Abstract
PURPOSE
We aimed to evaluate the success and failure rates and minor and major complications of percutaneous drainage of retroperitoneal abscesses.
METHODS
Between 1990 and 2010, percutaneously drained 170 retroperitoneal abscesses in 150 patients (83 males, 67 females, median age 44.2 years, age range, 1–86 years) were included retrospectively. Percutaneous drainage of retroperitoneal abscesses was performed under the guidance of ultrasonography and fluoroscopy or computed tomography. Six abscesses were drained via single needle aspiration whereas 164 abscesses were drained via catheters of 6–14 F caliber using the Seldinger technique.
RESULTS
When all retroperitoneal abscesses are considered, success rates were found as follows: 75.3% cure (128/170), 7.7% temporization (13/170), 4.1% palliation (7/170). Failure rate was 12.9% (22/170). Recurrence rate was 10.6% (18/170), and 13 of the recurred abscesses were treated via second session percutaneous drainage. Mortality rate was 2.7% (4/150).
CONCLUSION
Percutaneous drainage is the first treatment option for retroperitoneal abscesses due to procedural reliability, elimination of need for general anesthesia, better tolerability, and lower morbidity and mortality rates compared with the surgical methods. High cure, temporization, or palliation rates can be obtained via imaging-guided percutaneous drainage for all retroperitoneal abscesses with a safe access route.
Retroperitoneal abscesses are clinically indolent since they usually present with nonspesific symptoms (1–4); however, with the help of imaging modalities, retroperitoneal abcesses can be recognized earlier (4, 5). Retroperitoneal abscesses can develop as a result of trauma, intestinal perforation, contagious spread of infections such as pancreatitis, diverticulitis, pyelonephritis, osteomyelitis, or postsurgically due to anastomosis leak (2, 3).
Early diagnosis and treatment of retroperitoneal abscesses is crucial and diagnosis should be based on symptoms, physical examination, laboratory, and radiological findings (2). Ultrasonography (US) might be the first imaging option for diagnosis (6). US has sensitivity over 90% in diagnosis of retroperitoneal abscesses (1, 6, 7); meanwhile, computed tomography (CT) is superior to US to demonstrate the location of abscess and its neighbouring anatomical structures in detail and establish the conclusive diagnosis (2, 7–9).
In the past, surgery was the mainstay therapy for retroperitoneal abscesses until imaging-guided percutaneous drainage emerged as an alternative means of treatment. Percutaneous abscess drainage was first used for uniloculated nonfistulous abscesses having safe access routes; however, this approach has shifted to treat multiloculated, fistulous pancreatic, splenic or retroperitoneal abscesses via minimally invasive percutaneous techniques (4, 10, 11). Percutaneous drainage is especially useful in lowering mortality and morbidity rates for debilitated or postoperative patients compared with surgical techniques (11).
In the literature, studies related with percutaneous treatment of retroperitoneal abscesses are limited in number. The purpose of this study is to report and discuss our single center experience with US- or CT-guided percutaneous drainage of retroperitoneal abscesses. To the best of our knowledge, it is one of the largest series of retroperitoneal abscesses treated percutaneously in the literature.
Methods
Patients
The institutional ethics committee approved our study design (Decision date: 22.04.2010, Protocol no:1079). A total of 170 US- or CT-guided percutaneously drained retroperitoneal abscesses in 150 patients (83 men, 67 women) between 1990 and 2010 were retrospectively evaluated and included in the study. Median age of the patients were 44.2 years (range, 1–86 years) and 18 patients were younger than 18 years of age.
Certain predisposing and comorbid clinical conditions were found when detailed clinical history and laboratory values of the patients were evaluated. Main predisposing conditions were malignancies (20%), urolithiasis (22.6%), chronic renal failure (8%), and diabetes mellitus (6.6%) (Table 1). With regard to previously elaborated criteria (12), medical conditions predisposing patients to develop infections were determined. Subsequently, patients were divided into two groups: immunocompetent patients (Group 1) and immunosuppressed patients (Group 2). Group 1 comprised 115 retroperitoneal abscesses (67.6%) in 98 patients, while Group 2 comprised 55 retroperitoneal abscesses (32.4%) in 52 patients with malignancy (n=30), chronic renal failure (n=12), diabetes mellitus (n=10), prior splenectomy (n=1), and steroid use (n=2). Certain patients had more than one risk factor for immunosuppression.
Table 1.
Predisposing factors and comorbidities accompanying spontaneous and postoperative retroperitoneal abscesses
| Predisposing factors | Spontaneous abscesses | Postoperative abscesses |
|---|---|---|
| Malignancies | 9 | 21 |
| Diabetes mellitus | 6 | 4 |
| Chronic renal failure/ nephrectomy | 4 | 8 |
| Urolithiasis/ ureteropelvic junction obstruction surgery | 18 | 16 |
| Chronic osteomyelitis | 17 | 5 |
| Steroid use | 1 | 1 |
| Splenectomy | 0 | 1 |
| Crohn disease | 3 | 1 |
| Pancreatitis | 3 | 0 |
| Iatrogenic bowel perforation | 0 | 9 |
| Lombar disc operation | 0 | 7 |
| Hydatid cyst | 1 | 2 |
Some patients had more than one comorbidities.
Collections with proven infectious findings (positive culture or negative culture but having foul odor with polymorphonuclear leukocytes on microscopical evaluation) were included. A diagnosis of tuberculosis abscess was made based on demonstration of acidfast bacilli in drainage material.
Volume of abscesses was calculated with respect to standard ellipsoid formula (volume = 0.52×A×B×C). Informed consent was obtained from all patients or closest relatives of pediatric, debilitated and critically ill patients prior to percutaneous drainage procedure.
A total of 28 patients with sterile fluid collections such as simple cysts, lymphoceles, urinomas, hematomas and patients who had less than 1 year of follow-up after primary percutaneous drainage were excluded from the study.
Preprocedural preparation
Of 170 retroperitoneal abscesses, 148 (87.1%) were drained under US and floroscopy guidance, while 22 (12.9%) were drained under CT guidance. All procedures were performed in Interventional Radiology Department after 6–8 hours of fasting, following standard sterilization conditions. Patients with normal coagulation parameters (international normalized ratio less than 1.2 and thrombocyte count more than 80000/mm3) were accepted for drainage procedure. Conscious sedation was applied by the anesthesiology team with cardiac monitorization. Before initial percutaneous drainage for each patient, clinical forms including medical and surgical history, suspected cause of retroperitoneal abscess, immunologic status, laboratory findings, imaging techniques used for diagnosis, catheterization technique, catheter size and type were recorded. Location of retroperitoneal abscess and possible safe access routes were evaluated in detail via US and/or CT. Percutaneous drainage procedures were carried out with either US (Siemens or Toshiba Medical Systems) and floroscopy (Siemens Artis Zee and Siemens Megalix) or 4-detector CT (Siemens Volume Zoom).
Procedure
After localizing the abscess, we used a modified Seldinger technique and carried out first puncture under imaging guidance (either US or CT) by using 18 G Seldinger needle, followed by 5–10 cc fluid aspiration for Gram staining and culture. We introduced a 0.035-inch stiff guidewire (Amplatz Super Stiff, Boston Scientific), then a pigtail catheter of 6–14 F size (Flexima APDL, Boston Scientific or Skater, Angiotech) over the guidewire into the abscess cavity. Before advancing the catheter, we dilated the tract with necessary dilators. Catheter size was chosen based on viscosity and volume of abscess. After evacuation of abscess cavity as much as possible, we obtained cavitography with water soluble contrasts in order to demonstrate possible fistulous connections. Then, catheter was fixed with silk sutures to the skin and left for gravity drainage. Six abscesses were aspirated by a needle without catheter drainage (Figs. 1 and 2).
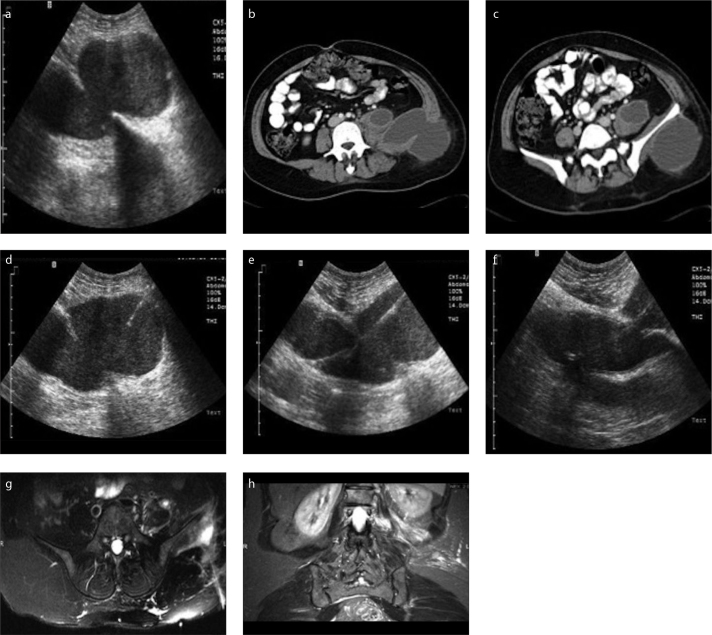
Figure 1. a–h.
Diagnostic, procedural and follow-up imaging of a percutaneously treated tuberculous psoas abscess. Preprocedural US (a) and contrastenhanced venous phase transverse abdominal CT (b, c) images reveal a multiloculated collection involving left psoas muscle extending to the left flank region, neighbouring left iliac bone. US images (d–f) captured during procedure show introduced 18 G Seldinger needle (d) and 0.035-inch guidewire (e), followed by insertion of 12 F catheter (f) into the abscess cavity. Follow-up T2-weighted fat saturated transverse and coronal MRI images (g, h) demonstrate minimally increased signal intensity in the left psoas muscle and soft tissues surrounding the left iliac bone with no residual or recurrent cavity.
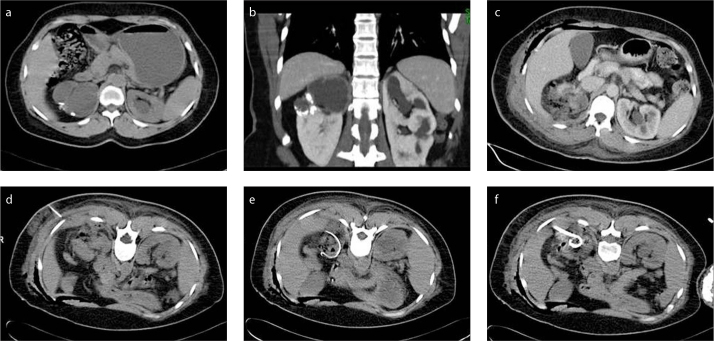
Figure 2. a–f.
Axial (a) and coronal (b) CT images reveal complicated cysts and accompanying calcification in the right kidney. An abscess cavity with increased surrounding fat density and air bubbles is seen in the operation area after partial nephrectomy (c). With patient in supine position, 18 G Chiba needle was advanced into the abscess localization under CT guidance (d). CT images (e, f) show loop appearance of the 0.035-inch guidewire in the abcess cavity (e) and insertion of 10F catheter into the abscess cavity over the wire (f).
Most of our patients had already been taking antibiotics as a medical therapy before the procedure. If not, one dose of intravenous (IV) antibiotics (cephazoline or cephtriaxone) was administered. We switched to appropriate antibiotics combinations based on subsequent results of Gram staining or culture and the underlying microorganism.
Follow-up
After the procedures, patients were followed-up in their relevant clinical wards. Clinical and laboratory findings, culture and smear results as well as catheterization periods were recorded on a daily basis in our standardized forms. Catheters were irrigated daily with 5–10 cc saline to prevent drainage blockage. If necessary, additional catheter maneuvers or drainage procedures were performed in the Interventional Radiology department. After sessation of drainage (less than 10 cc per day for at least 1 day), return of blood leukocyte levels to normal levels, regression of clinical symptoms, and radiological documentation of cavity shrinkage, catheters were removed. Patients were called back for further follow-up in order to confirm the absence of recurrence. For patients who failed to show up for routine follow-up, phone inquiries were made to contact the patient or their primary physician was notified.
Routine follow-up for effectiveness of treatment and detection of possible recurrences was performed periodically 1 and 12 months after procedure, then once a year via US and CT, if necessary. If recurrence was found, then a second percutaneous procedure was performed. Median follow-up period was 42 months (range, 1–74 months).
Procedural aim was cure, temporization, or palliation; and the outcome was evaluated as success or failure (12). Total cure was defined as total recovery with no further recurrence or infectious complications within 1 year following the initial procedure. Temporization was performed in patients that needed definitive surgery for primary pathologies (e.g., urolithiasis, chronic renal failure, Crohn disease, diverticulitis) in order to improve their clinical situation prior to surgery. Palliation was performed for patients having additional morbidities with short life expectancy such as widespread malignancy or severe immune deficiency in order to preclude the progression of clinical deterioration. We accepted percutaneous drainage procedure to be successful in all three groups. Patients who died due to septic complications regardless of their radiological improvement in retroperitoneal abscesses and patients with recurring abscesses during initial follow-up were included in the failure subgroup.
Statistical analysis
Descriptive statistics of the data are presented as number and frequency and median (min–max or 25–75 percentiles) for non-normalized variables, mean±SD for normal distributions. Relationship between categorical values were evaluated with chi square test (Pearson chi square, Yates correction chi square or Fisher exact test). The Kolmogorov-Smirnov and the Shapiro-Wilk W test were used for normal distribution. All analyses were done with respect to 95% confidence interval and P values lower than 0.050 were accepted as statistically significant. Statistical analysis was performed with SPSS 20.0 (IBM Corp.).
Results
Percutaneous abscess drainage was performed for 170 retroperitoneal abscesses of 150 patients. Patients usually presented with several signs and symptoms of infection such as fever, flank pain, leukocytosis, increased levels of erythrocyte sedimentation rate. Also, there were additional clinical findings, in particular those related with exact location of abscesses and neighbouring structures, such as difficulty in thigh movements for psoas abscesses. Among 170 abscesses, 164 (96.5%) were treated using the catheterization technique, whereas 6 (3.5%) were treated by needle aspiration only. Simple needle aspiration technique was used in cases with low viscosity and abscess diameter <4 cm. Median abscess volume was 520 cc (min–max, 25–4000 cc) before the procedure. Catheters with a median size of 8 F (min–max, 6–14 F) were used for abscess drainage. Mean postprocedural catheter drainage period was 13±10.6 days for 170 abscesses. With respect to anatomical locations, mean catheterization periods were 12±5.3 days for renal abscesses, 10±5.4 days for perirenal abscesses, 14±8.5 days for pararenal abscesses, 12±7.9 days for pyogenic psoas abscesses, and 16±9.5 days for tuberculous psoas abscesses (Table 2).
Table 2.
Total number, volumes, catheterization periods, success and failure rates of retroperitoneal abscesses with respect to anatomical locations
| Location | Total n |
Volume (mL) Median (min–max) |
Catheterization period (days) mean±SD | Cure n (%) |
Temporization & palliation n (%) | Success n (%) |
Failure n (%) |
|
|---|---|---|---|---|---|---|---|---|
| Renal | 13 | 153 (40–335) | 12±5.28 | 10 (76.9) | 1 (7.7) | 11 (84.6) | 2 (15.4) | |
|
| ||||||||
| Perirenal | 25 | 335 (25–2200) | 10±5.42 | 18 (72.0) | 3 (12.0) | 21 (84.0) | 4 (16.0) | |
|
| ||||||||
| Pararenal | 57 | 980 (50–4500) | 14±8.47 | 49 (86.0) | 5 (8.7) | 54 (94.7) | 3 (5.3) | |
|
| ||||||||
| Psoas | Pyogenic | 54 | 515 (60–4200) | 12±7.86 | 39 (72.2) | 9 (16.6) | 48 (88.9) | 6 (11.1) |
| Tuberculous | 21 | 550 (30–4000) | 16±9.46 | 12 (57.2) | 2 (9.5) | 14 (66.7) | 7 (33.3) | |
|
| ||||||||
| Total | 170 | 520 (25–4500) | 13±10.61 | 128 (75.3) | 20 (11.8) | 148 (87.1) | 22 (12.9) | |
In drainage materials sent to culture, Escherichia coli (22.3%, n=38) was the most common microorganism followed by Pseudomonas aeruginosa (15.8%, n=27) and Mycobacterium tuberculosis (14.7%, n=25).
When all retroperitoneal abscesses are considered, primary clinical success was obtained in 87.1% (75.3% cure, 7.7% temporization and 4.1% palliation), while percutaneous drainage ended in failure for 12.9% (n=22) (Table 2). Reasons for failure in our series were recurrence and mortality due to septicemia. Eighteen abscesses (10.6%) showed recurrence after successful primary drainage procedure in the first-year follow-up. Among them 2 (11.1%) were renal, 3 (16.7%) were perirenal, 1 (5.5%) was pararenal, and 12 (66.7%) were psoas abscesses (of which, 7 [38.9%] were tuberculosis and 5 [27.8%] were pyogenic abscesses). Of 18 recurred cases, 17 (94.4%) had been treated with catheterization and 1 (5.6%) with needle aspiration as initial procedure. Recurrent cases were treated by catheter drainage (n=10, 55.5%), simple aspiration (n=3, 16.7%), surgical drainage (n=3, 16.7%), and medical treatment (n=2, 11.1%). Secondary clinical succes rate after reintervention of the abcesses with recurrence was 94.7% (161/170). Cure was obtained in all successfully treated recurrent patients.
Anatomical location of the abscesses were psoas in 75 (44.1%), renal in 13 (7.7%), perirenal in 25 (14.7%), pararenal in 57 (33.5%) (Table 2). Success rates did not show statistically significant difference in terms of the anatomical locations of retroperitoneal abscesses (P = 0.296). Eighty-eight abscesses (51.8%) in 77 patients had developed postoperatively, whereas 82 abscesses (48.2%) in 73 patients were spontaneous.
Cure rates of postoperative retroperitoneal abscesses and spontaneous counterparts were 73.9% (n=65) and 76.8% (n=63), respectively (P = 0.440). Success rates among postoperative retroperitoneal abscesses did not show statistically significant difference with respect to their anatomical locations (P = 0.333). Cure rates were significantly lower in immunosuppressed patients compared with immunocompetent patients (61.8% [n=34] vs. 81.7% [n=94], P = 0.038). Failure rates were similar in both groups (15/115 [13%] vs. 7/55 [12.7%], P = 0.450). Palliation rates were significantly higher among the immunosuppressed patients (10.9% [n=6] vs. 0.9% [n=1], P = 0.042) since percutaneous treatment was usually performed for the purpose of palliation and temporization in these patients, based on lower life expectancy and present comorbidities (Table 3).
Table 3.
Cure, palliation and failure rates of Groups 1 and 2
| Group 1 (Immunocompetent) | Group 2 (Immunosuppressed) | P | |
|---|---|---|---|
| Cure | 94 (81.7) | 34 (61.8) | 0.038 |
| Palliation | 1 (0.9) | 6 (10.9) | 0.042 |
| Failure | 7 (12.7) | 15 (13) | 0.450 |
A total of 23 complications (13.5%) occurred in 22 patients, with 7 exitus (4.7%). Complications were classified and grouped according to CIRSE classification system for complications (13). Details of complications are given in Table 4. One complication (0.5%) (pleural effusion) was grade 2 whereas 18 (10.4%) complications were grade 3.
Table 4.
Complications of the abscess drainage procedures according to the CIRSE classification system for complications
| CIRSE grade | Complication | n (%) | Result |
|---|---|---|---|
| 2 | Pleural effusion | 1 (0.5) | Regression during watchful follow-up |
| 3 | Transient bacteremia | 3 (1.7) | Regression with antibiotic treatment |
| 3 | Venous hemorrhage | 1 (0.5) | IV fluid replacement |
| 3 | Accidental catheter removal | 2 (1.2) | New catheter was introduced via same access route |
| 3 | Catheter malposition | 4 (2.3) | Catheter was repositioned |
| 3 | Catheter break | 3 (1.8) | Exchanged with new catheter |
| 3 | Catheter occlusion | 5 (2.9) | Reopened with irrigation |
| 6 | Septicemia | 4 (2.6) | Exitus |
CIRSE, Cardiovascular and Interventional Radiological Society of Europe.
Four of our patients (2.7%) died as a result of septic complications within the first month following drainage whereas 3 (2%) died due to medical problems independent of procedure. Therefore 30-day mortality was 4.7% (7/150). Of 4 mortalities arising from sepsis, one had perirenal abscess, two had pararenal abscesses, and one had psoas abscess. Of these 4 patients, two had accompanying malignancies (one renal cell carcinoma, one liposarcoma), one had underlying chronic renal failure, and one had duodenum perforation due to prior endoscopic retrograde cholangiopancreatography. We did not observe any drainage related mortality in our cases for reasons other than septicemia. We had to change position of catheters in 9 cases (5.3%) due to blockage of drainage or catheter malposition. We had 3 cases (1.8%) with broken catheters in our series, but we did not encounter any spontaneous withdrawal of catheters.
Among 13 cases of renal abscess, cure was provided in 10 cases (76.9%), temporization in one case (7.7%). Outcome was failure in two cases (15.4%), success in 11 cases (84.6%), and grade 3 complications occurred in 3 cases (23.1%).
Among 25 cases of perirenal abscess, cure was obtained in 18 cases (72%), temporization in two cases (8%), palliation in one case (4%). Failure was encountered in 4 cases (16%), grade 6 complication (septicemia) in one case (4%), grade 3 complications in 4 cases (16%). Total success was reached in 21 cases (84%).
Among 57 cases of pararenal abscess, cure was obtained in 49 cases (85.9%), temporization in 3 cases (5.3%), palliation in two cases (3.5%). Failure was seen in 3 cases (5.3%), grade 6 complications in two cases (3.5%) and grade 1 complication in one case (1.8%). Success was achieved in 54 cases (94.7%).
Among 75 cases of psoas abscess, cure was achieved in 51 cases (68%), temporization in 7 cases (9.4%), palliation in 4 cases (5.3%); failure occurred in 13 cases (17.3%), grade 6 complication (septicemia) in one case (1.3%), grade 3 complication in 8 cases (10.7%). Success rate was 82.7% (62/75).
Among psoas abscesses, 21 (28%) were tuberculous, 54 (72%) were pyogenic abscesses. For 54 cases of pyogenic psoas abscesses, we had cure in 39 cases (72.2%), temporization in 5 cases (9.3%), palliation in 4 cases (7.4%); failure occurred in 6 cases (11.1%) with a total success rate of 88.9% (48/54). For 21 cases of tuberculous abscesses, cure was obtained in 12 cases (57.2%), temporization in two cases (9.5%); failure occurred in 7 cases (33.3%), with a total success rate of 66.7%. Success rates were significantly lower in the tuberculosis group compared with the pyogenic group (66.7% vs. 88.9%, P = 0.023).
Discussion
Percutaneous drainage has been accepted as the preferred method of treatment for retroperitoneal abscesses, as it is better tolerated by patients, eliminates the need for general anesthesia, and is associated with shorter hospital stay periods (2, 4, 14, 15). Mortality rate after surgical drainage of retroperitoneal abscesses is reported to be 39%–50% (1), while it is around 1.5%–10% for percutaneous drainage (2, 14–16). In our series, we had a 30-day mortality rate of 4.6% (n=7) due to septicemia (n=4) and subsequent multiorgan failure (n=3).
Given the substantial variability in patient groups and age selection, lack of long-term follow-up results, and differences in success evaluation criteria, there is limited chance of detailed comparison with similar series. In the literature, success rates of percutaneous therapy for abdominal abscesses vary between 70% and 100% (2, 4, 12, 18–22). In our study, we had a total success rate of 87.1%, which is comparable with or better than the success rates found in similar series in the literature.
For 6 abscesses treated via only needle aspiration, success rate was 83.3% (5/6) without any complication. Only one case of tuberculous abscess recurred after simple aspiration and was treated via percutaneous catheterization with no further complication. Although limited in number, our results suggest that for nonviscous and pyogenic retroperitoneal abscesses less than 4 cm in diameter, simple aspiration and systemic antibiotics would be sufficient, which is in concordance with similar series of percutaneously treated pyogenic liver abscesses (23). Being cheaper, easier, less invasive, and less prone to subsequent infectious complications, simple needle aspiration may be more advantageous than catheterization in certain clinical settings. However, tuberculous abscesses should be drained via catheterization due to higher recurrence rates.
There is limited data in the literature comparing mortality rates between spontaneous and postsurgical retroperitoneal abscesses. Schechter et al. (24) found higher morbidity and mortality rates in postsurgical intraabdominal abscess group. Higher rates of mortality in postsurgical retroperitoneal abscess group can be explained by their older age and underlying morbidities. The results in our study regarding success rates of postsurgical and spontaneous abscesses were in concordance with previously reported data (10, 20).
Lambiase et al. (12) reported lower cure rates for immunocompromised patients when compared to immunocompetent patients. Similarly, cure rates in our study were statistically significantly lower in immunocompromised patients compared with immunocompetent patients (61.8% vs. 81.7%). For immunocompromised patients, percutaneous drainage procedure is usually performed on a temporization or palliation basis due to lower life expectancy. Lower cure and success rates can be explained by their tendency to develop further infectious complications.
Possible failure reasons of percutaneous abscess drainage are clinical misdiagnosis of a tumor as a simple abscess, wrong drainage technique, presence of several complicating factors (e.g., malignancy, urolithiasis, chronic renal failure, diabetes) and fistulization of abscess cavity. Reasons for failure related with postprocedural follow-up are misdiagnosis of residual abscess cavity, early withdrawal of catheter, insufficient number and caliber of catheters, inappropriate entrance route and inappropriate concomitant use of antibiotics (18, 20, 25, 26).
No failures related to factors such as wrong choice of access route, insufficient number of catheters, use of small caliber catheter incompatible with abscess size, lack of gravitational utilization for optimal drainage were observed in our series. As soon as ineffective or inadequate drainage was observed during clinical follow-up, we performed a second percutaneous drainage session for either catheter exchange or additional catheter placement. Recurrence was identified as the main cause for failed procedures (10.5%) in our series. Recurrence rates in our study are comparable with similar retroperitoneal abscess series in the literature (2). Percutaneous drainage still remains as the most feasible option for recurrent retroperitoneal abscesses, as we treated 13 of 18 recurrent cases via a second session of percutaneous drainage successfully. Complications related with percutaneous drainage of abdominal abscesses are reported to be around 10%–15% in previous studies (11, 12, 18, 20) whereas it was 13.5% (23/170) in our series with a mean catheterization period of 13 days for all retroperitoneal abscesses; however, it should be noted that tuberculous abscesses require longer periods of catheterization (27).
Percutaneous drainage procedures result in shorter hospital stay periods compared with surgical drainage procedures. Procaccino et al. (28) reported 26 days of mean hospitalization period for surgically treated psoas abscesses, whereas Altemeier et al. (3) found mean hospitalization periods of 57 days and 80 days for surgically treated pararenal and perirenal abscesses. In our series, mean hospitalization period with catheterization was 6 days for all patients.
In the literature, success rates of percutaneous drainage for renal and perirenal abscesses vary between 61% and 93% (1, 12, 16, 18, 19). Cure and success rates were found to be comparable with the literature when renal and perirenal abscesses are considered. With a 68% cure rate and 82.7% success rate of psoas abscesses in our series, we achieved similar or better results compared with previous studies (2, 15, 17).
In a series, 67% of psoas abscesses were due to tuberculosis (29), while this rate was reported as 14.2% by Wong et al (30). As 28% (21/75) of psoas abscess cases in our series were due to tuberculosis, it can be concluded that tuberculosis still poses a significant risk factor for public health, particularly for development of psoas abscesses (29, 31–34). Therefore, Mycobacterium tuberculosis should also be considered as a possible infectious etiology of psoas abscesses, and antituberculosis drugs should be initiated in the presence of indicative culture and laboratory results. Gupta et al. (27) found a success rate of 70.3% for tuberculous abscesses. We had a success rate of 66.7% for tuberculous psoas abscesses, which is comparable with similar results in the literature.
To the best of our knowledge, our retrospective study is one of the largest series of percutaneously drained retroperitoneal abscesses in the literature. We could not find a statistically significant difference between the success rates of spontaneous and postoperative retroperitoneal abscesses. Percutaneous abscess drainage of immunocompromised patients resulted in significantly lower cure rates compared with the immunocompetent patient group. Though usually successfully performed, percutaneous drainage resulted in still higher failure rates in tuberculous psoas abscesses compared with pyogenic counterparts. Catheterization should be preferred in tuberculous abscesses instead of simple needle aspiration due to higher recurrence rates with the latter method. Mortality due to percutaneous drainage is mainly determined by underlying morbidities and immune status of the patient.
Our study has several limitations. First, the retrospective nature of the study is a limitation. Second, we did not have a separate group of surgically drained retroperitoneal abscess patients to reach further conclusions by comparison.
In conclusion, percutaneous drainage is a succesful and safe treatment method for retroperitoneal abscess independent of its anatomical location, with lower morbidity and mortality rates, shorter hospital stay, and better tolerability compared with the surgical techniques.
Main points.
US has sensitivity over 90% in diagnosis of retroperitoneal abscesses, while CT is superior to US to demonstrate the location of abscess and its neighboring anatomical structures in detail and establish conclusive diagnosis.
Catheterization should be preferred in tuberculous abscesses instead of simple needle aspiration, due to the higher recurrence rates observed with the latter method.
Percutaneous drainage of retroperitoneal abscesses is a succesful and safe treatment method with lower morbidity and mortality rates, shorter hospital stays, and better tolerability compared with the surgical techniques.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Coelho RF, Schneider-Monteiro ED, Mesquita JL, Mazzucchi E, Marmo Lucon A, Srougi M. Renal and perinephric abscesses: analysis of 65 consecutive cases. World J Surg. 2007;31:431–436. doi: 10.1007/s00268-006-0162-x. [DOI] [PubMed] [Google Scholar]
- 2.Manjón CC, Sánchez AT, Piedra Lara JD, et al. Retroperitoneal Abscesses, analysis of a series of 66 cases. Scand J Urol Nephrol. 2003;37:139–144. doi: 10.1080/00365590310008884. [DOI] [PubMed] [Google Scholar]
- 3.Altemeier WA, Culbertson WR, Fullen WD, Shook CD. Intraabdominal abscesses. Am J Surg. 1973;125:70–79. doi: 10.1016/0002-9610(73)90010-X. [DOI] [PubMed] [Google Scholar]
- 4.Gordon DH, Macchia RJ, Glanz S, Koser MW, Laungani GB. Percutaneous management of retroperitoneal abscesses. Urology. 1987;30:299–306. doi: 10.1016/0090-4295(87)90289-5. [DOI] [PubMed] [Google Scholar]
- 5.Crepps JT, Welch JP, Orlando R. Management and outcome of retroperitoneal abscesses. Ann Surg. 1987;205:276–281. doi: 10.1097/00000658-198703000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Men S, Akhan O, Köroğlu M. Percutaneous drainage of abdominal abscess. Eur J Radiol. 2002;43:204–218. doi: 10.1016/S0720-048X(02)00156-0. [DOI] [PubMed] [Google Scholar]
- 7.Ferruci JT, vanSonnenberg E. Intra-abdominal abscess: radiological diagnosis and treatment. JAMA. 1981;246:2728–2733. doi: 10.1001/jama.1981.03320230052028. [DOI] [PubMed] [Google Scholar]
- 8.Dobrin PB, Gully PH, Greenlee HB, et al. Radiological diagnosis of an intraabdominal abscess: do multiple tests help? Arch Surg. 1986;121:41–46. doi: 10.1001/archsurg.1986.01400010047005. [DOI] [PubMed] [Google Scholar]
- 9.Doust BD, Quiroz F, Stewart JM. Ultrasonographic distinction of abscesses from other intraabdominal fluid collections. Radiology. 1977;125:213–218. doi: 10.1148/125.1.213. [DOI] [PubMed] [Google Scholar]
- 10.Gerzof SG, Johnson WC, Robbins AH, et al. Expanded criteria for percutaneous abscess drainage. Arch Surg. 1985;120:227–232. doi: 10.1001/archsurg.1985.01390260085012. [DOI] [PubMed] [Google Scholar]
- 11.Vansonnenberg E, Wing VW, Casola G, et al. temporizing effect of percutaneous drainage of complicated abscesses in critically ill patients. AJR Am J Roentgenol. 1984;142:821–826. doi: 10.2214/ajr.142.4.821. [DOI] [PubMed] [Google Scholar]
- 12.Lambiase RE, Deyole L, Cronan JJ, Dorfman GS. Percutaneous drainage of 335 consecutive abscesses: Results of primary drainage with 1-year follow-up. Radiology. 1992;184:167–179. doi: 10.1148/radiology.184.1.1376932. [DOI] [PubMed] [Google Scholar]
- 13.Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse quality assurance document and standards for classification of complications: The Cirse classification system. Cardiovasc Intervent Radiol. 2017;40:1141–1146. doi: 10.1007/s00270-017-1703-4. [DOI] [PubMed] [Google Scholar]
- 14.Brolin RE, Nosher JL, Leiman S, et al. Percutaneous catheter versus open surgical drainage in the treatment of abdominal abscesses. Am Surg. 1984;50:102–108. [PubMed] [Google Scholar]
- 15.Dietrich A, Vaccarezza H, Vaccaro CA. Iliopsoas abscess: presentation, management, and outcomes. Surg Laparosc Endosc Percutan Tech. 2013;23:45–48. doi: 10.1097/SLE.0b013e31826e0ac9. [DOI] [PubMed] [Google Scholar]
- 16.Deyoe LA, Cronan JJ, Lambiase RE, Dorfman GS. Percutaneous drainage of renal and perirenal abscesses: results in 30 patients. AJR Am J Roentgenol. 1990;155:81–83. doi: 10.2214/ajr.155.1.2112870. [DOI] [PubMed] [Google Scholar]
- 17.Tejido Sánchez A, Jiménez de la Peña MM, Duarte Ojeda JM, et al. Percutaneous treatment of retroperitoneal abscesses. Actas Urol Esp. 2000;24:131–137. doi: 10.1016/S0210-4806(00)72419-3. [DOI] [PubMed] [Google Scholar]
- 18.Lang EK, Springer RM, Glorioso LW, Cammarata CA. Abdominal abscess drainage under radiologic guidance: Causes of failure. Radiology. 1986;159:329–336. doi: 10.1148/radiology.159.2.2421370. [DOI] [PubMed] [Google Scholar]
- 19.Sacks D, Banner MP, Meranze SG, Burke DR, Robinson M, McLean GK. Renal and related retroperitoneal abscesses: percutaneous drainage. Radiology. 1988;167:447–451. doi: 10.1148/radiology.167.2.3357954. [DOI] [PubMed] [Google Scholar]
- 20.Gerzoff SG, Robbins AH, Johnson WC, et al. Percutaneous catheter drainage of abdominal abscesses: a five-year experience. N Engl J Med. 1981;305:653–657. doi: 10.1056/NEJM198109173051201. [DOI] [PubMed] [Google Scholar]
- 21.Hélénon O, Cornud F, Di Stéfano D, et al. Percutaneous treatment of abscess of the kidney and retroperitoneum. J Radiol. 1989;70:541–548. [PubMed] [Google Scholar]
- 22.Asai N, Ohkuni Y, Yamazaki I, Kaneko N, Aoshima M, Kawamura Y. Therapeutic impact of CT-guided percutaneous catheter drainage in treatment of deep tissue abscesses. Braz J Infect Dis. 2013;17:483–486. doi: 10.1016/j.bjid.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zerem E, Hadzic A. Sonographically guided percutaneous catheter drainage versus needle aspiration in the management of pyogenic liver abscess. AJR Am J Roentgenol. 2007;189:138–142. doi: 10.2214/AJR.07.2173. [DOI] [PubMed] [Google Scholar]
- 24.Schechter S, Einsentat TE, Oliver GC, et al. Computerized tomographic scan guided drainage of inta-abdominal abscesses. Preoperative and postoperative modalities in colon and rectalsurgery. Dis Colon Rectum. 1994;37:984–988. doi: 10.1007/BF02049309. [DOI] [PubMed] [Google Scholar]
- 25.VanSonnenberg E, Mueller PR, Ferruci JT. Percutaneous drainage of 250 abdominal abscess and fluid collections. I. results, failures, and complications. Radiology. 1984;151:337–341. doi: 10.1148/radiology.151.2.6709901. [DOI] [PubMed] [Google Scholar]
- 26.Lang EK. Renal, perirenal, and pararenal abscesses: percutaneous drainage. Radiology. 1990;174:109–113. doi: 10.1148/radiology.174.1.2294535. [DOI] [PubMed] [Google Scholar]
- 27.Gupta S, Suri S, Gulati M, Singh P. Ilio-psoas abscesses: percutaneous drainage under image guidance. Clin Radiol. 1997;52:704–707. doi: 10.1016/S0009-9260(97)80036-0. [DOI] [PubMed] [Google Scholar]
- 28.Procaccino JA, Lavery JC, Fazio VW, Oakley JR. Psoas abscess: difficulties encountered. Dis Colon Rectum. 1991;34:784–789. doi: 10.1007/BF02051071. [DOI] [PubMed] [Google Scholar]
- 29.Dinç H, Önder C, Turhan U, et al. Percutaneous catheter drainage of tuberculous and nontuberculous psoas abscesses. Eur J Radiol. 1996;23:130–134. doi: 10.1016/0720-048X(96)01045-5. [DOI] [PubMed] [Google Scholar]
- 30.Wong OF, Ho PL, Lam SK. Retrospective review of clinical presentations, microbiology, and outcomes of patients with psoas abscess. Hong Kong Med J. 2013;19:416–423. doi: 10.12809/hkmj133793. [DOI] [PubMed] [Google Scholar]
- 31.Kim YJ, Yoon JH, Kim SI, Wie SH, Kim YR. Etiology and outcome of iliopsoas muscle abscess in Korea; changes over a decade. Int J Surg. 2013;11:1056–1059. doi: 10.1016/j.ijsu.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Akinci D, Ergun O, Topel Ç, Çiftçi, Akhan O. Pelvic abscess drainage: outcome with factors affecting the clinical success. Diagn Interv Radiol. 2018;24:146–152. doi: 10.5152/dir.2018.16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciftci TT, Akinci D, Akhan O. Percutaneous transhepatic drainage of inaccessible postoperative abdominal abscesses. AJR Am J Roentgenol. 2012;198:477–481. doi: 10.2214/AJR.11.6680. [DOI] [PubMed] [Google Scholar]
- 34.Akinci D, Akhan O, Ozmen MN, Karabulut N, Ozkan O, Cil BE, Karcaaltincaba M. Percutaneous drainage of 300 intraperitoneal abscesses with long-term follow-up. Cardiovasc Intervent Radiol. 2005;28:744–750. doi: 10.1007/s00270-004-0281-4. [DOI] [PubMed] [Google Scholar]