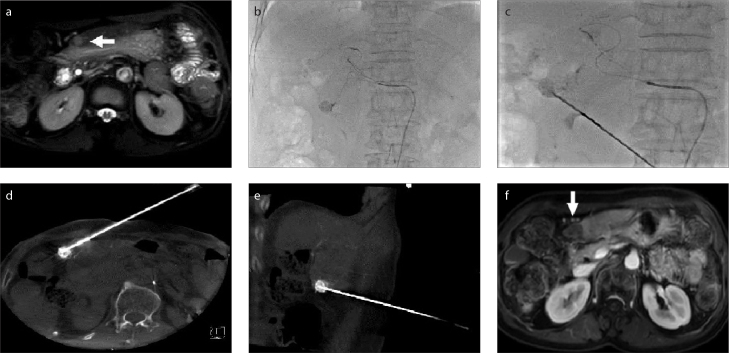
RFA was performed immediately after TACE with patients in the supine position. After planning the best needle access route according to pre-procedural contrast CT/MRI images, a 17-gauge electrode needle was introduced into the tumor under the guidance of fluoroscopy, US, or CBCT for RFA. The puncture route had to avoid the lung (lesions adjacent to the diaphragm, Fig. 1), large vessels (Fig. 2), gallbladder, and other organs (Figs. 3–5) if the lesion was near liver surface (Fig. 3), behind ribs (Fig. 4), or adjacent to gastrointestinal tract (Fig. 5).
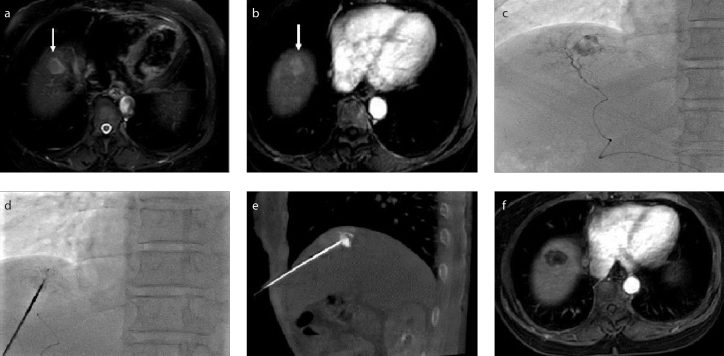
Figure 1. a–f.
A hepatocellular carcinoma (HCC) lesion near the diaphragm. Contrast-enhanced magnetic resonance images (a, b) demonstrate the lesion (arrow). Transarterial chemoembolization (TACE) (c) was performed to mark the lesion. Image (d) shows needle puncture performed under fluoroscopy. Cone beam CT (e) confirmed the position of the needle within the lesion. Contrast-enhanced MRI (f) confirmed complete necrosis of the lesion after radiofrequency ablation (RFA).
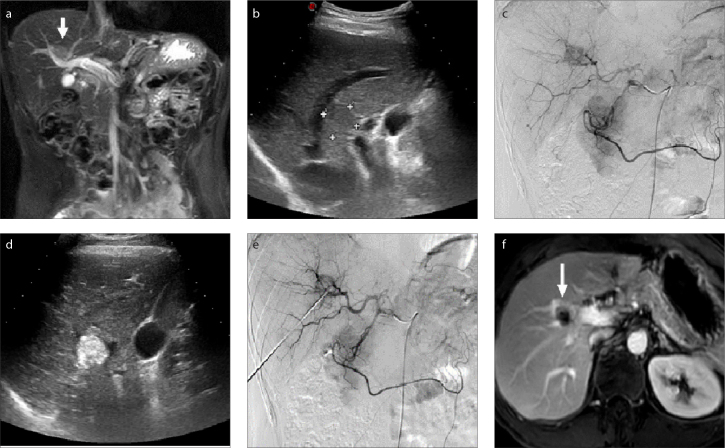
Figure 2. a–f.
Magnetic resonance (a) and US (b) images show a HCC lesion adjacent to large blood vessels (a, arrow). TACE (c) was performed to reduce the heat sink effect by embolization of the tumor artery. Real-time US guidance was performed during puncture (d). Transarterial angiography (e) shows that the blood supply of the tumor is cut off after RFA. Magnetic resonance image (f) shows complete necrosis of the lesion (arrow) following RFA.
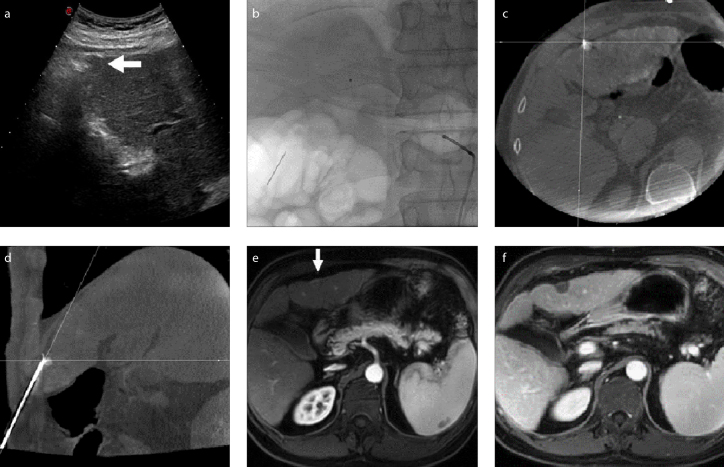
Figure 3. a–f.
US image (a) shows a HCC lesion near the liver surface (arrow). TACE (b, c) is used to mark the lesion and embolize its supply artery to reduce the risk of hemorrhage. Image (d) shows RFA performed with guidance of fluoroscopy, US and CBCT confirmation of the needle position within the lesion. Magnetic resonance images (e, f) show complete necrosis of the lesion (arrow) following RFA.
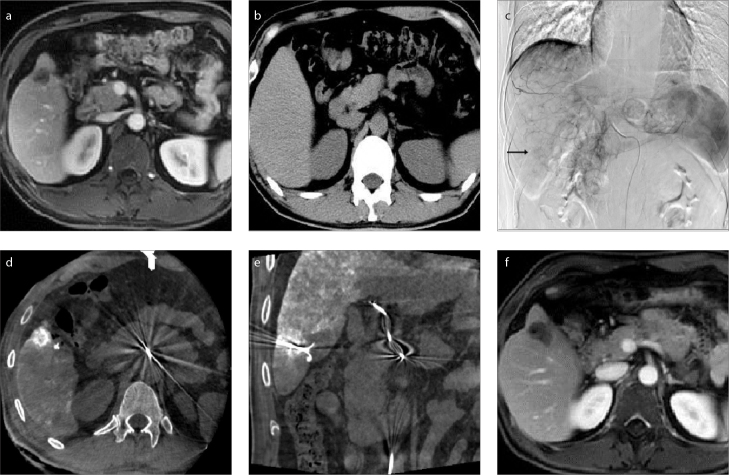
Figure 4. a–f.
Magnetic resonance image (a) shows a lesion behind the ribs. The lesion was invisible on US and conventional CT (b). TACE (c) was performed to mark the lesion (arrow) and embolize the lesion artery to reduce the risk of hemorrhage. Cone beam CT image (d) shows the lesion behind the ribs. In image (e), puncture was performed with a needle whose direction and position was adjusted, and ablation was started after confirmation of the needle position within the lesion by cone beam CT. Magnetic resonance image (f) shows complete necrosis achieved after ablation.
Figure 5. a–f.
Magnetic resonance image (a) shows a lesion (arrow) adjacent to the gastrointestinal tract. The lesion was marked with TACE (b), and the lesion supplying artery was embolized to reduce the risk of hemorrhage. Image (c) shows puncturing of the lesion under guidance of fluoroscopy and ultrasound. In images (d, e), the needle was confirmed to be within the lesion before RFA was started. Magnetic resonance image (f) shows complete necrosis (arrow) achieved after ablation.
To prevent complications, artificial ascites effusion was made using 5% dextrose water via a catheter or needle into the abdomen of patients if the tumors were close to the diaphragm, gallbladder or duodenum. For lesions adjacent to the stomach or gastrointestinal tract, patients would regularly fast 48 hours after the operation and have medications like antacids, antibiotic, and somatostatin in order to avoid complications such as perforation of the gallbladder or the stomach.
Follow-up and outcome evaluation
CT scan was performed on day 3 after RFA. Patients were followed up by contrast enhanced CT at 1 month and thereafter every 3 months. To evaluate the immediate response of lesions to ablation and immediate complications, CBCT was performed after removal of the electrode needle. Technique effectiveness was defined when complete ablation of the target lesion was evident on 1 month follow-up contrast imaging. The overall survival (OS), progression-free survival (PFS), and procedure-related adverse events were compared between conventional and special locations. The primary endpoint was to compare the OS and PFS at 1- and 2-year follow-up. The secondary endpoint was the occurrence of adverse events.
Statistical analysis
All statistical analyses were performed with SPSS 23.0 (IBM Corp.). Difference in means was analyzed by t-test, and the relationship between categorical variables was compared by Pearson chi-square test and Fisher Freeman Halton test. The OS and PFS were estimated with the Kaplan-Meier method. Cox proportional hazard models were used to assess the prognostic factors for OS and PFS. P values of <0.05 were considered statistically significant.
Results
Technical success of combined TACE and RFA was 100%. Complications occurred in 3 of 122 patients (2.5%). Liver capsule hemorrhage occurred in one patient in the special location not requiring management, and reactive pleural effusion took place in two patients with one in the conventional and the other in the special location, both requiring no further management. There were no procedure related mortalities. No significant (P = 0.218) difference existed in the complication rate between the conventional (n=1, 1.2%) and special location (n=2, 5.4%) groups. Post-ablation syndrome (flu-like symptoms comprising fever, malaise, chills, delayed pain, and nausea) occurred in 27 cases (32%) in the conventional location group and 12 cases (32%) in the special location group, with no significant difference (P = 0.942) between the two groups. Slight liver function damage was not significantly different between the two groups and recovered within 1 month (Table 3).
Table 3.
Changes of liver function during the perioperative period
| Conventional n=85 | Special n=37 | P | |
|---|---|---|---|
| ALT (9.00–50.00U/L) | |||
| Preoperation | 36.28±15.22 | 34.70±16.03 | 0.605 |
| 3-day postoperation | 159.92±109.04 | 132.30±66.07 | 0.155 |
| 1-month postoperation | 33.82±16.43 | 37.95±17.24 | 0.212 |
|
| |||
| AST (15.00–40.00 U/L) | |||
| Preoperation | 34.96±13.42 | 37.41±16.45 | 0.391 |
| 3-day postoperation | 137.73±84.75 | 155.35±106.79 | 0.332 |
| 1-month postoperation | 33.14±11.85 | 32.81±13.099 | 0.891 |
|
| |||
| Albumin (40.00–55.00 g/L) | |||
| Preoperation | 44.15±6.44 | 42.43±6.258 | 0.175 |
| 3-day postoperation | 35.64±6.07 | 34.04±5.71 | 0.177 |
| 1-month postoperation | 43.82±5.98 | 45.17±6.55 | 0.268 |
|
| |||
| Total bilirubin (3.40–20.50 μmol/L) | |||
| Preoperation | 19.42±6.01 | 18.41±6.29 | 0.400 |
| 3-day postoperation | 27.94±8.94 | 24.80±9.87 | 0.148 |
| 1-month postoperation | 18.70±6.03 | 19.21±6.75 | 0.677 |
|
| |||
| Prothrombin time (s) | |||
| Preoperation | 12.94±1.13 | 13.11±1.21 | 0.457 |
| 3-day postoperation | 13.34±1.33 | 13.32±1.50 | 0.984 |
| 1-month postoperation | 13.21±1.17 | 12.88±1.18 | 0.158 |
Normal ranges given in parentheses.
Data are presented as mean±standard deviation.
ALT, alanine transaminase; AST, aspartate transaminase.
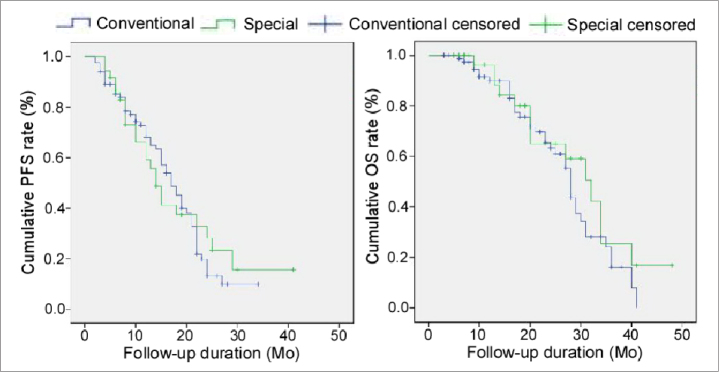
Complete tumor necrosis was observed in 73 cases (85.9%) in the conventional location group, while in 34 cases (91.9%) in the special location group at 1-month follow-up contrast enhanced imaging (P = 0.353). After 3–48 months (18±10.5 months) of follow-up, three patients were lost to follow-up, and 67 of 119 patients (56.3%) were alive. The PFS was 17 months in the conventional location group and 14 months in the special location group, while 1-year PFS rate was 68.1% in the conventional location group and 59.1% in the special location group, with no significant difference (P = 0.741) (Fig. 6). The OS was 28±0.66 months in the conventional location group and 32±3.76 months in the special location group. The cumulative 1- and 2-year OS rates were 89.9% and 63.3% for patients in the conventional location group and 96.3% and 65% for patients in the special location group, with no significant difference between groups (P = 0.273) (Fig. 6). Age (P = 0.043) and tumor size (P < 0.001) were the significant prognostic factors for OS, and tumor size (P < 0.001) was the only significant prognostic factor for PFS (Table 4).
Figure 6.
Kaplan-Meier curve of progression-free survival (PFS) and overall survival (OS).
Table 4.
Multivariate analysis on potential prognostic factors for overall survival and progression-free survival after therapy
| Variables | Overall survival | Progression-free survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| β | SE | Wald χ2 | HR (95% CI) | P | β | SE | Wald χ2 | HR (95% CI) | P | |
| Location | 0.060 | 0.350 | 0.030 | 1.06 (0.53–2.10) | 0.865 | −0.121 | 0.268 | 0.205 | 0.89 (0.52–1.50) | 0.651 |
|
| ||||||||||
| Sex | 0.375 | 0.334 | 1.260 | 1.47 (0.76–2.82) | 0.252 | −0.179 | 0.255 | 0.493 | 0.84 (0.51–1.38) | 0.483 |
|
| ||||||||||
| Age | −0.037 | 0.018 | 4.111 | 0.96 (0.93–0.99) | 0.043 | −0.021 | 0.014 | 2.419 | 0.98 (0.95–1.00) | 0.120 |
|
| ||||||||||
| Tumor size | 0.811 | 0.183 | 19.529 | 2.27 (1.58–3.24) | <0.001 | 0.764 | 0.147 | 26.965 | 2.15 (1.61–2.86) | <0.001 |
|
| ||||||||||
| Child-Pugh | 0.306 | 0.335 | 0.834 | 0.73 (0.38–1.41) | 0.351 | −0.031 | 0.279 | 0.013 | 0.97 (0.56–1.67) | 0.910 |
|
| ||||||||||
| AFP | −0.147 | 0.387 | 0.145 | 0.86 (0.441–1.84) | 0.694 | 0.145 | 0.290 | 0.250 | 0.87 (0.49–1.53) | 0.617 |
|
| ||||||||||
| Complete necrosis rate | 0.772 | 0.459 | 2.832 | 2.18 (0.89–5.36) | 0.092 | 0.624 | 0.368 | 2.866 | 1.87 (0.91–3.84) | 0.090 |
Overall survival: model χ2=25.261, P = 0.003; Progression free survival: model χ2=31.693, P < 0.001.
SE, standard error; HR, hazard ratio; CI, confidence interval; AFP, alpha fetal protein.
Discussion
Treatment of HCC has evolved over the past 10 years along with advances in interventional technology and equipment. Multiple imaging technologies can be used to guide percutaneous RFA combined with TACE in treating HCC lesions in high-risk or unfavorable locations adjacent to large vessels, biliary tracts, extrahepatic organs and ribs. Our study used percutaneous RFA guided with multiple imaging modalities combined with TACE and has achieved similar effectiveness and safety in treating tumors in special locations as in conventional sites.
Previous research has demonstrated that HCCs adjacent to large blood vessels have been inadequately ablated because of fear of vascular injury, especially portal vein injury, and heat loss caused by tissue convection from the blood flow acting as a “heat-sink” effect (21). Surgical resection of HCC near large vessels is also difficult with more blood loss, longer operation time, and more complications than in HCCs in the other segments. Early studies of RFA also believed that HCCs in the special locations were not indications for RFA therapy due to risk of ablating injury to important structures or incomplete ablation of the tumor lesions. Moreover, some small HCC lesions may be invisible on either US or the commonly used CT scan in RFA (13, 22). For these lesions, percutaneous US- or CT-guided ablation therapy is often limited because of tumor inconspicuity due to deep location, proximity to vessel, or location behind the ribs and may even result in pneumothorax, rupture, and bleeding of the tumor due to repeated puncture. However, all these patients urgently need a better treatment approach, and the combination of TACE with RFA has been suggested for this purpose. For tumor lesions near large vessels, the combination therapy can reduce the “heat-sink” effect by initially embolizing the nearby large vessels of hepatic arteries or possibly portal vein so as to improve the tumor necrosis rate during subsequent ablation (23, 24). For small lesions invisible on US or CT scan or in special locations, deposition of iodized oil within the tumor lesions through TACE can make the lesion visible on fluoroscopy and CBCT, facilitating needle puncture of the lesion under imaging guidance for successful subsequent ablation. TACE combined with RFA improved the technical success and effectiveness of ablation for tumor lesions invisible on US and CT as well as those found in special locations and may be used as a first-line treatment approach. Hyun et al. (18) reported superiority of TACE and RFA combination therapy to TACE monotherapy in terms of 1-month tumor response, time to progression, and OS when performed for early stage HCC infeasible for US-guided RFA.
The combination of RFA with TACE has advantages not only in marking tumor lesions, blocking blood supply, and preventing bleeding, but also in improving the OS rate and prognosis (25). In the combination approach, TACE plays a pivotal role in making CBCT-guided RFA possible. Individualized strategy was used for each location, and when the needle reached the lesion, CBCT was used to determine and adjust the needle location if necessary. As an image guidance technology for ablation, CBCT is superior to conventional CT because it can be performed immediately after TACE. Regarding the effect of combined TACE and RFA, an animal study investigating the ablation zone according to time interval between TACE and RFA reported that a single-session combination could create a greater ablation zone (26), which suggested that, when TACE combined with RFA therapy is considered, TACE should be followed by RFA as quickly as possible. Accordingly, we performed the RFA immediately after TACE. In our study, we used the combination of TACE and RFA in all patients so as to decrease possible bias in the treatment modality in HCCs in special and conventional locations.
In our study, we used multiple imaging modalities to guide percutaneous ablation. TACE was used in all patients to mark the HCC lesion when it was invisible on medical imaging, near the diaphragm and gastrointestinal tract, and behind the ribs, to prevent bleeding when the lesion was near the liver surface or to decrease the heat sink effect when the lesion was near large blood vessels. However, when the lesion was near the gallbladder, TACE was optional. US was applied to guide the puncture in most situations, but was not used when the lesion was near diaphragm or behind the ribs. Fluoroscopy was applied when the HCC lesion was near the diaphragm and the liver surface, behind the ribs or when the lesion was negative on medical imaging. CBCT was used in all cases in our study. The technical success of TACE combined with RFA was 100% in all cases. No major complications like large intrahepatic vessel or bile duct injury were seen, no procedure-related death occurred, and slight liver function damage recovered within 1 month for both groups. Since there were no significant differences in the complications between the two groups (one liver capsule hemorrhage and one reactive pleural effusion in the special location group and one reactive pleural effusion in the conventional group), it can be considered that TACE combined with multiple imaging modalities for guidance, reduced RFA complications in treating HCC lesions in special locations. Complete tumor necrosis rate was 85.9% in conventional locations, not significantly different from that in special locations (91.9%) on 1-month follow-up imaging. No significant difference was detected in the PFS or OS between the two groups. Age and tumor size were significant prognostic factors for OS, and tumor size was the only significant prognostic factor for PFS found in this study.
Teratani et al. (2) performed RFA for 231 HCC lesions in 207 patients in so-called high-risk locations adjacent to large blood vessels or extrahepatic organs with US guidance only; however, early major complications occurred in 8 patients (6.3%) with lesions adjacent to extrahepatic organs, in 3 patients (4.8%) with lesions near large vessels and in 2 patients (11%) with lesions adjacent to both large vessels and extrahepatic organs. Also, bile duct injury occurred in 6 of 79 lesions (7.6%) near a large portal vein in the study by Teratani et al. (2). In RFA treatment of HCC lesions in problematic locations by Chen et al. (12), US alone was applied for the guidance of RFA; however, 11 (5.1%) major complications took place among 215 patients, including hemorrhages in two patients, bowel perforation in one, jaundice due to biliary narrowing in one, large bloody pleural fluid in two, thoracic emphysema in one, and tumor-seeding in needle tract in four. Kelogrigoris et al. (27) reported the results of RFA for malignant liver tumors in challenging locations with the guidance of CT alone, where immediate complications after ablation took place in 8 of 84 patients (9.5%). These studies all used one modality of guidance which produced a higher complication rate. In our study, the use of multiple modalities of imaging decreased the ablation-related complication rate with no presence of major complications. Besides the use of multiple modalities of imaging guidance, the outcome of imaging-guided RFA is greatly affected by the experience and skill of the operators of ablation, and less-skilled operators may ablate the HCC lesions incompletely with a higher complication rate of ablation, leading to poor prognosis (27).
In the study by Chen et al. (12) who used RFA for problematically located HCC lesions with tailored approaches guided by US alone, the early tumor necrosis rate was 91.6%, and the 1-, 2- and 3-year survival rates were 81.6%, 63.8%, and 53.6%, respectively. In the report by Kelogrigoris et al. (27) using RFA for malignant liver tumors in challenging locations, the complete ablation rate was 89.7% (88/98) in the high-risk locations, and the 1-, 2- and 3-year survival rates were 82.6%, 67.3%, and 54.1%, respectively. In our study, complete tumor necrosis rate was 85.9% in conventional locations, 91.9% in special locations, and the 1-year PFS rate was 68.1% in conventional location and 59.1% in special locations, and the cumulative 1- and 2-year OS rates were 89.9% and 63.3% for tumors in the conventional location and 96.3% and 65% for tumors in the special location, with no significant difference between the two groups. Our study achieved similar or even better results compared with other reports treating HCC lesions at similar sites; however, this superiority of our study may be caused by the addition of TACE which allows selective delivery of chemotherapeutic agents to HCC lesions, reduces the viable tumor volume, decreases the heat sink effect of large vessels, and protects the rest of the liver against ischemic necrosis (6, 28). In combined TACE with RFA for HCC lesions, the OS rate was reported to be 80.1%, 55%, and 36.3% for 1, 2, and 3 years, respectively, in the study by Abdelaziz et al (6), 96.1%, 76.7%, and 41.3% for 1, 3, and 5 years, respectively, in the study by Pan et al. (28), and 100%, 78.6%, and 62.3% for 1, 3, and 5 years, respectively, in the study by Hirooka et al. (29). The OS rates of HCC patients may be incomparable between different studies because of varied severity and clinical conditions of the patients. However, multiple modalities of imaging for guidance of RFA combined with TACE may be an alternative for patients with unresectable HCC lesions in special locations, providing a safe, efficient therapeutic modality for these patients if performed by a well-trained and experienced interventional radiologist.
In our study, we used TACE combined with RFA guided by multiple imaging modalities for treating HCC lesions in special locations, achieving similarly good results as in conventional locations. TACE has also been combined with microwave ablation for treating HCC lesions in animal experiments (30) and in clinical application (31). After evaluating combined effects of TACE and open local thermal microwave ablation in a rat HCC model, Vogl et al. (30) found improved results of TACE followed by microwave ablation compared with single therapy regimen regarding the inhibition of growth rate and reduction of VEGF-level in peritumoral tissues. Compared with TACE monotherapy in BCLC stage B patients with HCC tumor size ≤7 cm and tumor number ≤5, the combined TACE with microwave ablation was found to have better clinical effects (31); however, this study did not specify the locations of the HCC lesions with the guidance of only CT scan before and after microwave ablation.
Our study has some limitations. First, the patient cohort is small and needs to be increased. Second, a high proportion of the patients received further treatment during follow-up, which makes it difficult to analyze the effects of combined therapy of TACE and RFA. Third, the follow-up duration was not long enough. A further prospective large-scale study with multiple centers is needed to validate the superiority of combined TACE with RFA for HCC in special locations.
In conclusion, the use of multiple imaging modalities in guiding RFA combined with TACE may be safe and effective for accurate ablation of HCCs in special high-risk locations.
Main points.
Radiofrequency ablation (RFA) combined with transarterial chemoembolization (TACE) is a feasible treatment option for hepatocellular carcinoma (HCC) patients who are not eligible for surgical resection.
However, treatment success is limited when lesions are in special (i.e., high-risk or unfavorable) locations adjacent to large vessels, biliary tracts, extrahepatic organs, and ribs.
Multiple imaging technologies can be used to guide percutaneous RFA combined with TACE in treating HCC lesions in these special locations, safely and effectively.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Peng Z, Chen S, Wei M, et al. Advanced recurrent hepatocellular carcinoma: treatment with sorafenib alone or in combination with transarterial chemoembolization and radiofrequency ablation. Radiology. 2018;287:705–714. doi: 10.1148/radiol.2018171541. [DOI] [PubMed] [Google Scholar]
- 2.Teratani T, Yoshida H, Shiina S, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43:1101–1108. doi: 10.1002/hep.21164. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M American Association for the Study of Liver Disease. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gane EJ, Portmann BC, Naoumov NV, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334:815–820. doi: 10.1056/NEJM199603283341302. [DOI] [PubMed] [Google Scholar]
- 5.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889–896. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 6.Abdelaziz AO, Abdelmaksoud AH, Nabeel MM, et al. Transarterial chemoembolization combined with either radiofrequency or microwave ablation in management of hepatocellular carcinoma. Asian Pac J Cancer Prev. 2017;18:189–194. doi: 10.22034/APJCP.2017.18.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 9.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 10.Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Shiina S. Image-guided percutaneous ablation therapies for hepatocellular carcinoma. J Gastroenterol. 2009;44(Suppl 19):122–131. doi: 10.1007/s00535-008-2263-9. [DOI] [PubMed] [Google Scholar]
- 12.Chen MH, Yang W, Yan K, et al. Radiofrequency ablation of problematically located hepatocellular carcinoma: tailored approach. Abdom Imaging. 2008;33:428–436. doi: 10.1007/s00261-007-9283-4. [DOI] [PubMed] [Google Scholar]
- 13.Kim PN, Choi D, Rhim H, et al. Planning ultrasound for percutaneous radiofrequency ablation to treat small (</= 3 cm) hepatocellular carcinomas detected on computed tomography or magnetic resonance imaging: a multicenter prospective study to assess factors affecting ultrasound visibility. J Vasc Interv Radiol. 2012;23:627–634. doi: 10.1016/j.jvir.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 14.Hyun D, Cho SK, Shin SW, et al. Combined transarterial chemoembolization and radiofrequency ablation for small treatment-naive hepatocellular carcinoma infeasible for ultrasound-guided radiofrequency ablation: long-term outcomes. Acta Radiol. 2018;59:773–781. doi: 10.1177/0284185117735349. [DOI] [PubMed] [Google Scholar]
- 15.Kim JW, Kim JH, Won HJ, et al. Hepatocellular carcinomas 2–3 cm in diameter: transarterial chemoembolization plus radiofrequency ablation vs. radiofrequency ablation alone. Eur J Radiol. 2012;81:e189–193. doi: 10.1016/j.ejrad.2011.01.122. [DOI] [PubMed] [Google Scholar]
- 16.Sugimori K, Morimoto M, Shirato K, et al. Radiofrequency ablation in a pig liver model: effect of transcatheter arterial embolization on coagulation diameter and histologic characteristics. Hepatol Res. 2002;24:164. doi: 10.1016/S1386-6346(02)00030-X. [DOI] [PubMed] [Google Scholar]
- 17.Lee MW, Kim YJ, Park SW, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma invisible on both ultrasonography and unenhanced CT: a preliminary study of combined treatment with transarterial chemoembolisation. Br J Radiol. 2009;82:908–915. doi: 10.1259/bjr/55877882. [DOI] [PubMed] [Google Scholar]
- 18.Hyun D, Cho SK, Shin SW, et al. Early stage hepatocellular carcinomas not feasible for ultrasound-guided radiofrequency ablation: comparison of transarterial chemoembolization alone and combined therapy with transarterial chemoembolization and radiofrequency ablation. Cardiovasc Interv Radiol. 2016;39:417–425. doi: 10.1007/s00270-015-1194-0. [DOI] [PubMed] [Google Scholar]
- 19.Hyun D, Cho SK, Shin SW, Rhim H, Koh KC, Paik SW. Treatment of small hepatocellular carcinoma (</=2 cm) in the caudate lobe with sequential transcatheter arterial chemoembolization and radiofrequency ablation. Cardiovasc Interv Radiol. 2016;39:1015–1022. doi: 10.1007/s00270-016-1314-5. [DOI] [PubMed] [Google Scholar]
- 20.Lee MW, Kim YJ, Park SW, Yu NC, Choe WH, Kwon SY, et al. Biplane fluoroscopy-guided radiofrequency ablation combined with chemoembolisation for hepatocellular carcinoma: initial experience. Br J Radiol. 2011;84:691–697. doi: 10.1259/bjr/27559204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217:633–646. doi: 10.1148/radiology.217.3.r00dc26633. [DOI] [PubMed] [Google Scholar]
- 22.Park BJ, Byun JH, Jin YH, Won HJ, Shin YM, Kim KW, et al. CT-guided radiofrequency ablation for hepatocellular carcinomas that were undetectable at US: therapeutic effectiveness and safety. J Vasc Interv Radiol. 2009;20:490–499. doi: 10.1016/j.jvir.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452–5460. doi: 10.1002/cncr.25314. [DOI] [PubMed] [Google Scholar]
- 24.Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262:689–700. doi: 10.1148/radiol.11110637. [DOI] [PubMed] [Google Scholar]
- 25.Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19:3872–3882. doi: 10.3748/wjg.v19.i24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee IJ, Kim YI, Kim KW, et al. Radiofrequency ablation combined with transcatheter arterial embolisation in rabbit liver: investigation of the ablation zone according to the time interval between the two therapies. Br J Radiol. 2012;85:e987–994. doi: 10.1259/bjr/90024696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelogrigoris M, Laspas F, Kyrkou K, Stathopoulos K, Georgiadou V, Thanos L. Percutaneous radiofrequency ablation for malignant liver tumours in challenging locations. J Med Imaging Radiat Oncol. 2012;56:48–54. doi: 10.1111/j.1754-9485.2011.02328.x. [DOI] [PubMed] [Google Scholar]
- 28.Pan TML, Wu C, Wu XQ, et al. Comparison of combined transcatheter arterial chemoembolization and CT-guided radiofrequency ablation with surgical resection in patients with hepatocellular carcinoma within the up-to-seven criteria: a multicenter case-matched study. J Cancer. 2017;8:3506–3513. doi: 10.7150/jca.19964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirooka MHA, Ochi H, Kisaka Y, Joko K, Michitaka K, Hiasa Y. Transcatheter arterial chemoembolization with or without radiofrequency ablation: outcomes in patients with barcelona clinic liver cancer stage b hepatocellular carcinoma. AJR Am J Roentgenol. 2018;210:891–898. doi: 10.2214/AJR.17.18177. [DOI] [PubMed] [Google Scholar]
- 30.Vogl TJ, Qian J, Tran A, et al. Study on the effect of chemoembolization combined with microwave ablation for the treatment of hepatocellular carcinoma in rats. Diagn Interv Radiol. 2017;23:150–155. doi: 10.5152/dir.2016.16617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang R, Shen L, Zhao L, Guan Z, Chen Q, Li W. Combined transarterial chemoembolization and microwave ablation versus transarterial chemoembolization in BCLC stage B hepatocellular carcinoma. Diagn Interv Radiol. 2018;24:219–224. doi: 10.5152/dir.2018.17528. [DOI] [PMC free article] [PubMed] [Google Scholar]