Abstract
A number of embolic agents are currently available each with their own properties. Precipitating hydrophobic injectable liquid (PHIL) is a new dimethyl sulfoxide (DMSO) compatible embolic agent with a number of specific properties which make it of interest to interventional radiologists. We review the use of PHIL in a non-neurointerventional setting, describing its use in a range of procedures such as trauma embolization, pseudoaneurysm embolization, and tumor embolization. PHIL’s properties include a lack of skin discoloration, the possibility of rapid injection and a lack of glare artifact on follow-up computed tomography imaging. These properties make it an important new tool in the armamentarium of the body interventional radiologist.
Many embolic agents are available to the interventional radiologist, each with its own characteristics. Agents based on the ethylene vinyl alcohol co-polymer (Onyx, Medtronic and SQUID, Emboflu) have gained popularity due to their “lava-like” flow enabling good control of the embolic material (1, 2). Here we describe the use of a novel liquid agent, with some similarities to Onyx/Squid in the vascular system.
Precipitating hydrophobic injectable liquid (PHIL) is a new liquid embolic agent. It has a CE marking for use in hypervascular lesions and arteriovenous malformations. The liquid agent consists of a co-polymer dissolved in dimethyl sulfoxide (DMSO). The monomer hydroxyethylmethacrylate is used to make the polymer (PHEMA). This is a non-adhesive hydrophobic polymer but when subjected to water, it swells due to the molecule’s hydrophilic pendant group. Iodine is covalently bonded to this to provide radio-opacity.
PHIL is delivered in liquid phase via a DMSO compatible microcatheter. Upon contact with blood, the DMSO solvent diffuses, leaving the PHIL to precipitate in situ immediately from the outside inwards. The distance travelled before solidification depends on the flow rate in the vessel, the position of the microcatheter in the lesion, the rate of injection and the viscosity of the product.
Like Onyx, there are currently 3 concentrations of PHIL (25%, 30%, and 35%), referring to the degree of DMSO solution, as well as a more recently introduced low viscosity (LV) version. There are, however, some key differences between the products. Unlike Onyx, PHIL does not require shaking prior to delivery. Also, iodine is covalently bonded in PHIL, rather than mixed with tantalum powder as in Onyx. This leads to less glare artifact on computed tomography (CT), which is often required for follow-up of complex embolization procedures. In addition, the nature of flow is fundamentally different between the products. Onyx demonstrates “lava-like” flow with a plug forming at the tip of the catheter before propagation forward whereas PHIL behaves more like a viscous liquid with “toothpaste-like” flow. Theoretically this implies that PHIL could be injected more rapidly than Onyx. Finally, PHIL is a yellow liquid rather than a black powdered material and so does not stain the skin, a desirable property in the context of superficial vascular lesions.
Technique
We describe the use of PHIL in the treatment of a variety of clinical presentations for non-neurointerventional radiologists. Written informed consent was obtained from all patients.
Trauma embolization
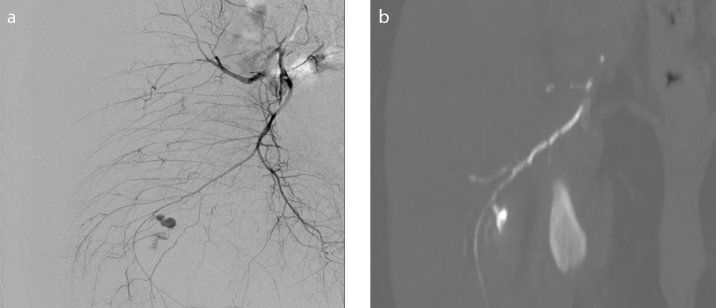
A 52-year-old male presented to the emergency department following a stab wound to the right buttock. Clinical examination demonstrated active bleeding from an open wound. Attempts at hemostasis using wound packs were unsuccessful. The patient was transferred to the interventional radiology suite for embolization. Angiogram demonstrated active bleeding from an inferior gluteal artery branch (Fig. 1a). Due to marked hemodynamic instability (blood pressure, 60/30 mmHg) and patient restlessness, a liquid embolic agent was chosen. The inferior gluteal branch was cannulated and embolized using 0.9 mL 30% PHIL through a Progreat 2.7 F microcatheter (Terumo Medical). Subsequent angiogram demonstrated good occlusive effect with preservation of the remaining internal iliac artery branches. The procedure time was 13 minutes. Follow-up CT a few hours later demonstrated good embolization of the branch both proximal and distal to the site of bleeding (Fig. 1b). The patient made a full recovery and was discharged five days later.
Figure 1. a, b.
Angiogram (a) demonstrating active bleeding from right inferior gluteal artery. CT reconstruction (b) demonstrating good embolization of the branch both proximal and distal to the site of bleeding.
Renal cell carcinoma (RCC) embolization
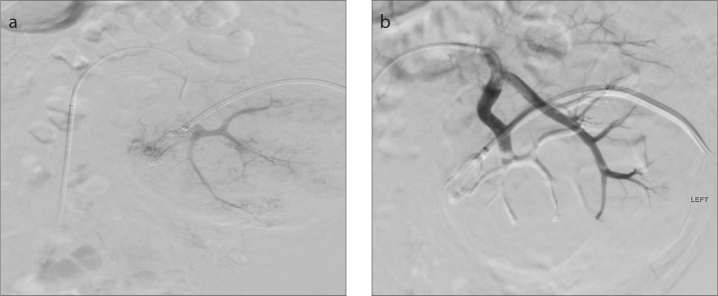
A 57-year-old male was diagnosed with a 10 cm left-sided RCC extending into the left renal vein, with lung metastases and paraaortic and thoracic lymphadenopathy. He had a congenitally absent right kidney. He was undergoing treatment with tyrosine kinase inhibitors when he developed intractable life-threatening hematuria. He developed acute kidney injury secondary to hydronephrosis, which was treated initially with nephrostomy insertion. Despite the administration of tranexamic acid he continued to bleed, so was referred for embolization. Selective cannulation (Fig. 2a) and subsequent embolization of the tumor-feeding arterial branches was performed using 1.8 mL 30% PHIL through a Progreat 2.7 F microcatheter. The hematuria was well-controlled with this intervention, with preservation of blood flow to the rest of the kidney (Fig. 2b) and a return to baseline renal function. He was subsequently discharged home and was able to continue treatment.
Figure 2. a, b.
Angiogram (a) demonstrating arterial supply to the renal tumor. Angiogram (b) showing embolization with PHIL with preservation of blood flow to the remaining functioning renal parenchyma.
Hepatic artery pseudoaneurysm
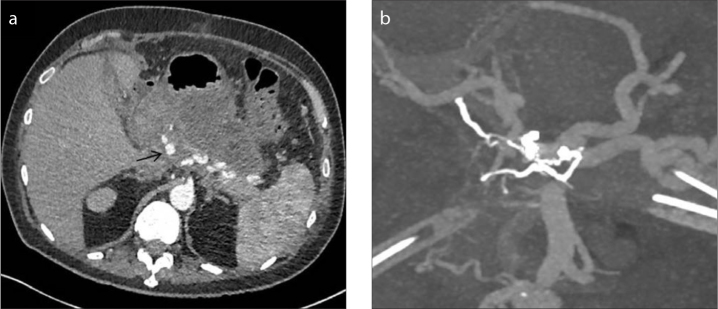
A 73-year-old male with a background of endoscopic retrograde cholangiopancreatography (ERCP) induced pancreatitis had a large gastrointestinal bleed. CT demonstrated a superior mesenteric artery (SMA) pseudoaneurysm, which was coiled. Subsequent CT demonstrated a proximal common hepatic artery (CHA) pseudoaneurysm (Fig. 3a). Given the earlier bleed, it was decided to perform an endovascular occlusion.
Figure 3. a, b.
CT image (a) demonstrating pseudoaneurysm arising from the proximal common hepatic artery. CT reconstruction (b) demonstrating embolization performed from a small celiac collateral with closure of both afferent and efferent supply to the pseudoaneurysm, whilst preserving the main right hepatic artery.
There was an anatomical variant, with the left hepatic artery arising from the left gastric artery. The gastroduodenal artery was thread-like as a result of ongoing pancreatitis, meaning that retrograde access to the hepatic circulation from the SMA was not possible. An initial attempt at coil embolization of the CHA pseudoaneurysm was unsuccessful, with residual filling seen from tiny collaterals arising from the proximal celiac artery.
It was felt that only a liquid embolic agent would be suitable to embolize both afferent and efferent supply to the pseudoaneurysm. However, there was a complicating factor in that the efferent supply to the pseudoaneurysm communicated with the main right hepatic artery, thereby putting this vessel at risk. 0.8 mL 25% PHIL was carefully injected using a Headway 17 (Microvention) microcatheter from the origin of the feeding collateral. The ability to control the propagation of the embolic agent meant it was possible to close both afferent and efferent supply to the pseudoaneurysm with precise control of the “stop” point, enabling preservation of the main right hepatic artery (Fig. 3b). The patient experienced no further bleeding complications and was eventually discharged from hospital some weeks later.
Discussion
PHIL is a relatively new liquid embolic agent. It has primarily been used in neurointervention, including for the treatment of cerebral arteriovenous malformations (AVM) and dural fistulae (3, 4). The use of PHIL is not established in body interventional radiology. To date, the only publication in this setting is a technical note relating to the use of this agent in endoleak management (5).
PHIL has advantages over some conventional embolic agents. A time saving advantage, particularly useful in a trauma setting, is its delivery in pre-filled glass syringes that do not require shaking prior to use. Also, as described in the trauma and hepatic pseudoaneurysm cases above, hemorrhage control often relies upon successful embolization of both the afferent and efferent vessels. It is not always possible to reach the efferent supply due to progressive decrease in size of the vessels or vasospasm at the bleeding site. The ability of a liquid agent in this situation is therefore clear in that it enables injection from a more proximal site. However, care must be taken with glue injection to avoid inadvertent non-target embolization. Conversely, if using Onyx or Squid, one must allow enough forward propagation of the embolic agent. PHIL represents an “in-between” agent which is liquid enough to propagate forward easily but in a controlled manner. Distal delivery can be altered by choosing a different concentration or viscosity agent. For example, PHIL LV has been effectively used where the microcatheter cannot be placed close to the embolization point (6).
Tantalum contained within Onyx has led to glare artifact on follow-up imaging, hampering further assessment with CT. Whilst “tantalum-light” versions of Onyx are now available, PHIL, with its radio-opacity dependent on covalently bound iodine, may be the preferable option where CT follow-up is likely to be required. Furthermore, no significant acoustic shadowing is seen following PHIL embolization, so ultrasound surveillance may also be an option.
In superficial AVM embolization, PHIL has the advantage of being pale in color, preferable to Onyx, which can stain the skin. Also, deeper penetration with different concentrations, means it is possible to get PHIL into the nidus and reflux back into arterial feeders when approaching from the venous side. Where future surgical intervention is planned, it should be noted that intraoperative combustion of Onyx has been reported (7).
PHIL’s efficacy in embolic terms appears comparable to other agents, with no bleeding channel around the embolic cast seen in our cases. The cost of PHIL is also broadly comparable to Onyx, in that although a standard vial of PHIL comes as 1 mL as compared to 1.5 mL Onyx per standard vial, evidence from in vitro studies (8) indicate that less PHIL is required to obtain an equivalent embolic effect.
Conclusion
PHIL is a new embolic agent that has primarily been used in the neurointerventional setting. It has some specific properties which are likely to be useful for body interventional radiologists, including controllable but excellent distal penetration, lack of glare artifact on CT and no skin discoloration. Given these properties it is recommended that body interventionalists have an awareness of this new embolic agent.
Main points.
PHIL is a new embolic agent that has primarily been used in the neurointerventional setting.
Properties which are likely to be useful for body interventional radiologists include
○ controllable but excellent distal penetration
○ lack of glare artifact on CT follow-up
○ no skin discoloration.
Given these properties, it is recommended that body interventionalists have an awareness of this new embolic agent.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Taki W, Yonekawa Y, Iwata H, et al. A new liquid material for embolization of arteriovenous malformations. AJNR Am J Neuroradiol. 1990;11:163–168. [PMC free article] [PubMed] [Google Scholar]
- 2.Akmangit DI, Daglioglu E, Kaya T, et al. Preliminary experience with Squid: A new liquid embolizing agent for AVM, AV fistulas and tumors. Turk Neurosurg. 2014;24:565–570. doi: 10.5137/1019-5149.JTN.11179-14.0. [DOI] [PubMed] [Google Scholar]
- 3.Leyon JJ, Chavda S, Thomas A, Lamin S. Preliminary experience with the liquid embolic material agent PHIL (Precipitating Hydrophobic Injectable Liquid) in treating cranial and spinal dural arteriovenous fistulas: technical note. J Neurointerv Surg. 2016;8:596–602. doi: 10.1136/neurintsurg-2015-011684. [DOI] [PubMed] [Google Scholar]
- 4.Samaniego E, Kalousek V, Abdo G, Ortega-Gutierrez SJ. Pre-liminary experience with precipitating Hydrophobic Injectable Liquid (PHIL) in treating cerebral AVMs. Neurointerv Surg. 2016;8:1253–1255. doi: 10.1136/neurintsurg-2015-012210. [DOI] [PubMed] [Google Scholar]
- 5.Helmy A, Shaida N. Treatment of type 2 endoleak with a novel agent: precipitating hydrophobic injectable liquid (PHIL) Cardiovasc Intervent Radiol. 2017;40:1094–1098. doi: 10.1007/s00270-017-1603-7. [DOI] [PubMed] [Google Scholar]
- 6.Samaniego EA, Derdeyn CP, Hayakawa M, Hasan D, Ortega-Gutierrez S. In vivo evaluation of the new PHIL low viscosity in a swine rete mirabile model. Interv Neuroradiol. 2018;24:706–712. doi: 10.1177/1591019918784915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith SJ, Thomas A, Ashpole RD. Intra-operative combustion of Onyx embolic material. Br J Neurosurg. 2009;23:76–78. doi: 10.1080/02688690802512866. [DOI] [PubMed] [Google Scholar]
- 8.Vollherbst DF, Sommer CM, Ulfert C, Pfaff J, Bendszus M, Möhlenbruch MA. Liquid embolic agents for endovascular embolization: evaluation of an established (Onyx) and a novel (PHIL) embolic agent in an in vitro AVM model. AJNR Am J Neuroradiol. 2017;38:1377–1382. doi: 10.3174/ajnr.A5203. [DOI] [PMC free article] [PubMed] [Google Scholar]