Abstract
BACKGROUND AND PURPOSE:
It has been shown that the brain of a preterm infant develops differently from that of a term infant, but little is known about the neonatal cerebrovascular anatomy. Our aims were to establish reference data for the prevalence of the anatomic variations of the neonatal circle of Willis (CoW) and to explore the effect of prematurity, MR imaging abnormality, vascular-related abnormality, laterality, and sex on these findings.
MATERIALS AND METHODS:
We scanned 103 infants with an optimized MR angiography (MRA) protocol. Images were analyzed for different variations of the CoW, and results were compared for the following: 1) preterm-at-term and term-born infants, 2) infants with normal and abnormal MR imaging, 3) infants with and without a vascular-related abnormality, 4) boys and girls, and 5) left- and right-sided occurrence.
RESULTS:
The most common anatomic variation was absence/hypoplasia of the posterior communicating artery. Preterm infants at term had a higher prevalence of a complete CoW and a lower prevalence of anatomic variations compared with term-born infants; this finding was significant for the anterior cerebral artery (P = .02). There was increased prevalence of variations of the major cerebral arteries in those infants with vascular-related abnormalities, statistically significant for the posterior cerebral artery (P = .004). There was no statistically significant difference between boys and girls and left/right variations.
CONCLUSIONS:
Prematurity is associated with more complete CoWs and fewer anatomic variations. In vascular-related abnormalities, more variations involved major arterial segments, but fewer variations occurred in the communicating arteries. Overall reference values of the variations match those of the general adult population.
The circle of Willis (CoW) is a ringlike arterial structure, which, when complete,1 consists of 9 component vessels (Fig 1A, -B). However, as both autopsy2 and imaging3 studies in adults have reported, the CoW may be incomplete and may present different anatomic variations, depending on the presence or absence and the size (hypoplasia/hyperplasia) of its component vessels (Fig 2A, -D). In the past, the origin of these variations was thought to involve hereditary factors4; later studies reported that these altered CoW configurations are the result of developmental modifications driven by the functional demand of the growing brain, with a change of their prevalence during the human lifespan.5–7
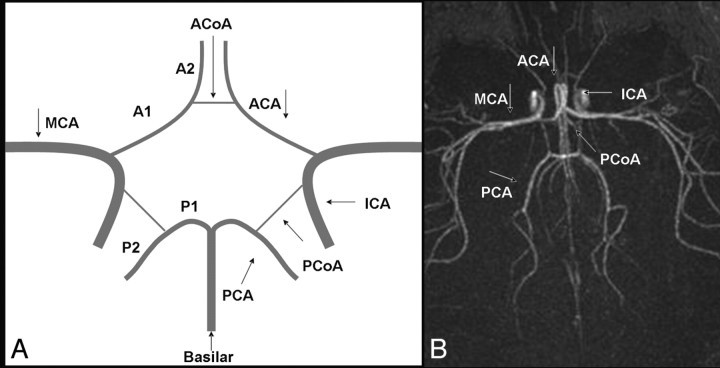
Fig 1.
A, Graph of the CoW with its component vessels: A1 segments of the ACAs, AcomA, internal carotid arteries (ICAs), PcomAs, and P1 segments of PCAs. The middle cerebral artery (MCA) and the basilar artery are not structurally part of the CoW. B, Complete CoW in a preterm neonate on an axial 3D TOF MRA image.
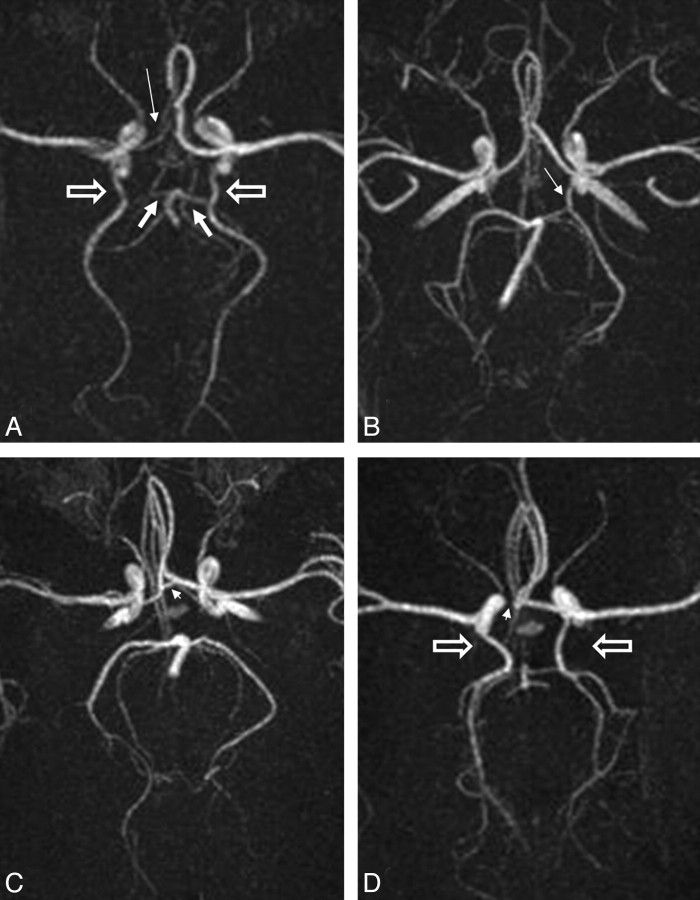
Fig 2.
Axial MIP images of different anatomic variations of the CoW in the neonatal brain. A, Hypoplastic right A1 segment of the ACA (thin arrow) and bilateral fetal-type origin of the PCA with bilaterally hypoplastic P1 segments (thick arrows) and enlarged PcomAs (block arrows). B, Unilateral (left) fetal-type origin of the PCA with the PcomA diameter (thin arrow) larger than the P1 diameter. C, Clearly present AcomA (arrowhead) with bilaterally absent PcomAs. D, Absent right A1 segment (arrowhead), and bilateral fetal-type origin of the PCA (block arrows) with absent P1 segments. The whole of the anterior circulation originates from the enlarged left ACA.
The arteries of the CoW are the major blood suppliers of the brain. The CoW can serve potentially as a primary collateral pathway in cases of impaired or decreased flow within 1 or more of the major cerebral vessels.8 Its ability to redistribute blood flow to hemodynamically deprived areas and to operate as a natural protective mechanism in vascular accidents depends largely on the presence and size of its component vessels.
Previous MR imaging studies in term-born infants and preterm infants at term-equivalent age have shown that premature exposure to the ex utero environment alters brain development in the white matter,9–11 cortex,12,13 and deep gray matter.14,15 It is also known that certain cerebral pathologies with a strong vascular component, such as periventricular leucomalacia and intraventricular hemorrhage, present more often in preterm than term-born infants.16,17 We have previously shown that the proximal cerebral arteries of preterm infants imaged at term-equivalent age have a straighter pattern compared with those in infants born at term and that this difference persists well into infancy.18
The aims of this study were to establish reference data for the prevalence of the anatomic variations in a neonatal population; and to explore the effect of prematurity, degree of prematurity, MR imaging-detected brain abnormality, vascular-related abnormality, sex, and laterality on these findings.
Materials and Methods
Inclusion Criteria
Infants scanned with a high-resolution dedicated MR angiography (MRA) protocol at 3T were included in this study (n = 103). We excluded 9 of these infants due to motion artifacts because these may hamper the detection of anatomic variations, especially for the small communicating cerebral arteries. Of the 94 remaining infants, 44 were preterm and imaged at term-equivalent age and 50 were term-born and scanned soon after birth. There were 50 boys and 44 girls. Of the preterm infants, 23 were born before 30 weeks of gestation and 21 were born after 30 weeks of gestation.
Of the 94 infants, 16 presented with vascular-related abnormalities, such as facial port-wine stain (n = 4), perinatal arterial territory ischemic stroke (n = 7), or cerebral hemorrhage (n = 2). Three preterm infants had retinopathy of prematurity (ROP).
All structural T1- and T2-weighted images were assessed by an experienced pediatric neuroradiologist for the presence of overt white matter or basal ganglia focal lesions. MR imaging findings were classified as abnormal if there was evidence of infarction, porencephalic cyst, parenchymal hemorrhage, or other overt focal lesions for the preterm infant; and abnormal signal intensities in the basal ganglia, loss of gray/white matter differentiation, infarction, and parenchymal hemorrhage in the term infant. In total, 24 term infants and 41 preterm infants had an MR image finding that was considered normal at term or term-equivalent age respectively. The detailed demographic data for those infants is given in Table 1.
Table 1:
Summary of the demographic data of the preterm-at-term and term-born infants taking part in this study
| Demographic Parameters | Preterm Infants at Term |
Term infants |
Statistical Difference between Preterm-at-Term and Term Infants |
|
|---|---|---|---|---|
| Median (range) | Median (range) | P Value | ||
| Gestational age (weeks) | 29.5 (25.7–34.7) | 40 (36.4–42.3) | ||
| Postmenstrual age at scanning (weeks) | 41.1 (36.3–45.3) | 41.6 (37.1–42.8) | .13 | |
| Birth weight (kg) | 1.29 (0.51–2.69) | 3.29 (1.85–4.50) | ||
| Weight at scanning (kg) | 3.06 (1.70–5.5) | 3.48 (1.93–5.28) | .11 | |
| Head circumference at birth (cm) | 27.1 (22.1–34.5) | 34.4 (31–38) | ||
| Head circumference at scanning (cm) | 35.4 (31.2–40) | 36.4 (32–39.4) | .02 | |
Patient Preparation
Approval was granted by the Research Ethics Committee (2003/6564 and 06/Q0406/14) of the hospital, and informed parental consent was obtained before each scanning. Infants were imaged either in natural sleep or, where necessary, after sedation with oral chloral hydrate (20–30 mg/kg) to prevent image degradation from motion artifacts. Each infant was positioned supine, and the head was immobilized by using a pillow evacuated by suction to fit snugly around it. Additional ear protection was used for each infant. Temperature was maintained and monitoring of infants' vital signs was performed throughout the scanning. A neonatologist experienced in MR imaging was present throughout the examination.19
MR Imaging Data Acquisition
All infants were scanned by using a 3T scanner (Achieva; Philips Medical Systems, Best, the Netherlands) with a dedicated high-resolution 3D time-of-flight (TOF) MRA protocol with TR/TE/flip angle of 19/5.7ms/16°, respectively, and true isotropic resolution of 0.6 × 0.6 × 0.6 mm3. This protocol has been specifically optimized for use in a neonatal population, in which vascular flow is slower and vessels are narrower.20 Standard anatomic T1- and T2-weighted images were also acquired; more specifically T1-weighted volume scans and T2-weighted multisection fast-field echo anatomic scans were obtained for the detection of brain abnormalities.
Image Analysis
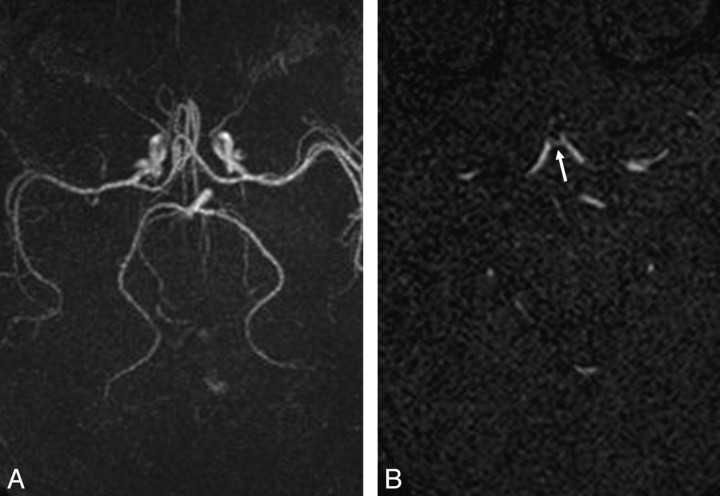
Qualitative assessment of angiographic images was performed by an experienced observer blinded to the clinical and demographic details of the subjects, by using ImageJ software (Version 1.32, National Institutes of Health, Bethesda, Md; http://rsb.info.nih.gov/ij/download.html). A second observer confirmed the findings with substantial interobserver agreement (Cohen κ coefficient, κ = 0.79). The aim of the image assessment was to confirm the completeness or incompleteness of the CoW and to identify and record the prevalence of different anatomic variations (absence/hypoplasia of the posterior communicating artery [PcomA], anterior communicating artery [AcomA], proximal A1 and P1 segments of the anterior cerebral artery [ACA], and posterior cerebral artery [PCA] respectively; and fetal-, transitional-, or adult-type origin of the PCA). Both maximum intensity projections (MIPs) in all imaging planes and source images were used to confirm the findings (Fig 3A, -B).
Fig 3.
A, Axial MIP image of a term-born infant in which the AcomA cannot be clearly seen due to excessive vessel overlap of the pericallosal branches of the ACA. B, Axial source image of the same infant in which the AcomA can be clearly seen (arrow), to exclude AcomA absence/hypoplasia.
Completeness of the CoW and anatomic variations were classified on the basis of the arterial configurations described in previous adult studies.3,7 Segments of the communicating arteries visualized only in the source images but not in the MIP images were reported as hypoplastic; segments visualized in neither the source nor the MIP images were reported as absent. Both hypoplastic and absent segments were considered not present when determining the completeness or incompleteness of the CoW because visibility was used as a surrogate measure of the functionality of the vasculature21; therefore, absent or hypoplastic vessels were considered not functional.
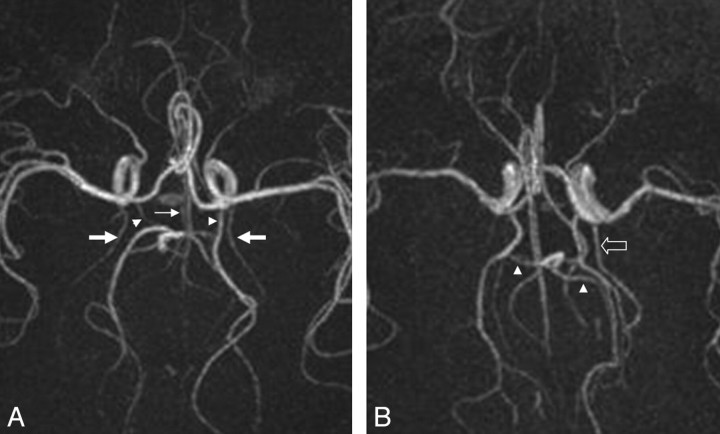
Care was taken to differentiate the PcomAs from the anterior choroidal and overlapping pericallosal branches of the ACA on the axial MIP (Fig 4A). This was achieved by scrolling through the sections and judging the courses of the arteries in sequential display. The communication of the PcomA with the PCA had to be visualized to determine the identity of the vessel. The same method was used to help differentiate the PCAs from the superior cerebellar segments and the anatomic variants of enlarged anterior choroidal branches (Fig 4B).
Fig 4.
A, Axial MIP image in which the anterior choroidal arteries (thick arrows) and pericallosal branches of the ACA (thin arrow) overlap the PcomAs (arrowheads). B, Axial MIP image in which the superior cerebellar arteries (arrowheads) can be mistaken for PCA branches and an abnormally enlarged anterior choroidal artery can be mistaken for fetal-type origin of the PCA (block arrow).
A P1 segment of the PCA larger than the ipsilateral PcomA as visualized on the MIP was classified as adult-type origin of the PCA, a P1 segment with the same size as the PcomA was classified as a transitional-type origin of the PCA, and a P1 with a smaller size than the PcomA (or totally absent) was classified as fetal-type origin of the PCA.
Statistical Considerations
Infants were subdivided in the following subgroups: 1) preterm at term (n = 41) and term-born (n = 24) with normal MR imaging findings; 2) preterm-born before 30 weeks' gestational age (GA, n = 22) and those born after 30 weeks' GA (n = 19) with normal MR imaging findings; 3) term infants with normal MR imaging findings (n = 24) and abnormal MR imaging findings (n = 26); 4) boys (n = 34) and girls (n = 31) with normal MR imaging findings and infants with (n = 16) and without (n = 78) evidence of vascular-related abnormality. Laterality (left- or right-side occurrence) was also examined in those vessels that had bilateral representation in the CoW.
The StatsDirect package (http://www.statsdirect.com/) was used. The Shapiro Wilk test was applied to test the normality of the demographic data, and the unpaired t test, to identify differences between the groups. The Fisher exact test was used to explore associations between prematurity, degrees of prematurity, MR imaging abnormality, sex, vessel laterality, and vascular-related abnormality with the anatomic variations and the completeness of the CoW. The r by c χ2 test (StatsDirect) was used to explore whether the laterality of vascular variations correlated with the laterality of the brain parenchymal or other vascular abnormalities. A P value < .05 was considered to indicate a statistically significant difference.
Results
Reference Values of CoW Anatomic Variations in Neonates with Normal MR Imaging Findings
In the 65 infants (including both term and preterm-at term infants) with normal MR imaging, the CoW was complete in 27 (41.5%). The AcomA was absent or hypoplastic in 12 (18.5%). For the vessels with bilateral representation and hence 130 (2 × 65) potential examples, the PcomA was absent or hypoplastic in 39 of 130 cases (30%), the A1 segment of the ACA was absent or hypoplastic in 6 of 130 cases (4.6%), and the P1 segment of the PCA was absent or hypoplastic in 8 of 130 cases (6.1%). Fetal-type origin of the PCA was observed in 36 of 130 cases (27.7%), and the transitional configuration of the posterior part of the CoW was present in 7 of 130 cases (5.4%). The most common variation was the absence of the PcomA (30%). More details can be found in Table 2.
Table 2:
Reference values of the prevalence of anatomic variations of the CoW in a group of 65 infants (24 term-born, 41 preterm-at-term) with normal MR imaging findings
| Term (normal MRI) n = 24 | Preterm (normal MRI) n = 41 | P Value | Mean | |
|---|---|---|---|---|
| Completeness of CoW | 9 in 24 (37.5%) | 18 in 41 (44%) | .405 | 41.50% |
| AcomA absence/hypoplasia | 6 in 24 (25%) | 6 in 41 (14.6%) | .237 | 18.50% |
| PcomA absence/hypoplasia | 13 in 48 (27%) | 26 in 82 (31.7%) | .363 | 30% |
| A1 segment of ACA absence/hypoplasia | 5 in 48 (10.4%) | 1 in 82 (1.2%) | .026* | 4.60% |
| P1 segment of PCA absence/hypoplasia | 4 in 48 (8.3%) | 4 in 82 (4.8%) | .332 | 6.10% |
| Fetal-type origin of PCA | 14 in 48 (29%) | 22 in 82 (26.8%) | .463 | 27.70% |
| Transitional-configuration origin of PCA | 6 in 48 (12.5%) | 4 in 82 (4.8%) | .11 | 5.40% |
| Bilateral fetal-type origin | 8 in 14 (57.1%) | 14 in 22 (63.6%) | .482 | 61.10% |
| Bilaterally absent PcomA | 6 in 13 (46.2%) | 14 in 26 (53.8%) | .455 | 51.30% |
Note:—CoW indicates circle of Willis; MRI, MR imaging; AcomA, anterior communicating artery; PcomA, posterior communicating artery; ACA, anterior cerebral artery; PCA, posterior cerebral artery.
Statistically significant.
Effect of Prematurity in Neonates with Normal MR Imaging Findings
Preterm infants had a higher prevalence of a complete CoW of 44% relative to term-born infants with 37.5%, but this difference was not statistically significant (P = .41). Preterm infants had a generally lower prevalence of CoW anatomic variations (Table 2). The term-born infants had a significantly higher prevalence (P = .02) of absence/hypoplasia of the A1 segment (10.4%) compared with the preterm infants (1.2%).
The preterm infants born at <30 weeks' GA (n = 22) had a higher prevalence of CoW completeness (50%) compared with the preterm infants born after 30 weeks (n = 19, 36.8%), but this difference was not statistically significant (P = .30). Overall, they had a lower prevalence of anatomic variations (Table 3). The preterm infants born after 30 weeks' GA had more significantly increased prevalence (P = .04) in the bilateral fetal-type origin of the PCA (83.3%) than those infants born before 30 weeks' GA (40%).
Table 3:
Prevalence of anatomic variations of the CoW in a group of 41 preterm-at-term infants (19 born after 30 weeks' GA, 22 born before 30 weeks' GA) with normal MR imaging findings
| Preterm (>30 weeks) | Preterm (<30 weeks) | P Value | |
|---|---|---|---|
| Completeness of CoW | 7 in 19 (36.8%) | 11 in 22 (50%) | .298 |
| AcomA absence/hypoplasia | 4 in 19 (21%) | 2 in 22 (9%) | .262 |
| PcomA absence/hypoplasia | 15 in 38 (39.5%) | 11 in 44 (25%) | .122 |
| A1 segment of ACA absence/hypoplasia | 0 in 38 (0%) | 1 in 44 (2%) | .537 |
| P1 segment of PCA absence/hypoplasia | 2 in 38 (5.2%) | 2 in 44 (4.5%) | .635 |
| Fetal-type origin of PCA | 12 in 38 (31.6%) | 10 in 44 (22.7%) | .257 |
| Transitional-configuration origin of PCA | 1 in 38 (2.6%) | 3 in 44 (6.8%) | .365 |
| Bilateral fetal-type origin | 10 in 12 (83.3%) | 4 in 10 (40%) | .048* |
| Bilaterally absent PcomA | 10 in 15 (66.7%) | 4 in 11 (36.4%) | .129 |
Note:—GA indicates gestational age.
Statistically significant.
There was no statistically significant difference between the early (<30 weeks' GA) infants and the term ones, neither in the completeness of the CoW (P = .29) nor in the incidence of specific anatomic variations.
Effect of MR Imaging Abnormalities
Term infants with abnormal MR imaging findings had a slightly higher prevalence of complete CoW and subsequently a lower prevalence of anatomic variations compared with those with normal MR imaging findings, but this difference did not reach statistical significance. However, A1 segment absence/hypoplasia was observed less frequently in term-born infants with abnormal MR imaging findings than in those with normal MR imaging findings (P = .05). The same was true for the bilaterally absent PcomA (P = .07), approaching statistical significance, as seen in Table 4.
Table 4:
Prevalence of anatomic variations of the CoW in a group of 50 term-born infants (24 with normal MR imaging findings and 26 with abnormal MR imaging findings)
| Term (normal MRI) | Term (abnormal MRI) | P Value | |
|---|---|---|---|
| Completeness of CoW | 9 in 24 (37.5%) | 11 in 26 (42.3%) | .477 |
| AcomA absence/hypoplasia | 6 in 24 (25%) | 6 in 26 (23.1%) | .567 |
| PcomA absence/hypoplasia | 13 in 48 (27%) | 18 in 52 (34.6%) | .276 |
| A1 segment of ACA absence/hypoplasia | 5 in 48 (10.4%) | 1 in 52 (1.9%) | .053* |
| P1 segment of PCA absence/hypoplasia | 4 in 48 (8.3%) | 3 in 52 (5.7%) | .455 |
| Fetal-type origin of PCA | 14 in 48 (29%) | 12 in 52 (23%) | .321 |
| Transitional-configuration origin of PCA | 6 in 48 (12.5%) | 5 in 52 (9.6%) | .443 |
| Bilateral fetal-type origin | 8 in 14 (57.1%) | 6 in 12 (50%) | .512 |
| Bilaterally absent PcomA | 6 in 13 (46.2%) | 14 in 18 (77.8%) | .076 |
Statistically significant.
It was not feasible to apply similar statistical tests in the total preterm population (n = 44) because there were only 3 preterm infants scanned at term with abnormal MR imaging findings and 41 preterm at term with normal MR imaging findings.
In total, 26 term infants and 3 preterm infants had an MR imaging finding that was considered abnormal at term or term-equivalent age, respectively. Some infants had >1 parenchymal abnormality. In total, abnormalities on structural MR imaging consisted of abnormal signal intensity of the basal ganglia (n = 17), infarction (n = 10), loss of gray/white matter differentiation (n = 3), periventricular cyst (n = 2), cerebral hemorrhage (n = 2), and porencephalic cyst (n = 1). The detailed prevalence of anatomic variations of the CoW in relation to each type of parenchymal abnormality is presented in Table 5. Because >1 anatomic variation and/or >1 parenchymal abnormality could be simultaneously present in the same infant, it was not feasible to associate a specific variation with a specific parenchymal abnormality.
Table 5:
Prevalence of anatomic variations of the CoW in relation to MR imaging—detected parenchymal abnormalities in a group of 29 infants (26 term-born and 3 preterm infants imaged at term)
| Parenchymal Abnormalities | Complete CoW | Fetal-Type Origin of PCA | PcomA Absence/Hypo | AcomA Absence/Hypo | P1 Absence/Hypo | A1 Absence/Hypo |
|---|---|---|---|---|---|---|
| Abnormal signal intensity in basal ganglia (n = 17) | 4 | 6 | 7 | 4 | 2 | 1 |
| Infarction (n = 10) | 2 | 4 | 3 | 2 | 3 | 0 |
| Loss of grey/white matter differentiation (n = 3) | 0 | 2 | 2 | 1 | 1 | 0 |
| Hemorrhage (n = 2) | 1 | 0 | 1 | 0 | 0 | 0 |
| Periventricular cysts (n = 2) | 1 | 0 | 1 | 0 | 0 | 0 |
| Porencephalic cysts (n = 1) | 1 | 0 | 0 | 0 | 0 | 0 |
Note:—Hypo indicates hypoplasia.
Effects of Vascular-Related Abnormalities
There was no difference in the completeness of the CoW between infants with (n = 16) and without (n = 78) evidence of a vascular-related abnormalities. There were, however, significant differences in the prevalence of some anatomic variations between the 2 groups (Table 6). More specifically, there was significantly increased (P = .004) absence/hypoplasia of the P1 segment of the PCA in the group with the vascular-related abnormalities (18.8%) compared with the group without them (3.2%). The absence of the A1 segment was seen more often in the group with pathology (9.3%) compared with the group without (2.5%), approaching but not reaching significance (P = .09). Absence/hypoplasia of the PcomA, however, was seen less often in the group with pathology (P = .07). Prevalence for the other anatomic variations remained similar between the 2 groups.
Table 6:
Prevalence of anatomic variations of the CoW in relation to vascular-related abnormalities in a group of 94 infants (16 with a vascular-related abnormality and 78 without)
| With Vascular-Related Abnormality | Without Vascular-Related Abnormality | P Value | |
|---|---|---|---|
| Completeness of CoW | 7 in 16 (43.7%) | 33 in 78 (42.3%) | .564 |
| AcomA absence/hypoplasia | 4 in 16 (25%) | 14 in 78 (17.9%) | .364 |
| PcomA absence/hypoplasia | 6 in 32 (18.8%) | 52 in 156 (33.3%) | .075 |
| A1 segment of ACA absence/hypoplasia | 3 in 32 (9.3%) | 4 in 156 (2.5%) | .097 |
| P1 segment of PCA absence/hypoplasia | 6 in 32 (18.8%) | 5 in 156 (3.2%) | .004* |
| Fetal-type origin of PCA | 9 in 32 (28.1%) | 39 in 156 (25%) | .432 |
| Transitional-configuration origin of PCA | 3 in 32 (9.3%) | 12 in 156 (7.7%) | .487 |
XXX.
The most common anatomic variation in the group with vascular-related abnormalities was the fetal-type origin of the PCA (28.1%). The most common anatomic variation in the group without vascular-related abnormalities was the absence/hypoplasia of the PcomA (33.3%).
Effect of Sex
There were no significant differences in CoW completeness or prevalence of anatomic variations when comparing boys (n = 34) and girls (n = 31) with normal MR imaging findings. Overall, girls presented with more complete CoWs (45.2%) than boys (38.2%), but this difference was not significant. The prevalence of the AcomA absence/hypoplasia occurred almost twice as often in term-born girls (37.5%) as in term-born boys (18.8%), but this difference was not statistically significant. Absence/hypoplasia of the PcomA was more common in preterm boys (38.9%) than in girls (26%). Absence/hypoplasia of the A1 segment of the ACA was 4 times more frequent in boys (7.4%) than in girls (1.6%). Absence/hypoplasia of the P1 segment of the PCA occurred twice as often in term-born girls (12.5%) than in boys (6.3%).
Effect of Laterality (Left- or Right-Sided Prevalence)
There were generally no differences in laterality of the lateralized anatomic variations, namely of absence/hypoplasia of the A1 segment, P1 segment, and PcomA in infants with normal MR imaging findings. However, there were twice as many left-sided PCAs with a fetal-type origin (8 in 12 or 66.7%) as right-sided ones (4 in 12 or 33.3%) in the term-born infants with abnormal MR imaging findings. There was no correlation between the laterality of the parenchymal and vascular-related abnormalities and the laterality of the anatomic variations (P = .43).
Discussion
Reference values on the prevalence of the anatomic variations of the CoW have been systematically described in a neonatal population by using high-resolution MRA at 3T. The results are in keeping with the adult literature and are within the range cited in the adult studies.3,22–24 These variations have been examined in relation to prematurity, MR imaging abnormality, vascular-related abnormality, sex, and laterality in neonates.
Preterm infants had a higher, though not significantly so, prevalence of a complete CoW and overall fewer anatomic variations of the CoW compared with the term-born infants. Similarly, more very preterm infants (50%), born at <30 weeks' GA, had a complete CoW compared with the moderately preterm infants (36.8%). It is tempting to hypothesize that this trend might reflect a well-instructed reaction (vascular remodelling) to protect the most potentially vulnerable populations by maintaining adequate blood supply to their brain. In keeping with these findings, term-born infants with normal MR imaging findings had more anatomic variations of the CoW, with significantly increased prevalence (P = .02) of the absence/hypoplasia of the A1 segment.
The significantly increased prevalence (P = .04) of the bilateral fetal-type origin of the PCA in preterm infants born after 30 weeks' GA matched with a decreased occurrence of the transitional-type configuration may relate to the chronologic order of the relative changes in metabolic demand in the fetal brain. After 20–21 weeks of gestation, there is a period of accelerated growth of the occipital lobes, associated with increasing metabolic demand.7,25 Therefore, this increase in fetal-type origins of the PCA with the relative decrease of the transitional-type origins, in which all vessels appear to be of equal size, may be a response to this increasing metabolic demand.
Sex and laterality did not influence the prevalence of the anatomic variations in the neonatal population, and this was in line with most of the adult literature.3–5 Some studies, however, report an increased prevalence of anatomic variations on the left side.26
Most interesting, the comparison of the groups with and without vascular-related abnormalities revealed that the major cerebral arteries (A1, P1) were more often absent/hypoplastic in the affected group (P = .09 and 0.004, respectively). This might not be surprising given the medical histories of those infants. The PcomA, however, was more often present (P = .07) in the affected group (infants with vascular-related abnormality). This finding was similar to those in previous adult studies in which there was a significantly higher percentage of entirely complete CoW in patients with internal carotid artery obstruction compared with control subjects, with the communicating arteries playing a major role in supplying bypass routes.21,27 Nevertheless, the variety of the vascular-related abnormalities included in this subgroup (cerebral infarction, ROP, facial port-wine stain) makes it difficult to draw any definitive conclusions at this stage. A larger scale study focused on a specific cerebrovascular pathology could perhaps offer more categoric results.
Vessel hypoplasia or absence may render the CoW nonfunctional and leave the brain more exposed to a potential blood-supply deprivation and, therefore, more prone to injury. Several studies have explored the correlation between certain types of CoW anatomic variations with a variety of adult brain pathologies. It has been shown that hypoplasia or absence of the PcomA in patients with angiographically proved internal carotid artery occlusion is considered a risk factor for ischemic stroke.28,29 In our study, MR imaging abnormality did not generally correlate with the presence of anatomic variations, but A1 segment hypoplasia was more frequently seen in infants with normal MR imaging findings, whereas bilateral absence/hypoplasia of the PcomA was more frequently seen in infants with MR imaging abnormality. It was not possible to attribute MR imaging abnormality to a poorly functioning CoW because a larger sample size with a narrower definition of MR imaging abnormality would be required. Additionally, the etiology of vascular disorders such as stroke occurring perinatally is often different from that in adult.
The TOF MRA is adequately sensitive and specific for the detection of the CoW vessels, but it does have its limitations. Some of the vessels that cannot be seen with TOF MRA are not truly absent or hypoplastic; they are just too small to be detected, given the spatial resolution.30,31 This study was performed with a dedicated neonatal MRA protocol, in which the acquired resolution was 0.6 × 0.6 × 0.6 mm3 isotropic to maximize the potential to visualize the small neonatal vessels uniformly in all planes relative to the 0.80 × 0.80 × 1.20 mm3, which has been used in corresponding adult studies.3–5 With a mean diameter of approximately 1 mm for the middle cerebral arteries of our neonatal population,18 it would be reasonable to assume that the size of the communicating arteries would lie at the limits of the acquired resolution. Therefore, the results we report in this study are expected to underestimate slightly the true prevalence of a complete CoW because some of the smaller communicating arteries will be too small to be detectable.
The physiologic significance and possible brain perfusion effects of the differences in the prevalence of the anatomic variations of the CoW between the preterm and term group have yet to be determined. It remains unclear whether the differences that we have observed between preterm and term infants and in infants with vascular-related disease represent cause or effect. Further longitudinal MRA studies could also explore the dynamic nature of the completeness of the CoW over the human lifespan and in response to different functional demands.
Conclusions
The anatomic variations and completeness of the CoW have been systematically studied in a group of 94 infants by using a high-resolution dedicated neonatal protocol at 3T. The most common anatomic variation was the absence of the PcomA, accounting for 30% of the studied neonatal population. There was no statistically significant difference between boys and girls and left/right anatomic variations. Preterm infants imaged at term-equivalent age presented with more complete CoWs and fewer anatomic variations than term-born infants. This was statistically significant for the A1 segment (P = .02). Similarly, preterm infants born at <30-weeks' GA had a more complete CoW and a lower prevalence of anatomic variations compared with those born after 30-weeks' GA. Additionally, infants with vascular-related abnormalities had more absent/hypoplastic major arterial segments but fewer variations in the communicating arteries, with the absence/hypoplasia of the P1 seen more often in the group with pathology (P = .004). Further studies are needed to explore the functional significance of these findings in a neonatal population.
Acknowledgments
We acknowledge all the staff at the Department of Imaging Sciences of the Hammersmith Hospital for their endless support. Also, we thank all the parents and children who made this study possible.
Footnotes
This work was supported by a research grant from the Greek State Scholarships Foundation (IKY), the Health Foundation, the Academy of Medical Sciences, and Philips Medical Systems.
References
- 1. Gray H. Gray's Anatomy. Philadelphia: Lea & Febiger, 1918 [Google Scholar]
- 2. Padget DH. The development of the cranial arteries in the human embryo. Contributions in Embryology 1948;32:205–61 [Google Scholar]
- 3. Hartkamp-Krabbe MJ, van der Grond J, de Leeuw FE, et al. Circle of Willis: morphologic variation on three dimensional time-of-flight MR angiograms. Radiology 1998;207:103–11 [DOI] [PubMed] [Google Scholar]
- 4. Milenkovic Z, Vutevic R, Puzic M. Asymmetry and anomalies of the circle of Willis in fetal brain: microsurgical study and functional remarks. Surgical Neurology 1985;24:563–70 [DOI] [PubMed] [Google Scholar]
- 5. Hillen B. The variability of the circle of Willis: univariate and bivariate analysis. Acta Morphol Neerl Scand 1986;24:87–101 [PubMed] [Google Scholar]
- 6. Hillen B. The variability of the circulus arteriosus (Willisii): order or anarchy? Acta Anat (Basel) 1987;129:74–80 [DOI] [PubMed] [Google Scholar]
- 7. Van Overbeeke JJ, Hillen B, Tulleken CAF. A comparative study of the circle of Willis in fetal and adult life: the configuration of the posterior bifurcation of the posterior communicating artery. J Anat 1991;176:45–54 [PMC free article] [PubMed] [Google Scholar]
- 8. Osborn AG. Introduction to Cerebral Angiography. Philadelphia: Harper and Row; 1980 [Google Scholar]
- 9. Huppi PS, Warfield S, Kikinis R, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol 1998;43:224–35 [DOI] [PubMed] [Google Scholar]
- 10. Maalouf EF, Duggan PJ, Rutherford MA, et al. Magnetic resonance imaging of the brain in a cohort of extremely preterm infants. J Pediatr 1999;135:351–57 [DOI] [PubMed] [Google Scholar]
- 11. Counsell SJ, Allsop JM, Harrison MC, et al. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics 2003;112(1 Pt 1):1–7 [DOI] [PubMed] [Google Scholar]
- 12. Inder TE, Huppi PS, Warfield S, et al. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol 1999;46:755–60 [DOI] [PubMed] [Google Scholar]
- 13. Ajayi-Obe M, Saeed N, Cowan FM, et al. Reduced development of cerebral cortex in extremely preterm infants. Lancet 2000;356:1162–63 [DOI] [PubMed] [Google Scholar]
- 14. Inder TE, Warfield SK, Wang H, et al. Abnormal cerebral structure is present at term in premature infants. Pediatrics 2005;115:286–94 [DOI] [PubMed] [Google Scholar]
- 15. Boardman JP, Counsell SJ, Rueckert D, et al. Abnormal deep gray matter development following preterm birth detected using deformation-based morphometry. Neuroimage 2006;32:70–78 [DOI] [PubMed] [Google Scholar]
- 16. Crawford MA, Golfetto I, Ghebremeskel K, et al. The potential role for arachidonic and docosahexaenoic acids in protection against some central nervous system injuries in preterm infants. Lipids 2003;3:303–15 [DOI] [PubMed] [Google Scholar]
- 17. Ward RM, Beachy JC. Neonatal complications following preterm birth. BJOG 2003;110(suppl 20):8–16 [DOI] [PubMed] [Google Scholar]
- 18. Malamateniou C, Counsell SJ, Allsop JM, et al. The effect of preterm birth on neonatal cerebral vasculature studied with magnetic resonance angiography at 3.0 Tesla. Neuroimage 2006;32:1050–59 [DOI] [PubMed] [Google Scholar]
- 19. Rutherford MA. MRI of the Neonatal Brain. London: WB Saunders; 2002:17–21 [Google Scholar]
- 20. Malamateniou C, Counsell SJ, Allsop JM, et al. Optimized magnetic resonance angiography at 3.0 Tesla for neonates. In: Proceedings of 13th Scientific Meeting & Exhibition, International Society for Magnetic Resonance in Medicine, Miami, Fla. May 7–13, 2005 [Google Scholar]
- 21. Hoksbergen AW, Legemate DA, Csiba L, et al. Absent collateral function of the circle of Willis as risk factor for ischemic stroke. Cerebrovasc Dis 2003;16:191–98 [DOI] [PubMed] [Google Scholar]
- 22. Hartkamp MJ, van Der Grond J, van Everdingen KJ, et al. Circle of Willis collateral flow investigated by magnetic resonance angiography. Stroke 1999;30:2671–78 [DOI] [PubMed] [Google Scholar]
- 23. Krabbe-Hartkamp MJ, van der Grond J, de Leeuw FE, et al. Circle of Willis: morphologic variation on three-dimensional time-of-flight MR angiograms. Radiology 1998;207:103–11 [DOI] [PubMed] [Google Scholar]
- 24. Kapoor K, Singh B, Dewan LI. Variations in the configuration of the circle of Willis. Ana Sci Int 2008;83:96–106 [DOI] [PubMed] [Google Scholar]
- 25. Moffat DB. The development of the posterior cerebral artery. J Anat 1961;95:485–94 [PMC free article] [PubMed] [Google Scholar]
- 26. Macchi C, Lova RM, Miniati B, et al. The circle of Willis in healthy older persons. J Cardiovasc Surg (Torino) 2002;43:887–90 [PubMed] [Google Scholar]
- 27. Miralles M, Dolz JL, Cotillas J, et al. The role of the circle of Willis in carotid occlusion: assessment with phase contrast MR angiography and transcranial duplex. Eur J Vasc Endovasc Surg 1995;10:424–30 [DOI] [PubMed] [Google Scholar]
- 28. Schomer DF, Marks MP, Steinberg GK, et al. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med 1994;330:1565–70 [DOI] [PubMed] [Google Scholar]
- 29. Jongen JC, Franke CL, Ramos LM, et al. Direction of flow in posterior communicating artery on magnetic resonance angiography in patients with occipital lobe infarcts. Stroke 2004;35:104–08 [DOI] [PubMed] [Google Scholar]
- 30. Laub GA. Time-of-flight method of MR angiography. Magn Reson Imaging Clin N Am 1995;3:391–98 [PubMed] [Google Scholar]
- 31. Parker DL, Parker DJ, Blatter DD, et al. The effect of image resolution on vessel signal in high resolution magnetic resonance angiography. J Magn Reson Imaging 1996;6:632–41 [DOI] [PubMed] [Google Scholar]