Abstract
BACKGROUND AND PURPOSE:
White matter hyperintensities (WMHs) are frequently characterized as markers of cerebrovascular disease, whereas medial temporal atrophy (MTA) is a recognized marker of Alzheimer disease (AD). Our purpose was to test the reliability of a visual rating system (VRS) in evaluating WMHs and MTA and in distinguishing healthy from cognitively impaired subjects.
MATERIALS AND METHODS:
Subjects (n = 192) enrolled in the Florida Alzheimer's Disease Research Center were diagnosed with no cognitive impairment, nonamnestic mild cognitive impairment (na-MCI), amnestic MCI (a-MCI), or probable AD. The severity of WMHs was assessed on T2-weighted fluid-attenuated inversion recovery axial MR images, and the severity of MTA was evaluated on 1.5-mm-thick coronal MR images by using a computer-based visual rating system. Cardiovascular risk factor scores were calculated as the sum of 10 independent cardiovascular risk factors.
RESULTS:
WMH and MTA scores were greater in subjects with probable AD, relative to those with no cognitive impairment and na-MCI. MTA scores differentiated subjects with a-MCI from those with no cognitive impairment and na-MCI. The total WMH score was significantly related to MTA (r = 0.39; P < .001) but not to cardiovascular risk factor scores (r = 0.07; P = not significant). The overall correct classification rate of probable AD versus no cognitive impairment by using MTA scores was 81.8%, improving to 86.5% when combined with WMH scores.
CONCLUSIONS:
Both MTA and WMH scores distinguished subjects with no cognitive impairment and probable AD. Combining MTA and WMH scores improved the correct classification rate, whereas WMH scores were significantly related to MTA scores, but not to cardiovascular risk factor scores. This finding suggests that among subjects with a-MCI and probable AD, WMHs on MR images are primarily associated with neurodegenerative disease.
The presence of white matter hyperintensities (WMHs), observed as bright foci on T2-weighted MR images,1 occurs commonly among elderly cognitively healthy subjects and in those with mild cognitive impairment (MCI) and a variety of dementias, including Alzheimer disease (AD).2–6 The etiology of these WMHs has most frequently been ascribed to normal aging and cerebrovascular disease, even among subjects with dementia diagnosed with probable Alzheimer disease.6,7 There is growing evidence, however, that neurodegeneration or associated processes, such as gliosis, microglial infiltration, inflammation, and amyloid angiopathy, may also result in WMHs.8–13
The pathologic process characteristic of AD begins in the medial temporal structures, and the attenuation of this pathology has a proportional effect on the degree of atrophy in these structures (especially the hippocampus and entorhinal cortex).14 Medial temporal atrophy (MTA) can be identified accurately from MR images and correlates with the severity of AD-related neuropathologic changes at autopsy.15 The severity of MTA can serve as a substitute for the severity of AD and other degenerative pathology in the medial temporal lobe.16–18
We have developed a computer-assisted visual rating system (VRS) that uses drop-down reference images to illustrate the anatomy and demonstrate different levels of atrophy of medial temporal structures.19,20 This system has high inter-rater and intrarater reliability and distinguishes subjects with no cognitive impairment from those with amnestic MCI (a-MCI) and probable AD purely on the basis of measurements of MTA.19,20 We have now adjusted the VRS so that procedures analogous to those used for MTA measurements can be used to rate WMHs in the periventricular and centrum semiovale regions of the brain.
In this study, we tested inter- and intrarater reliability of the VRS in evaluating WMHs in the periventricular and centrum semiovale regions. We also examined the ability of VRS-WMH ratings to distinguish subjects diagnosed with no cognitive impairment, nonamnestic mild cognitive impairment (na-MCI), a-MCI, and probable AD, among 192 subjects enrolled in the Florida Alzheimer's Disease Research Center. Recent studies have linked cerebrovascular risk factors such as diabetes,21 coronary heart disease,22 and stroke23 with a-MCI, which is a known precursor of AD.24 We assessed the associations between WMHs, MTA, and cardiovascular risk factor so as to obtain clues about the possible etiology of WMHs.4 We also assessed whether WMHs were additive with MTA ratings in correctly classifying diagnostic groups, such as healthy patients and those with a-MCI and probable AD. The purpose of this study was to test the reliability of the VRS in evaluating WMHs and to test the ability of combined MTA and WMHs assessed by the VRS in distinguishing healthy and cognitively impaired subjects.
Materials and Methods
Subject Recruitment.
Subjects (N = 192) were recruited during a period of 3 years into the Florida Alzheimer's Disease Research Center Clinical Core in Miami and Tampa, Fla, through advertisements, health fairs, community memory screening programs, and memory disorder clinics. The Mount Sinai Medical Center and the University of South Florida Institutional Review Boards approved this study, and all subjects or a legal representative gave informed consent.
Subject Evaluation.
All subjects in this study received the following evaluations: 1) full clinical history; 2) neurologic assessment; and 3) the Mini-Mental State Examination (MMSE)25 (an MMSE score of ≥18 was required for eligibility4); a neuropsychological test battery,26 which included the Clinical Dementia Rating scale 27 according to National Alzheimer's Coordinating Center (NACC) protocol (http://www.alz.washington.edu/); the Three Trial Fuld Object-Memory Evaluation28; the Hopkins Verbal Learning Test29; and the Stroop Color and Word Test.30
Diagnostic Classification.
The Florida Alzheimer's Disease Research Center consensus diagnosis followed the NACC protocols. The probable AD diagnosis was made according to National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria for Alzheimer disease.31 The a-MCI diagnosis was made according to the criteria of Petersen et al.32 The diagnosis of no cognitive impairment required that informants report “no significant decline in cognition” and that no cognitive test scores were more than 1.5 SD below age- and education-corrected means. The na-MCI diagnosis required that no memory test score be more than 1.5 SD below education-corrected normative values but that 1 or more nonmemory test score be greater than 1.5 SD below normative values.
MR Imaging Procedures
Siemens Symphony 1.5T.
Brain MR imaging scans were obtained on a Symphony 1.5T scanner (Siemens, Erlangen, Germany) by using a proprietary 3D magnetization-prepared rapid acquisition of gradient echo (3D MPRAGE) protocol. Images were obtained from the vertex of the skull to the foramen magnum and from the occipital poles to the temporal poles. Specifications for 3D MPRAGE were the following: 120 contiguous coronal sections with a 1.5-mm gap in thickness; section interval, 0.75 mm; TR, 2190 ms; TE, 4.38 ms; TI, 1100 ms; FA, 15°; NEX, 1; matrix, 256 × 256; FOV, 260 mm; bandwidth, 130 Hz/pixel; acquisition time, 9 minutes; phase-encoding direction, right to left. MTA was evaluated by atrophy rating of the hippocampus, entorhinal cortex, and the perirhinal cortex on a coronal section intersecting the mamillary bodies.
For WMH assessment, images were obtained by using a fluid-attenuated inversion recovery (FLAIR) turbo spin-echo protocol. Images were obtained from the vertex of the skull to the foramen magnum. Specifications for FLAIR sequences were the following: 20 contiguous axial sections of 5.5-mm thickness; section interval, 1.9 mm; TR, 9000 ms; TE, 109 ms; TI, 2500 ms; flip of preparation pulse, 150°; echo-train length, 19; NEX, 1; matrix, 144 × 256; FOV, 230 mm; phase FOV, 75%; distance factor, 35%; bandwidth, 130 Hz/pixel; acquisition time, 2–3 minutes; phase-encoding direction, right to left.
GE 3T Signa HDX.
Brain MR images were obtained on a 3T Signa HDX (GE Healthcare, Milwaukee, Wis) by using a proprietary 3D fast-spoiled gradient (FSPGR) magnetization-prepared protocol. Images were obtained from the vertex of the skull to the foramen magnum and from the occipital poles to the temporal poles. Specifications for 3D FSPGR were the following: 140 contiguous coronal sections of 1.2-mm thickness; contiguous images with no section interval; TR, 7.8 ms; TE, 3.0 ms; inversion recovery preparation time, 450 ms; flip angle, 12°; NEX, 1; matrix, 256 × 256; FOV, 240 mm; bandwidth, 31.25 Hz/pixel; acquisition time, 6–7 minutes; phase-encoding direction, right to left.
For WMH assessment, images were obtained by using an axial T2-weighted FLAIR fast spin-echo protocol. Images were obtained from the vertex of the skull to the foramen magnum. Specifications for FLAIR sequences were the following: 20 contiguous axial sections of 5.0-mm thickness; section interval, 1.0 mm; TR, 9500 ms; TE, 120 ms; TI, 2250 ms; flip to projection pulse, 90°; NEX, 1; matrix, 352 × 224; FOV, 240 mm; phase FOV, 75%; bandwidth, 130 Hz/pixel; acquisition time, 3 minutes; phase-encoding direction, right to left.
VRS in Assessing MTA.
We developed the VRS to promote standardization, increase reliability, and prevent reliability drift among raters for a semiquantitative visual rating of various radiologic features of interest on brain MR images.19 VRS uses a 0–4 scale to rate atrophy of the medial temporal lobe structures, including the hippocampus, entorhinal cortex, and perirhinal cortex as follows: 0 = no atrophy, 1 = minimal atrophy, 2 = mild atrophy, 3 = moderate atrophy, 4 = severe atrophy. The VRS program includes drop-down menus linked to reference images that visualize the anatomy of the brain structures and provide examples of different levels of atrophy or abnormality of radiologic features based on a scale of 0–4. After the rater determines ratings by comparing MR images to VRS reference images, the program provides a data base in which VRS ratings are catalogued and stored. VRS also provides a training module that contains a series of normal and abnormal findings on scans, which can be rated and compared with the reference ratings. This feature provides feedback to the rater and improves the accuracy and reliability of VRS data.19 We have previously reported that MTA ratings distinguish subjects with probable AD from those with a-MCI and those with a-MCI from those with no cognitive impairment.19,20
VRS Assessment of WMHs
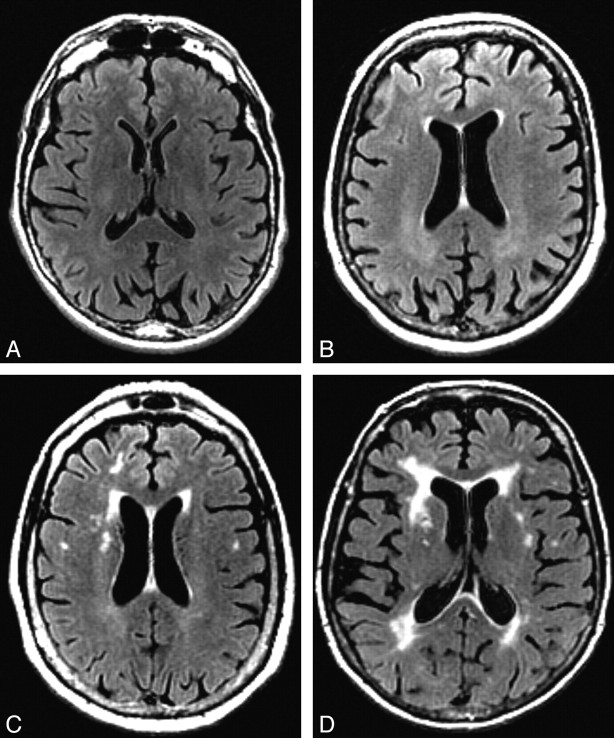
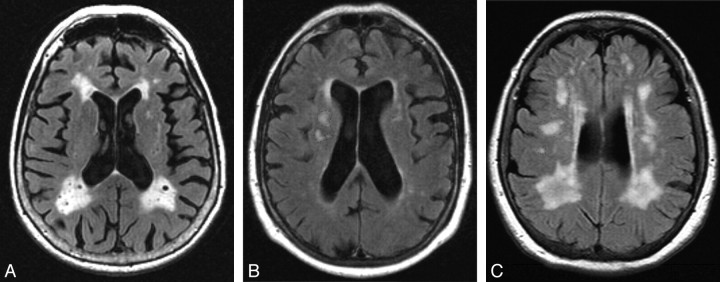
VRS was used to evaluate WMHs on FLAIR sequences in 4 periventricular WMH regions (frontal, parietal, occipital, and temporal), and the centrum semiovale WMH region, by using a 0–4 severity scale (Figs 1 and 2). Criteria for periventricular WMH ratings were based on extension of WMHs from the lateral ventricle to the cerebral cortex as follows: 0 = no detectable WMH, 1 = thin rim (pencil edge) of hyperintensity adjacent to the ventricle, 2 = extension of WMHs to one third of the distance to the cerebral cortex, 3 = extension of WMHs to two thirds of the distance to the cerebral cortex, 4 = extension of WMHs to the cerebral cortex. Criteria for centrum semiovale WMHs were based on the anteroposterior extent of WMHs in the centrum semiovale as follows: 0 = no centrum semiovale WMHs, 1 = at least 1 centrum semiovale WMH ≤1 cm in greatest dimension, 2 = at least 1 centrum semiovale WMH >1 cm in dimension, 3 = multiple coalescing centrum semiovale WMHs occupying less than two thirds of the anteroposterior extent of WMHs in the centrum semiovale, 4 = multiple coalescing centrum semiovale WMHs occupying more than two thirds of the anteroposterior extent of WMHs in the centrum semiovale.
Fig 1.
WMHs in prodromal regions. A−D, Examples of WMHs in the bilateral periventricular frontal regions on FLAIR axial brain MR images that would be visually rated at a level of 0 (A), 1 (B), 2 (C), and 3 (D).
Fig 2.
WMHs in occipital and centrum semiovale regions. A, WMHs in the occipital bilateral regions would be visually rated at a level of 4. B and C, WMHs in the centrum semiovale regions would be rated at a level of 2 (B, right), 1 (B, left), and 3 (C, right and left).
Justification for the use of severity ratings to judge WMHs, rather than using terminology such as “punctuate” or “confluent,” is that this a simple and direct measure of the extent of abnormal hyperintensity and, therefore, very likely pathologic white matter on the brain MR imaging. There is no evidence that terms such as “punctuate” or “confluent” have specific biologic significance or are superior to severity measures. In fact, automated procedures have been developed to measure the area or volume of white matter that reaches a specific threshold of hyperintensity on MR imaging. These measures, which are more quantitative than our measures but are essentially severity measures, have been shown to be related to cognitive status.33,34
Cardiovascular Risk Factor Assessment
The cardiovascular risk factor score was calculated as a sum of 10 independent risk factors selected from the NACC/Uniform Data Set assessment protocol (Form A5: “Subject Health History”; www.alz.washington.edu/MOVIES/UDSdemoforms.pdf). These factors were rated as “present” or “absent” and included the following: 1) “Heart attack/cardiac arrest,” 2) “Atrial fibrillation,” 3) “Angioplasty/endarterectomy/coronary artery bypass surgery,” 4) “Congestive Heart failure,” 5) “Stroke/TIA” (transient ischemic attack), 6) “Hypertension,” 7) “Hypercholesterolemia,” 8) “Diabetes,” 9) “Smoked within last 30 days,” and 10) “Smoked more than 100 cigarettes in his/her life.”
Statistical Methods
Statistical Analyses.
To test interrater reliability of the VRS in evaluating WMHs, 2 raters evaluated axial FLAIR brain MR images of 30 subjects diagnosed with no cognitive impairment (n = 10), MCI (n = 10), or AD (n = 10). WMHs were assessed in the periventricular frontal, parietal, occipital, and temporal regions and in the centrum semiovale region. VRS raters were blinded to the identity and diagnosis of the subjects. To test intrarater reliability of the VRS, a single rater re-evaluated axial FLAIR brain MR images in 21 of the original 30 subjects after an interval of approximately 3 weeks. Group comparisons of atrophy and WMH scores were analyzed by using a series of 1-way analyses of variance (ANOVA). The Scheffe post hoc procedure was used to examine differences between means. Logistic regression was used to determine the correct classification of specific subject groups on the basis of WMH scores.
Results
Weighted κ exhibited a high degree of correspondence with respect to interrater and intrarater reliability for bilateral frontal, parietal, occipital, and centrum semiovale brain regions and a moderate degree of correspondence for temporal regions (Table 1). For all brain regions, intrarater reliabilities were achieved in the good-to-excellent range. There were group differences (Table 2) with regard to age, F[(3,171) = 4.17; P < .008]; years of education, F[(3,167) = 5.47; P < .002]; and average MMSE score, F[(3,171) = 49.15; P < .001]. Scheffe post hoc procedure tests indicated that subjects with no cognitive impairment were younger than patients with a-MCI and dementia. Subjects classified as having no cognitive impairment were also more highly educated than subjects in the na-MCI group. Subjects with no cognitive impairment had the highest mean MMSE scores, followed by a-MCI and na-MCI groups. The lowest average MMSE scores were obtained by the probable AD group. There were no group differences with regard to sex distribution [X2 (df = 3) = 4.89; P > .18].
Table 1.
Inter-rater and intrarater reliability of WMH ratings
| Brain Structure | Interrater κ Values | Intrarater κ Values |
|---|---|---|
| Frontal (right) | .72 | .77 |
| Frontal (left) | .73 | .66 |
| Parietal (right) | .73 | .70 |
| Parietal (left) | .70 | .80 |
| Temporal (right) | .57 | .70 |
| Temporal (left) | .57 | .73 |
| Occipital (right) | .75 | .69 |
| Occipital (left) | .82 | .77 |
| Centrum semiovale (right) | .71 | .77 |
| Centrum semiovale (left) | .73 | .74 |
Note:—WMH indicate white matter hyperintensity.
Table 2.
Demographics: samples of subjects with NCI, na-MCI, a-MCI, and probable AD*
| NCI | na-MCI | a-MCI | Probable AD | |
|---|---|---|---|---|
| Sample size | n = 40 | n = 53 | n = 65 | n = 34 |
| Age (SD) | 71.0a (5.6) | 74.2ab (6.1) | 75.3b (6.6) | 76.6b (6.5) |
| Years of education (SD) | 14.6b (3.9) | 11.7a (3.4) | 13.8ab (3.0) | 13.1ab (3.7) |
| % Female | 67.5% | 40.7% | 46.7% | 53.3% |
| MMSE (SD) | 29.3c (.9) | 27.0b (2.4) | 26.8b (2.3) | 22.7a (2.8) |
Note:—NCI indicates no cognitive impairment; na-MCI, nonamnestic mild cognitive impairment; a-MCI, amnestic mild cognitive impairment; AD, Alzheimer disease; MMSE, Mini-Mental State Examination.
Means with different alphabetic superscripts (a,b,c) are significantly different at P < .05 by the Scheffe post hoc procedure.
Although there were differences in age between the groups, we chose not to covary by age in the ANOVA models. Because age is a risk factor for both AD and a-MCI, whereas AD and MCI are associated with increased MTA, covarying for age can obscure a true effect of the disease state on outcome measures. Miller and Chapman35 concluded that ANOVA was not designed to correct for systematic differences in natural unrandomized groups and can lead to spurious findings. Subjects with probable AD had higher average WMH scores in all brain regions relative to subjects with no cognitive impairment (Table 3). In addition, subjects with probable AD had higher WMH scores in all regions, relative to subjects with na-MCI, except in the right frontal and right occipital lobes. Although subjects with probable AD had higher mean WMH scores than subjects with a-MCI in the left centrum semiovale, they did not differ with respect to WMH scores in other brain regions.
Table 3:
WMH scores for each diagnostic group study*
| NCI |
na-MCI |
a-MCI |
Probable AD (N = 34) | F Values | |
|---|---|---|---|---|---|
| (n = 40) | MCI (n = 53) | MCI (n = 65) | |||
| Frontal (right) (SD) [95% CI] | .90a (0.8) | .83a (0.8) | 1.22ab (.9) | 1.44b (1.1) | 4.21‡ |
| [.64–1.16] | [.62–1.04] | [.98–1.45] | [1.06–1.83] | ||
| Frontal (left) (SD) [95% CI] | .73a (0.6) | .81ab (.8) | 1.14ab (1.0) | 1.26b (1.1) | 3.54† |
| [.52–.93] | [.59–1.03] | [.89–1.39] | [.90–1.63] | ||
| Parietal (right) (SD) [95% CI] | .45a (0.6) | .79ab (1.0) | 1.02ab (1.1) | 1.38b (1.2) | 5.90† |
| [.26–.64] | [.53–1.06] | [.75–1.28] | [.96–1.80] | ||
| Parietal (left) (SD) [95% CI] | .43a (0.6) | .77ab (.9) | 1.06bc (1.1) | 1.35c (1.3) | 6.30§ |
| [.25–.60] | [.52–1.03] | [.78–1.34] | [.92–1.78] | ||
| Occipital (right) (SD) [95% CI] | .25a (0.6) | .47ab (0.8) | .97b (1.2) | 1.12bc (1.4) | 6.35§ |
| [.05–.45] | [.24–.70] | [.66–1.28] | [.64–1.59] | ||
| Occipital (left) | .38a (0.7) | .45a (0.8) | 1.02ab (1.3) | 1.12b (1.3) | 5.57§ |
| (SD) [95% CI] | [.15–.60] | [.23–.68] | [.70–1.33] | [.65–1.59] | |
| Centrum semiovale | .57a (0.7) | .92a (1.0) | 1.15ab (1.1) | 1.68b (1.3) | 7.34§ |
| (right) (SD) [95% CI] | [.36–.79] | [.66–.1.19] | [.87–1.44] | [1.23–2.12] | |
| Centrum semiovale | .55a (0.7) | .83a (.9) | 1.12a (1.1) | 1.74b (1.2) | 9.65§ |
| (left) (SD) [95% CI] | [.32–.78] | [.58–1.08] | [.85–1.39] | [1.30–2.17] |
Note:—95% CI refers to the 95th confidence interval for the mean.
Means with different alphabetic superscripts (a, b, c) are significantly different at P < .05 by the Scheffe post hoc procedure.
P < .05.
P < .01.
P < .001.
Average MTA scores differentiated subjects with no cognitive impairment and those with na-MCI from those with a-MCI (Table 4). Furthermore, those subjects with probable AD had higher average MTA scores in comparison with subjects in other diagnostic groups. Subjects with probable AD were differentiated from those with na-MCI and MCI on the basis of total WMH scores but did not differ from subjects with a-MCI. Average cardiovascular risk factor scores did not differ across the diagnostic groups. MTA ratings generated by the VRS did correlate with total WMH (r = 0.39; P < .001) ratings because WMHs in all regions were significantly associated with MTA scores (Table 5). There were no significant associations between total WMH scores and total cardiovascular risk factor scores (r = 0.07, P = ns). Although a weak association was noted between cardiovascular risk factor scores and periventricular WMH ratings in the left occipital region (r = 0.15; P < .05), it is very likely that this represented a spurious finding associated with multiple comparisons.
Table 4:
MTA, total WMH score, and CVRF score for each diagnostic group*
| NCI | na-MCI | a-MCI | Probable AD | F Values | |
|---|---|---|---|---|---|
| MTA score | .54a (0.5) | .50a (0.5) | 1.18b (1.0) | 1.98c (1.1) | 28.46‡ |
| [.37–.71] | [.35–.64] | [.93–1.44] | [1.59–2.35] | ||
| CVRF score | 2.16 (1.5) | 2.85 (1.7) | 2.75 (1.4) | 2.44 (1.7) | 1.68 |
| [1.67–2.65] | [2.3–3.33] | [2.40–3.10] | [1.83–3.05] | ||
| Total WMH score | 4.25a (3.7) | 5.89ab (5.6) | 8.69bc (7.6) | 11.09c (8.7) | 8.21† |
| [3.07–5.43] | [4.34–7.42] | [6.81–10.57] | [8.06–14.11] |
Note:—MTA indicates medial temporal atrophy; CVRF, cardiovascular risk factor.
Means with different alphabetic superscripts (a,b,c) are significantly different at P < .05 by the Scheffe post hoc procedure.
P ≤ .01.
P < .001.
Table 5:
Correlation between WMH scores and MTA ratings
| MTA (r) | |
|---|---|
| Frontal (right) | 0.36 (P < .001) |
| Frontal (left) | 0.29 (P < .001) |
| Parietal (right) | 0.36 (P < .001) |
| Parietal (left) | 0.35 (P < .001) |
| Occipital (right) | 0.34 (P < .001) |
| Occipital (left) | 0.23 (P < .001) |
| Centrum semiovale (right) | 0.30 (P < .001) |
| Centrum semiovale (left) | 0.34 (P < .001) |
With logistic regression, correct classification rates for no cognitive impairment versus a-MCI were 62% for centrum semiovale WMHs, 60% for periventricular WMHs, and 62% for total WMHs, respectively. When classification accuracy of no cognitive impairment versus AD subjects was computed, ratings for centrum semiovale WMH, periventricular WMH, and total WMH yielded correct classification of 70%, 70%, and 69% of subjects, respectively.
Stepwise logistic regression was used to determine the extent that cardiovascular risk factors, MTA, centrum semiovale WMHs, and age discriminated between subjects with probable AD versus no cognitive impairment and those with a-MCI versus no cognitive impairment. Results indicated that the overall MTA score distinguished subjects with probable AD from those with no cognitive impairment with a 75.9% sensitivity and 86.5% specificity, with an overall correct classification rate of 81.8%. Addition of centrum semiovale WMHs averaged over the left and right cerebral hemispheres resulted in a significant increase in a correct classification rate to 84.8%, with an 82.8% sensitivity and 86.5% specificity. If the total WMH score was substituted for centrum semiovale WMHs, a combination of MTA and WMH scores yielded an 86.2% sensitivity and 86.5% specificity, with an overall correct classification rate of 86.4%. Age and cardiovascular risk factor score did not enter into the logistic regression model. For subjects with no cognitive impairment versus those with a-MCI, MTA first entered into the model with an overall classification rate of 65.7%, with a 76.3% sensitivity and 50.0% specificity. The only other variable that entered into the regression model was total WMHs, which resulted in a significant increase in the classification rate to 71.7%, with a 76.3% sensitivity and 65.0% specificity.
Discussion
In clinical neurologic and radiologic practice, “WMHs on MR images” and “ischemic microvascular lesions” are frequently considered to be overlapping terms.8,9,34 As a consequence, many clinicians diagnose vascular dementia8 or exclude a diagnosis of AD on the basis of evidence of widespread WMHs on MR images. However, among elderly individuals with cognitive impairment and dementia, neuropathologic studies provide a much different etiologic perspective for WMHs, demonstrating the heterogeneity of these lesions.10–13 Among patients with AD, the most representative neuropathologic correlates of WMHs on MR imaging are microglial activation, gliosis, rarefaction of white matter, wallerian degeneration, nonamyloid sclerosis, and reduced attenuation of blood vessels.10–13 The results of the current study also suggest that degenerative disease or conditions associated with degenerative disease are the principal etiologic factors related to WMHs, rather than vascular disease.
In our study, both periventricular WMHs and centrum semiovale WMHs were consistently associated with MTA scores, which, in turn, were strongly correlated with the severity of cognitive impairment. MTA scores are a substitute for the severity of pathology in medial temporal regions14–17 and can be used to assist in the diagnosis of AD, even in a predementia stage.20 MR imaging−based measurements of hippocampal volume loss are highly correlated with the rate of progression of MCI to AD.36,37 In our study, WMH and MTA scores did not distinguish subjects with no cognitive impairment and those with na-MCI but did distinguish subjects with probable AD, who had higher WMH and MTA scores, from those with no cognitive impairment and those with na-MCI. Similarly, subjects with a-MCI had higher centrum semiovale WMHs and MTA scores than those with no cognitive impairment, but subjects with probable AD and a-MCI were indistinguishable by MTA and WMH scores.
Although the severity of WMHs was independently related to MTA scores, WMH scores had no correlation to cardiovascular risk factor scores, other than very weak correlations to single periventricular WMH and centrum semiovale WMH regions. Studies that have examined the relationship of WMHs on brain MR imaging to MR imaging diffusion characteristics among patients diagnosed with AD show that an increase in the apparent diffusion coefficient in water appears to be the underlying cause of the signal-intensity hyperintensity in these subjects.38 The underlying neuropathologic correlate that is specific to WMHs among patients with a pathologic diagnosis of AD is microglial activation. Changes in myelin and axonal attenuation seen in areas involved with WMHs may be related to this inflammatory process.10
The association of WMHs to cardiovascular risk factors in unselected generally cognitively healthy elderly individuals without dementia is well known. However, among individuals with dementia, the vast majority of whom have underlying AD and WMHs, it would seem that there is very little evidence to support a vascular etiology as a cause for the greater severity of WMHs on MR imaging in these individuals. The notion that WMHs are usually related to microvascular disease may not apply to patients with MCI and dementia but may apply to individuals with vascular dementia or with no cognitive impairment at the time of death.11,12,37 Similarly, it would seem that in the absence of any clinical evidence of cerebrovascular disease, clinical logic should argue that in patients who are diagnosed with AD, the presence of WMHs would suggest a white matter pathology caused by the underlying disease that we know is present, rather than by a disease that we speculate might be present. The relatively limited pathologic studies that have been performed to investigate the etiology of WMHs in patients with AD suggest that inflammation with microglial activation may be the underlying etiology of these WMHs, rather than cerebrovascular disease. Similar to the findings of authors from other studies, we did not find a particular location of the WMHs to be specifically associated with degenerative or vascular markers.39,40
In this cross-sectional study, we found that the severity of periventricular WMHs, centrum semiovale WMHs, and MTA generally increased with the severity of cognitive impairment, from no cognitive impairment to probable AD. However, we were unable to show any independent relationship of the severity of WMHs to cognitive function in this study. Other cross-sectional studies have also not found an independent association of WMHs to cognitive function.41,42 Nevertheless, several longitudinal studies do show that the severity of WMHs at baseline is associated with the rate of progression of cognitive impairment,43–45 and at least 1 study has demonstrated that this association is independent of the effects of MTA.43 However, it is not possible to conclude from these results, as has been done previously,43 that the faster rate of progression associated with WMHs is related to the synergistic combination of vascular disease, represented by the WMHs, and degenerative disease. In fact, the propensity of subjects who progress faster to have more WMHs may merely indicate that these subjects have more severe and complicated disease, with multiple factors associated with degeneration.10–13
Conclusions
Elderly patients presenting with MCI and dementia, regardless of whether they are diagnosed with probable or prodromal AD, often have evidence of cerebrovascular disease, in addition to the pathologic features of AD on neuropathologic examination.46,47 It is possible that WMHs on MR images of patients with a primary neuropathologic diagnosis of AD represent the presence of vascular lesions, which may not by themselves contribute to the severity of cognitive and functional impairment. However, in our study, we found that WMH scores increased progressively from no cognitive impairment to a-MCI to probable AD. We also found that MTA scores, but not WMH scores, were independently and strongly related to the severity of cognitive impairment. Most interesting, mean WMH scores in na-MCI and no cognitive impairment were not different, even though it would have been expected that if WMHs were associated with vascular pathology, then subjects with na-MCI, who tend to have more vascular disease than those with a-MCI,31,32,35 would have higher WMH scores than those with a-MCI. In fact, we found that the opposite was the case, suggesting that WMHs in subjects who are cognitively impaired are more strongly associated with neurodegenerative disease than with vascular disease. The strengths of the current study are the large number of subjects evaluated and the detailed neuropsychological, clinical, and MR imaging studies of both WMHs and MTA in the same subjects. Furthermore, the inclusion of subjects with both a-MCI and na-MCI provided the opportunity to evaluate WMH and MTA scores in the earliest stages of dementia, presumably caused by both AD (a-MCI) and non-AD (na-MCI) pathology. Limitations of this study include the lack of longitudinal data, which would have added another dimension to interpretation of the results, and lack of clinicopathologic correlations between WMHs and pathologic findings. Nevertheless, the current study does provide important support for the hypothesis that WMHs are most likely related to degenerative rather than vascular pathology among subjects diagnosed with na-MCI, a-MCI, and probable AD.
Acknowledgments
We are grateful to Christopher Woodhouse, MD, at Mount Sinai Medical Center, Department of Radiology, for providing detailed information on the protocols for MR images used in this study.
Footnotes
This research was supported the National Institute on Aging, National Institutes of Health, Bethesda, Md (1P50 AG025711-01), and a grant from the Johnnie B. Byrd, Sr. Alzheimer's Center and Research Institute, Tampa, Fla. Mount Sinai Medical Center, Miami Beach, Fla., provided financial support for this study.
References
- 1. Kapeller P, Barber R, Vermeulen RJ, et al. Visual rating of age-related white matter changes on magnetic resonance imaging. Stroke 2003;34:441–45 [DOI] [PubMed] [Google Scholar]
- 2. Fazekas F, Kapeller P, Schmidt R, et al. The relation of cerebral magnetic resonance signal hyperintensities to Alzheimer's disease. J Neurolog Sci 1996;142:121–25 [DOI] [PubMed] [Google Scholar]
- 3. Wahlund LO, Barkhof F, Fazekas F, et al. A new scale for age-related white matter changes applicable to MRI and CT. Stroke 2001;32:1318–22 [DOI] [PubMed] [Google Scholar]
- 4. Flier WM, Barkhof F, Scheltens P. Shifting paradigms in dementia. Ann NY Acad Sci 2007;1097:215–24 [DOI] [PubMed] [Google Scholar]
- 5. Rosano C, Alzenstein H, Wu M, et al. Focal atrophy and cerebrovascular disease increase dementia risk among cognitively normal older adults. J Neuroimaging 2007;17:148–55 [DOI] [PubMed] [Google Scholar]
- 6. Golomb J, Kluger A, Gionutsos , et al. Nonspecific leukoencephalopathy associated with aging. Neuroimaging Clin N Am 1995;5:33–44 [PubMed] [Google Scholar]
- 7. Stolp H, Dziegielewska K. Role of developmental inflammation and blood-brain barrier dysfunction in neurodevelopmental and neurodegenerative diseases. Neuropathol Appl Neurobiol 2009;35:132–46 [DOI] [PubMed] [Google Scholar]
- 8. Black S, Gao FQ, Bilbao J. Understanding white matter disease. Imaging-pathological correlations in vascular cognitive impairment. Stroke 2008;40:S48–52 [DOI] [PubMed] [Google Scholar]
- 9. Du AT, Schuff N, Chao LL, et al. White matter lesions are associated with cortical atrophy more than entorhinal and hippocampal atrophy. Neurobiol Aging 2005;26:553–59 [DOI] [PubMed] [Google Scholar]
- 10. Gouw A, Seewann H, Vrenken WM, et al. Heterogeneity of white matter hyperintensities in Alzheimer's disease: post-mortem quantitative MRI and neuropathology. Brain 2008;131:3286–98 [DOI] [PubMed] [Google Scholar]
- 11. Englund E. Neuropathology of white matter changes in Alzheimer's disease and vascular dementia. Dement Geriatr Cogn Disord 1998;9:6–12 [DOI] [PubMed] [Google Scholar]
- 12. Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology 2008;71:804–11 [DOI] [PubMed] [Google Scholar]
- 13. Bronge L, Bogdanovic N, Wahlund L-O. Postmortem MRI and histopathology of white matter changes in Alzheimer brains: a quantitative, comparative study. Dement Geriatr Cogn Disord 2002;13:205–12 [DOI] [PubMed] [Google Scholar]
- 14. Jack CR, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology 2002;58:750–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bobinski M, de Leon MJ, Wegiel J, et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer's disease. Neuroscience 2000;95:721–25 [DOI] [PubMed] [Google Scholar]
- 16. Gosche KM, Mortimer JA, Smith CD, et al. Hippocampal volume as an index of Alzheimer neuropathology: findings from the Nun Study. Neurology 2002;58:1476–82 [DOI] [PubMed] [Google Scholar]
- 17. Likeman M, Anderson VM, Stevens JM, et al. Visual assessment of atrophy on magnetic resonance imaging in the diagnosis of pathologically confirmed young-onset dementias. Arch Neurol 2005;62:1410–15 [DOI] [PubMed] [Google Scholar]
- 18. Barkhof F, Polvikoski TM, van Straaten EC, et al. The significance of medial temporal lobe atrophy: a postmortem MRI study in the very old. Neurology 2007;69:1521–27 [DOI] [PubMed] [Google Scholar]
- 19. Urs R, Potter E, Barker W, et al. Visual rating system for assessing magnetic resonance images: a tool in the diagnosis of mild cognitive impairment and Alzheimer Disease. J Comput Assist Tomogr 2009;33:73–78 [DOI] [PubMed] [Google Scholar]
- 20. Duara R, Loewenstein DA, Potter E, et al. Medial temporal lobe atrophy on MRI scans and the diagnosis of Alzheimer's disease. Neurology 2008;71:1986–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roberts RO, Geda YE, Knopman DS, et al. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol 2008;65:1066–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roberts RO, Knopman DS, Geda YE, et al. Coronary heart disease is associated with non-amnestic mild cognitive impairment. Neurobiol Aging December 15 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knopman DS, Roberts RO, Geda YE, et al. Association of prior stroke with cognitive function and cognitive impairment: a population-based study. Arch Neurol 2009;66:614–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petersen RD, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–92 [DOI] [PubMed] [Google Scholar]
- 25. Folstein M, Folstein S, McHugh P. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98 [DOI] [PubMed] [Google Scholar]
- 26. Loewenstein DA, Barker WW, Harwood DG, et al. Utility of a modified Mini-Mental State Examination with extended delayed recall in screening for mild cognitive impairment and dementia among community dwelling elders. Int J Geriatr Psychiatry 2000;15:434–40 [DOI] [PubMed] [Google Scholar]
- 27. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–14 [DOI] [PubMed] [Google Scholar]
- 28. Fuld PA. Fuld Object-Memory Evaluation. Wood Dale, Ill: Stoelting Co; 1981 [Google Scholar]
- 29. Lacritz LH, Cullum CM, Weiner M., et al. Comparison of the Hopkins Verbal Learning Test Revised to the California Verbal Learning Test in Alzheimer's Disease. Appl Neuropsychol 2001;8:180–84 [DOI] [PubMed] [Google Scholar]
- 30. Golden JC. Stroop Color and Word Test. Chicago: Stoelting Co; 1978 [Google Scholar]
- 31. McKhann G, Drachman DA, Folstein MF, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984;34:939–44 [DOI] [PubMed] [Google Scholar]
- 32. Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–08 [DOI] [PubMed] [Google Scholar]
- 33. de Carli C, Murphy DG, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology 1995;45:2077–84 [DOI] [PubMed] [Google Scholar]
- 34. Bombois S, Debette S, Delbeuck X, et al. Prevalence of subcortical vascular lesions and association with executive function in mild cognitive impairment subtypes. Stroke 2007;38:2595–97 [DOI] [PubMed] [Google Scholar]
- 35. Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol 2001;110:40–48 [DOI] [PubMed] [Google Scholar]
- 36. de Leon MJ, Golomb J, George AE, et al. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. AJNR Am J Neuroradiol 1993;14:897–906 [PMC free article] [PubMed] [Google Scholar]
- 37. de Leon MJ, George AE, Golomb J, et al. Frequency of hippocampal formation atrophy in normal aging and Alzheimer's disease. Neurobiol Aging 1997;18:1–11 [DOI] [PubMed] [Google Scholar]
- 38. Kantarci K, Jack CR, Xu YC, et al. Mild cognitive impairment and Alzheimer disease: regional diffusivity of water. Radiology 2001;219:101–07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van den Heuvel DM, ten Dam VH, de Craen AJ, et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry 2006;77:149–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wright CB, Festa JR, Paik MC, et al. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke 2008;39:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Firbank MJ, Burton EJ, Barber R, et al. Medial temporal atrophy rather than white matter hyperintensities predict cognitive decline in stroke survivors. Neurobiol Aging 2007;11:1664–69 [DOI] [PubMed] [Google Scholar]
- 42. van de Pol LA, Korf ES, van der Flier WM, et al. Magnetic resonance imaging predictors of cognition in mild cognitive impairment. Arch Neurol 2007;64:1023–28 [DOI] [PubMed] [Google Scholar]
- 43. van Straaten EC, Harvey D, Scheltens P, et al. Alzheimer's Disease Cooperative Study Group: periventricular white matter hyperintensities increase the likelihood of progression from amnestic mild cognitive impairment to dementia. J Neurol 2008;255:1302–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Modrego PJ, Rios C, Pérez Trullen JM, et al. The cerebrovascular pathology in Alzheimer's disease and its influence on clinical variables. Am J Alzheimers Dis Other Demen 2008;23:91–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Debette S, Bombois S, Bruandet A, et al. Subcortical hyperintensities are associated with cognitive decline in patients with mild cognitive impairment. Stroke 2007;38:2924–30 [DOI] [PubMed] [Google Scholar]
- 46. Schneider JA, Arvanitakis Z, Bang W, et al. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–204 [DOI] [PubMed] [Google Scholar]
- 47. Reitz C, Tang MX, Manly J, et al. Hypertension and the risk of mild cognitive impairment. Arch Neurol 2007;67:1734–40 [DOI] [PMC free article] [PubMed] [Google Scholar]