Abstract
BACKGROUND AND PURPOSE:
Functional MR imaging (fMRI) is a promising but, in some aspects, still debated noninvasive tool for functional language mapping. We developed a clinical fMRI overt language design at the sentential level to optimize sensitivity for language-related areas of the brain. To evaluate applicability and sensitivity, we investigated a consecutive series of presurgical patients with epilepsy with minimal morphologic brain abnormalities.
MATERIALS AND METHODS:
Thirty right-handed patients with temporal lobe epilepsy (TLE) and a control group of 23 right-handed healthy subjects participated in the study. The language design included semantic and syntactic error-detection tasks and was constructed to represent the most relevant aspects of everyday language demands. It was applied during block-designed fMRI runs. We performed image preprocessing and statistical analysis with SPM5 at a group level, applying widely used statistical criteria. The study was approved by the local ethics committee, and all participants gave written informed consent.
RESULTS:
Given the strict statistical criteria, the sensitivity for inferior frontal and posterior temporal activations (comprising Broca and Wernicke regions) was improved relative to previous findings in the literature. For both language areas, we found 100% sensitivity in healthy subjects (Brodmann areas, BA22 and BA44) and 97% sensitivity in patients (when including BA47). Lateralization results demonstrated the capability to detect atypical language lateralizations in patients, which were more frequent in than those in healthy subjects.
CONCLUSIONS:
We developed a clinical language fMRI design that integrates various relevant aspects of everyday language demands and provides robust localization of core language areas.
Preoperative functional MR imaging (fMRI) localization of language functions is a promising tool to improve the treatment of pre- and postsurgical patients.1,2 Although neuropsychological assessment can contribute to determination of language lateralization,3 neuropsychological tests alone are not sufficient to determine functional brain organization. Therefore, neuropsychological assessment is often combined with the invasive intracarotid amobarbital test, also known as the Wada test.4 In contrast to the Wada test, fMRI is a noninvasive technique, and some clinical studies on the topic of presurgical language fMRI have already been published.5–7 However, a still-unsolved issue is the optimal design of a robust and feasible clinical fMRI language task that reliably detects essential language areas and is applicable to all types of patients.8
Because the primary goal of presurgical language mapping is to provide a comprehensive picture of all brain areas that are essential for everyday language abilities, the application of blocked overt-speech sentences seems promising. With this rationale, we developed an overt language design for patient applications, integrating a wide range of everyday language aspects such as full sentences, reading, talking, and syntactic/semantic decisions. Our language paradigm includes a semantic and a syntactic task, to evaluate which kind of task specification is superior in a clinical context.
The goal of the present study was to evaluate the language design in a population of presurgical patients with temporal lobe epilepsy (TLE) who were referred for an fMRI language examination by their responsible physicians. We did not exclude any patient on the basis of clinical symptoms or neuropsychological criteria and applied widely used statistical criteria for analysis. This process allowed testing the general applicability of the design and the sensitivity in a typical clinical population because patients with TLE are one of the major patient groups prospectively profiting from the new fMRI technique. We hypothesize that the new overt language design is applicable to a clinical population and will show a higher sensitivity for major language areas than previously published designs.
Materials and Methods
Participants
Thirty right-handed consecutive presurgical patients (ie, examined in the order of referral) with TLE and a control group of 23 right-handed healthy subjects participated in the study. Seventeen patients (7 men; mean age, 35.1 years; 10 women; mean age, 32.4 years; Table 1) were diagnosed with left TLE and 13 (7 men; mean age, 30.1 years; 6 women; mean age, 43.7 years; Table 1), with right TLE. The diagnosis of TLE was accomplished by an extensive evaluation with selected modalities such as clinical symptoms, electroencephalography (EEG), MR imaging, single-photon emission CT, positron-emission tomography, video-monitored EEG, invasive EEG, and Wada testing in different combinations by a specialized clinical epilepsy unit. Six patients included in the study demonstrated hippocampal sclerosis in the anatomic MR imaging. Patients showing extended morphologic brain abnormalities such as tumors were excluded from the study to avoid problems with spatial normalization. The 23 control subjects (12 men; mean age, 31.8 years; 11 women; mean age, 30.0 years; Table 2) had no history of neurologic disease and were the same as the subjects in Foki et al.7 The study was approved by the local ethics committee. All participants were native German speakers and gave written informed consent according to the Helsinki Declaration.
Table 1:
LIs of patients with left and right TLE*
| Patients with TLE | Sex | Age (yr) | LI |
|
|---|---|---|---|---|
| Broca | Wernicke | |||
| Left TLE | ||||
| L1 | F | 26 | −0.357† | +0.523 |
| L2 | F | 46 | +0.823 | +0.507 |
| L3 | M | 31 | +0.149† | +0.539 |
| L4 | M | 28 | +0.552 | +0.442 |
| L5 | M | 48 | +0.404 | +0.199† |
| L6 | F | 37 | +0.244 | +0.374 |
| L7 | F | 32 | +0.140† | +0.679 |
| L8 | F | 25 | +0.189† | +0.572 |
| L9 | M | 39 | +0.829 | −0.179† |
| L10 | M | 20 | +0.521 | −0.074† |
| L11 | M | 21 | −0.780† | +0.049† |
| L12 | F | 41 | +0.038† | +0.567 |
| L13 | F | 27 | +0.500 | +0.511 |
| L14 | F | 25 | +0.374 | +0.774 |
| L15 | F | 16 | +0.986 | +0.867 |
| L16 | M | 59 | +0.618 | +0.967 |
| L17 | F | 49 | +0.179† | +0.233 |
| Right TLE | ||||
| R1 | M | 25 | +0.001† | +0.230 |
| R2 | F | 29 | +0.240 | −0.106† |
| R3 | M | 29 | +0.059† | +0.509 |
| R4 | M | 45 | +0.124† | +0.158† |
| R5 | F | 53 | +0.157† | +0.005† |
| R6 | M | 22 | +0.213 | +0.375 |
| R7 | F | 51 | +0.298 | +0.600 |
| R8 | M | 36 | +0.413 | +0.857 |
| R9 | F | 54 | +0.802 | +0.772 |
| R10 | M | 32 | +0.674 | +0.842 |
| R11 | F | 25 | +0.777 | +0.857 |
| R12 | M | 22 | −0.147† | +0.065† |
| R13 | F | 50 | +0.433 | +0.706 |
Note:—LI indicates laterality index; TLE, temporal lobe epilepsy.
LIs of patients with left and right TLE for the anterior (Broca) and the posterior (Wernicke) regions of interest using [semantic + syntactic] tasks.
Atypical LIs (LI ≤ 0.20).
Table 2:
LIs of healthy subjects*
| Healthy Subjects | Sex | Age (yr) | LI |
|
|---|---|---|---|---|
| Broca | Wernicke | |||
| S1 | F | 28 | +0.196† | +0.596 |
| S2 | F | 24 | +0.477 | +0.465 |
| S3 | F | 25 | +0.327 | +0.517 |
| S4 | M | 26 | +0.469 | +0.876 |
| S5 | F | 55 | +0.008† | +0.048† |
| S6 | M | 24 | +0.387 | +0.949 |
| S7 | M | 52 | +0.104† | +0.585 |
| S8 | F | 48 | +0.207 | +0.824 |
| S9 | M | 25 | +0.053† | +0.288 |
| S10 | F | 25 | +0.674 | +0.689 |
| S11 | M | 26 | +0.984 | +0.592 |
| S12 | M | 46 | +0.186† | +0.716 |
| S13 | M | 27 | +0.661 | +0.418 |
| S14 | F | 28 | +0.173† | +0.033† |
| S15 | F | 26 | +0.500 | +0.331 |
| S16 | F | 26 | +0.311 | +0.306 |
| S17 | M | 25 | +0.362 | +0.954 |
| S18 | M | 35 | +0.706 | +0.989 |
| S19 | F | 23 | −0.614† | +0.143† |
| S20 | M | 29 | +0.182† | +0.663 |
| S21 | F | 22 | +0.366 | +0.776 |
| S22 | M | 34 | +0.542 | +0.290 |
| S23 | M | 33 | +0.333 | −0.013† |
LIs of healthy subjects for the anterior (Broca) and the posterior (Wernicke) regions of interest using [semantic + syntactic] tasks.
Atypical LIs (LI ≤ 0.20).
Tasks
The investigation comprised 20 pseudorandomized block-designed fMRI runs of a syntactic (10 runs) and a semantic task (10 runs). After an initial 10 seconds of dummy scans, each run consisted of 4 control (C) and 3 activation (A) periods with a duration of 20 seconds each in the order of CACACAC, resulting in a total duration of 150 seconds per run (Fig 1). Each experimental run was performed separately, interrupted by short rest periods according to individual needs of the participants. During every activation period, 2 German sentences consisting of 4 + 2 words were presented word by word. Each new word was underlined and presented in the middle of the screen. The final word consisted of 2 alternative verbs that were either syntactically or semantically correct or incorrect. The instruction was to read overtly every word and to repeat the correct final sentence. The correct sentence had to be repeated at a convenient speed until the presentation of the next sentence or the control condition started. The sentences remained visible during this time. Subjects were trained to speak softly and to minimize movements of head and facial muscles.9
Fig 1.
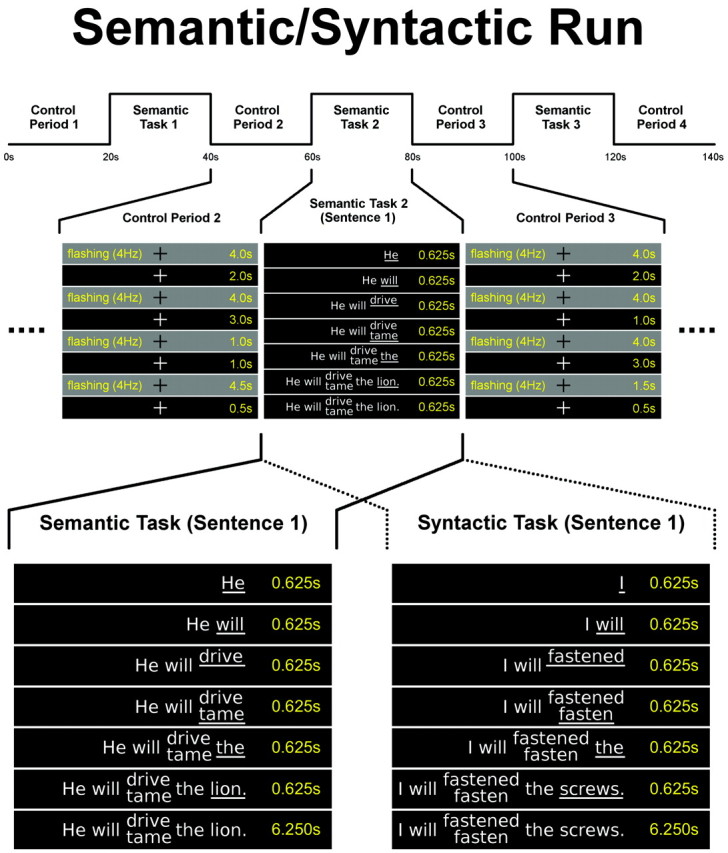
Schematic of a language paradigm. First row, Schematic illustration of a semantic run (duration, 140 seconds [s]). Syntactic runs comprised syntactic tasks instead of semantic tasks. Second row, Construction of pseudorandomized control periods (duration, 20s) and the first sentence of the semantic task 2 (duration: 20s) shown in detail. Every task comprises 2 sentences. Third row, Comparison of the construction of a semantic and a syntactic error sentence (as appearing in semantic/syntactic runs). Note that due to the translation into English, the verbs are no longer at the end of the sentences. The original German sentences read “Er wird den Löwen fahren/zähmen” (semantic error) and “Ich werde die Schrauben befestigt/befestigen” (syntactic error).
At the start of the control period, the participants were shown a white fixation cross in the middle of the screen. In addition, during the whole control period, 4 blocks of pseudorandomized visual stimulation (black/gray flashing of the whole screen at 4 Hz) were presented. Participants were instructed to touch the palate with their tongue every time a flashing visual stimulus sequence appeared (to provide control of non-language-related motor aspects). To achieve good individual performance, the participants had to practice 2–4 runs outside the scanner. During measurement, the participants were monitored to control cooperation and verbal output.
MR Imaging Acquisition
To minimize head movements, we used an optimized plaster cast helmet for comfortable and safe head fixation.7,10 Whole-head blood oxygen level–dependent fMRI was performed on a 3T scanner by using a phase-corrected blipped single-shot gradient-echo echo-planar imaging sequence optimized for the local scanner (sinc pulse excitation; TE/TR; 55.5/5000 ms; flip angle, 90°; 128 × 128 matrix, 230 × 230 mm FOV; 35 anterior/posterior commissure sections covering the whole brain; in-plane resolution, 1.8 × 1.8 mm; section thickness, 3 mm; no intersection gap; interleaved acquisition of sections).
fMRI Data Processing
Preprocessing and General Aspects.
Image preprocessing and statistical analysis were performed by using statistical parametric mapping 5 (SPM5; Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm5), applying standard head registration (rigid-body 6-parameter model, 3D sinc interpolation),11 spatial normalization to the functional Montreal Neurologic Institute (MNI) template (2-mm isotropic voxels, trilinear interpolation), spatial smoothing (isotropic gaussian filter kernel; full width at half maximum, 8 mm), first-level analysis by using high-pass filtering (80 seconds), canonical hemodynamic response function, and default correction for autocorrelation.
We performed single-subject and group analyses. For single-subject analysis, the contrast images generated during the first-level analyses were followed by a family-wise error (FEW)–corrected individual SPM5 analysis (P < .05). For group analysis, the contrast images generated during the first-level analyses were submitted to a second-level random-effects analysis, and a false discovery rate (FDR, P < .05) was applied.12
Determination of the General Activation Patterns.
A 1-sample t test using both language tasks (ie, [semantic + syntactic] tasks) for the whole group of patients with left and right TLE and healthy subjects was used to calculate an activation map showing all regions in the whole brain that are involved in processing the language paradigm (FDR, P < .05).
Specific Activation Patterns in the Temporofrontal Region of Interest.
For further analysis, we generated a bilateral temporofrontal region of interest (Fig 2C) with the SPM toolbox MARINA,13 containing left and right Broca and Wernicke areas as generated for the calculation of laterality indices (LIs, see below). This temporofrontal region of interest contained all bilateral regions of primary interest concerning language processing.
Fig 2.
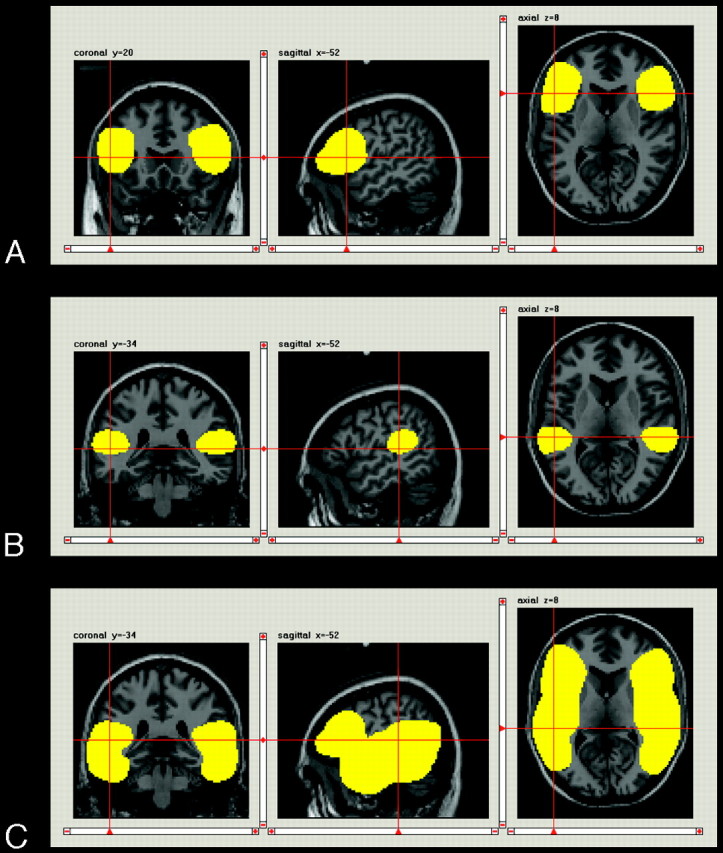
Coronal, sagittal, and axial views of the applied regions of interest as displayed by the program MARINA: A, Anterior (Broca) region of interest. B, Posterior (Wernicke) region of interest. C, Temporofrontal region of interest.
Temporofrontal Region of Interest: Patients with Left-versus-Right TLE.
With region-of-interest data, a 1-sided 2-sample t test using both language tasks (ie, [semantic + syntactic] tasks) was calculated to detect differences between the groups of patients with left and right TLE (temporofrontal region of interest; FDR, P < .05).
Temporofrontal Region of Interest: Semantic-versus-Syntactic Tasks.
In addition, a 1-sided paired t test for the whole group of patients with left and right TLE and the group of healthy subjects was used to assess the differences in the semantic and syntactic tasks (ie, [semantic − syntactic] and [syntactic − semantic] tasks; temporofrontal region of interest; FDR, P < .05).
Correlation of Activation Patterns with Brodmann Areas.
To classify all activated regions of [semantic + syntactic] group results and [semantic − syntactic] group results for patients and healthy subjects in terms of Brodmann areas (BAs), the MNI Space Utility (MSU) (http://www.ihb.spb.ru/∼pet_lab/MSU/MSUMain.html) was applied with a cluster criterion of k = 5 voxels on the group data. Subsequently, the number of significant voxels per BA was determined.
Number of Participants Showing Activity in Specific BAs.
The MSU was used to count activity in all BAs for every single patient and healthy subject (by using the [semantic + syntactic] first-level contrast images) to achieve a measure of assumed sensitivity of our tasks. For this purpose, we used an FEW-corrected individual SPM5 analysis (P < .05), and the MSU was applied on the resulting data with a cluster criterion of k = 5 voxels. Then for each BA, it was determined how many subjects showed activity for each of the patient and healthy-subjects groups.
LIs
For each participant, anterior (Broca) and posterior (Wernicke) LIs were calculated according to a previously published approach.6,14 This rather robust procedure first calculates a mean maximum t value defined as the mean of those 5% of voxels showing the highest activation in each region of interest of both hemispheres. The threshold for inclusion in the calculation is then set to 50% of the mean maximum t value. Finally, the sum of t values above the threshold is entered in the following formula for calculating LIs:
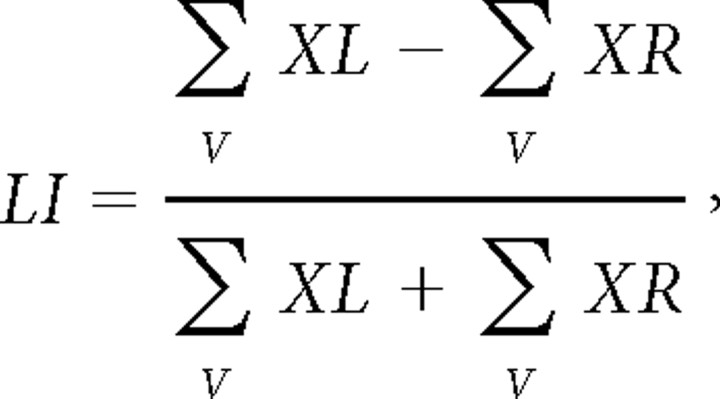 |
where V is the set of activated suprathreshold voxels within the region of interest, XL is the t value of left-hemispheric voxels, and XR is the t value of right-hemispheric voxels.
The latest version of the SPM toolbox MARINA13 was used to generate the anterior (Broca) and posterior (Wernicke) regions of interest in MNI space for each hemisphere.15 The anterior region of interest (Broca, Fig 2A) covered the pars opercularis and pars triangularis of the inferior frontal gyrus (IFG); the posterior region of interest (Wernicke, Fig 2B) covered the posterior half of the superior temporal gyrus.16
Results
Performance Monitoring
All patients and subjects performed the task successfully (>90% correct answers determined from prior practice and monitoring during the measurements). Because no performance problem was expected and found, no quantitative recording of patient monitoring was performed. Only 1 fMRI run in 1 patient had to be excluded due to severe artifacts.
Activation Patterns
General Activation Patterns in Patients and in Healthy Subjects.
The group activation pattern produced by both parts of the language task (ie, [semantic + syntactic] tasks) and calculated by a 1-sample t test showed a large number of brain regions known to be involved in different subprocesses of language. This was true for both groups of participants (healthy subjects and patients, Fig 3 and Tables 3 and 4). It comprised 2 tests: 1) patients with left and right TLE: [semantic + syntactic] tasks versus controls; 2) healthy subjects: [semantic + syntactic] tasks versus controls.
Fig 3.
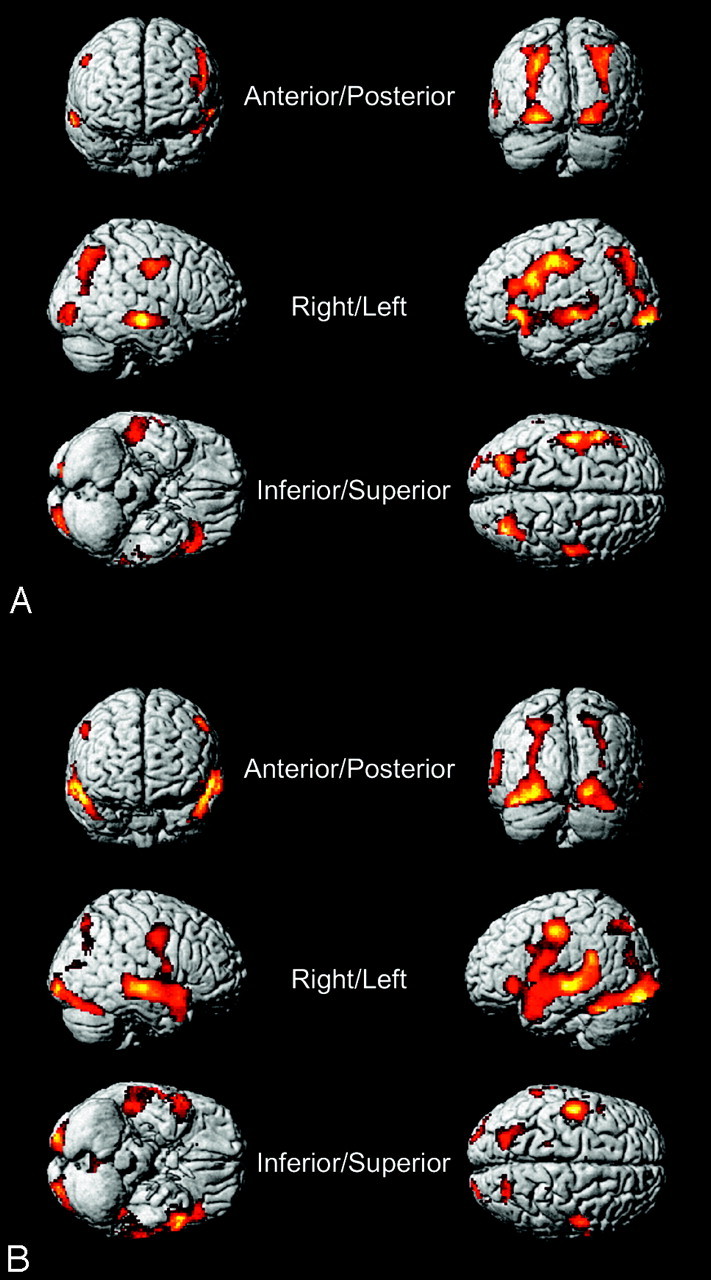
A, Patients with TLE: semantic + syntactic task. 3D view of the language-activation map of both tasks (ie, [semantic + syntactic] tasks) across the whole group of patients with TLE (no region of interest; FDR, P < .05; threshold [T] = 2.879) overlaid on SPM5 standard brain in MNI space. B, Healthy subjects: semantic + syntactic task. 3D view of a language-activation map of both tasks (ie, [semantic + syntactic] tasks) across the whole group of healthy subjects (no region of interest; FDR, P < .05; T = 2.834) overlaid on SPM5 standard brain in MNI space.
Table 3:
Active Brodmann areas of the semantic + syntactic task in patients with TLE*
| Brodmann Area | No. Voxels | % Total Activation |
|---|---|---|
| Whole brain | 9672 | 100.00 |
| Left brain | 1694 | 17.51 |
| Brodmann area 6 (L) | 302 | 3.12 |
| Brodmann area 7 (L) | 273 | 2.82 |
| Brodmann area 9 (L) | 154 | 1.59 |
| Brodmann area 21 (L) | 116 | 1.20 |
| Brodmann area 47 (L) | 110 | 1.14 |
| Brodmann area 19 (L) | 101 | 1.04 |
| Brodmann area 4 (L) | 99 | 1.02 |
| Brodmann area 22 (L) | 96 | 0.99 |
| Brodmann area 18 (L) | 95 | 0.98 |
| Brodmann area 38 (L) | 70 | 0.72 |
| Brodmann area 3 (L) | 67 | 0.69 |
| Brodmann area 46 (L) | 54 | 0.56 |
| Brodmann area 2 (L) | 36 | 0.37 |
| Brodmann area 40 (L) | 28 | 0.29 |
| Brodmann area 17 (L) | 21 | 0.22 |
| Brodmann area 44 (L) | 18 | 0.19 |
| Brodmann area 45 (L) | 16 | 0.17 |
| Brodmann area 39 (L) | 16 | 0.17 |
| Brodmann area 8 (L) | 13 | 0.13 |
| Brodmann area 13 (L) | 5 | 0.05 |
| Brodmann area 37 (L) | 2 | 0.02 |
| Corpus callosum (L) | 1 | 0.01 |
| Brodmann area 42 (L) | 1 | 0.01 |
| Right brain | 735 | 7.60 |
| Brodmann area 7 (R) | 214 | 2.21 |
| Brodmann area 21 (R) | 163 | 1.69 |
| Brodmann area 6 (R) | 130 | 1.34 |
| Brodmann area 19 (R) | 59 | 0.61 |
| Brodmann area 4 (R) | 38 | 0.39 |
| Brodmann area 40 (R) | 29 | 0.30 |
| Brodmann area 18 (R) | 28 | 0.29 |
| Brodmann area 3 (R) | 25 | 0.26 |
| Brodmann area 39 (R) | 19 | 0.20 |
| Brodmann area 17 (R) | 19 | 0.20 |
| Brodmann area 22 (R) | 10 | 0.10 |
| Corpus callosum (R) | 1 | 0.01 |
| Other locations | 7243 | 74.89 |
| Unidentified | 7242 | 74.88 |
| Corpus callosum (C) | 1 | 0.01 |
Note:—L indicates left; R, right; C, central.
Active Brodmann areas of the [semantic + syntactic] task in the whole brain with false discovery rate correction (P < .05) and cluster criterion of 5 voxels ordered by the number of active voxels in patients with TLE, showing the Brodmann areas with the highest number of active voxels on top (see “Materials and Methods” section for details of calculation).
Table 4:
Active Brodmann areas of the semantic + syntactic task in healthy subjects*
| Brodmann Area | No. Voxels | % Total Activation |
|---|---|---|
| Whole brain | 15051 | 100.0 |
| Left brain | 2719 | 18.07 |
| Brodmann area 21 (L) | 412 | 2.7 |
| Brodmann area 22 (L) | 358 | 2.38 |
| Brodmann area 6 (L) | 354 | 2.35 |
| Brodmann area 7 (L) | 261 | 1.73 |
| Brodmann area 37 (L) | 233 | 1.5 |
| Brodmann area 18 (L) | 169 | 1.12 |
| Brodmann area 38 (L) | 163 | 1.08 |
| Brodmann area 19 (L) | 149 | 0.99 |
| Brodmann area 4 (L) | 139 | 0.92 |
| Brodmann area 3 (L) | 90 | 0.60 |
| Brodmann area 9 (L) | 63 | 0.42 |
| Brodmann area 13 (L) | 61 | 0.4 |
| Brodmann area 44 (L) | 60 | 0.40 |
| Brodmann area 47 (L) | 51 | 0.34 |
| Brodmann area 40 (L) | 41 | 0.27 |
| Brodmann area 20 (L) | 37 | 0.25 |
| Dentate (L) | 18 | 0.12 |
| Brodmann area 17 (L) | 14 | 0.09 |
| Brodmann area 42 (L) | 13 | 0.09 |
| Brodmann area 41 (L) | 9 | 0.06 |
| Brodmann area 43 (L) | 7 | 0.05 |
| Brodmann area 29 (L) | 6 | 0.04 |
| Brodmann area 36 (L) | 4 | 0.03 |
| Corpus callosum (L) | 2 | 0.01 |
| Brodmann area 2 (L) | 2 | 0.01 |
| Brodmann area 1 (L) | 2 | 0.01 |
| Brodmann area 45 (L) | 1 | 0.01 |
| Right brain | 1462 | 9.71 |
| Brodmann area 21 (R) | 378 | 2.51 |
| Brodmann area 6 (R) | 262 | 1.74 |
| Brodmann area 22 (R) | 195 | 1.30 |
| Brodmann area 38 (R) | 187 | 1.24 |
| Brodmann area 18 (R) | 118 | 0.78 |
| Brodmann area 7 (R) | 105 | 0.70 |
| Brodmann area 19 (R) | 74 | 0.49 |
| Brodmann area 17 (R) | 58 | 0.39 |
| Dentate (R) | 33 | 0.22 |
| Brodmann area 4 (R) | 21 | 0.14 |
| Brodmann area 47 (R) | 10 | 0.07 |
| Brodmann area 44 (R) | 10 | 0.07 |
| Corpus callosum (R) | 3 | 0.02 |
| Brodmann area 39 (R) | 3 | 0.02 |
| Brodmann area 3 (R) | 2 | 0.01 |
| Brodmann area 9 (R) | 1 | 0.01 |
| Brodmann area 37 (R) | 1 | 0.01 |
| Brodmann area 13 (R) | 1 | 0.01 |
| Other Locations | 10870 | 72.22 |
| Unidentified | 10867 | 72.20 |
| Corpus callosum (C) | 3 | 0.02 |
Active Brodmann areas of the [semantic + syntactic] task in the whole brain with false discovery rate correction (P < .05) and cluster criterion of 5 voxels ordered by the number of active voxels in healthy subjects, showing the Brodmann areas with the highest number of active voxels on top (see “Materials and Methods” section for details of calculation).
In summary, we found active clusters in the following BAs: BA47, often associated with semantic processing; and BA44, generally considered to be related to syntactic and/or phonologic processing.17,18 BA45 is associated with syntactic as well as with semantic processing.17,18 Additionally, the IFG is also involved in verbal working memory functions during sentence tasks.18 Active regions were also found in the left (and partly right) insula (BA13). In previous reports, the insula has been associated with object naming relative to object decision tasks and with articulatory processes during productive speech.19,20 Significant contribution to the overall activation pattern was likewise found in the temporal lobes.21 These regions are likely to be involved in word perception20 and lexicosemantic21 and phonological processing.22 The middle temporal gyrus seems to be particularly relevant in sentence tasks,23 whereas the superior temporal gyrus is more frequently activated during word tasks.24 In the posterosuperior temporal gyrus (including the Wernicke area [BA22]), a clearly left lateralized activation cluster was found. Most subcomponents of language depend on the proper function of this region.25
Temporofrontal Region of Interest: Patients with Left-versus-Right TLE.
Concerning patients with left and right TLE, the 2-sample t test revealed no differences. This comprised 1 test: [semantic + syntactic] tasks in patients with left TLE versus [semantic + syntactic] tasks in patients with right TLE.
Temporofrontal Region of Interest: Semantic-versus-Syntactic Tasks.
Although the paired t test (applied to the temporofrontal region of interest) revealed no difference for the [syntactic − semantic] analysis in both groups of participants, a subtle difference for the [semantic − syntactic] analysis could be found. In patients with TLE, this difference was rather small, consisting of the left insula (BA13) and the left orbital part of the IFG (BA47, compare Table 5 and Fig 4). In the healthy subjects, the difference in [semantic − syntactic] tasks was much stronger, emphasizing temporal activation (BA21 and BA22) and mostly bilateral BA13 and BA47 (to some extent also BA45, compare Table 6 and Fig 4). This analysis comprised 4 tests: 1) [semantic − syntactic] tasks in patients with left and right TLE, 2) [semantic − syntactic] tasks in healthy subjects, 3) [syntactic − semantic] tasks in patients with left and right TLE, and 4) [syntactic − semantic] tasks in healthy subjects. To analyze the activated BAs, we used a cluster criterion of k = 5 voxels (ie, only clusters containing ≥5 voxels were used to calculate Tables 3–6). Note, that despite the applied cluster criterion of k = 5 voxels, in case a cluster overlapped a BA with only a few voxels, it was possible that this BA included less <5 voxels.
Table 5:
Active Brodmann areas of the semantic − syntactic task in patients with TLE*
| Brodmann Area | No. voxels | % Total Activation |
|---|---|---|
| Temporofrontal ROI | 219 | 100.00 |
| Left brain | 30 | 13.70 |
| Brodmann area 47 (L) | 16 | 7.31 |
| Brodmann area 13 (L) | 14 | 6.39 |
| Right brain | 0 | 0.00 |
| Other locations | 189 | 86.30 |
| Unidentified | 189 | 86.30 |
Note:—ROI indicates region of interest.
Active Brodmann areas of the [semantic − syntactic] task in a temporofrontal ROI with false discovery rate correction (P < .05) and cluster criterion of 5 voxels ordered by the number of active voxels in patients with TLE, showing the Brodmann area with the highest number of active voxels on top (see “Materials and Methods” section for details of calculation).
Fig 4.
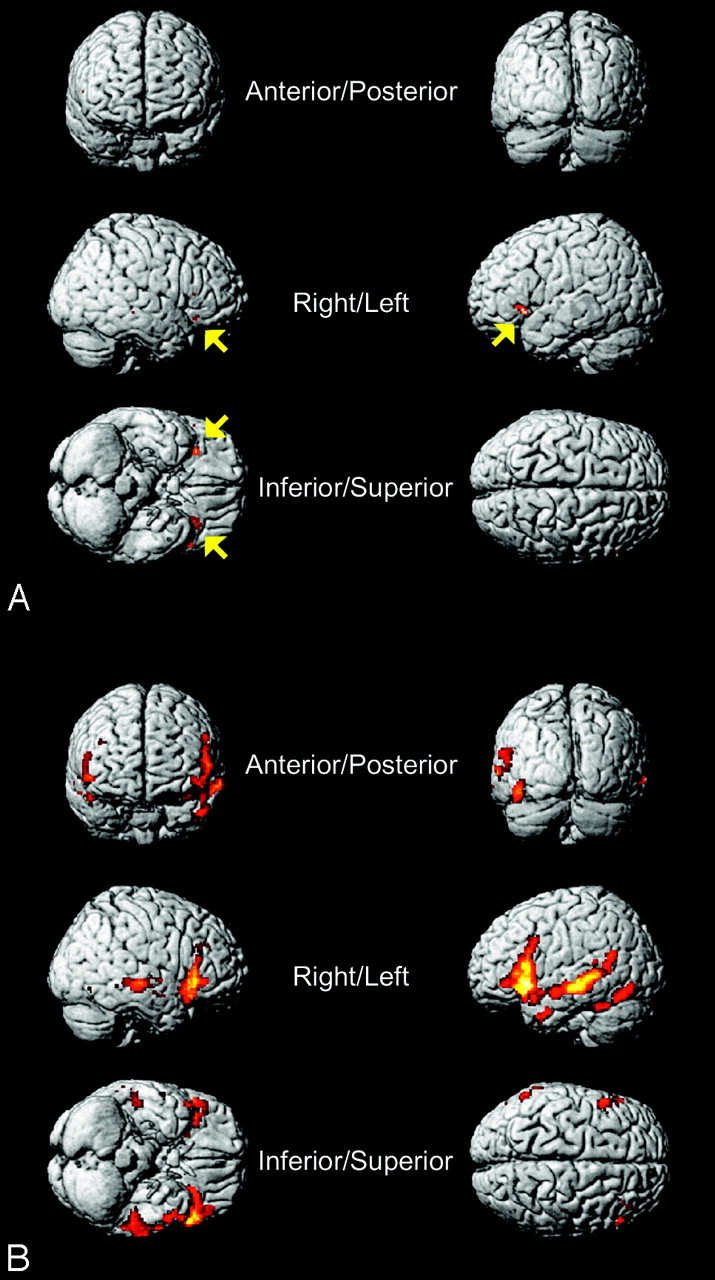
A, Patients with TLE: semantic − syntactic task. 3D view of a language-activation map shows the differences between the [semantic − syntactic] task across the whole group of patients with TLE (temporofrontal region of interest; FDR, P < .05; threshold [T] = 3.838) overlaid on SPM5 standard brain in MNI space. Note the small activation in the left and right IFGs (BA47) and the left insula (BA13) (yellow arrows, compare with Table 5). B, Healthy subjects: semantic − syntactic task. 3D view of a language-activation map shows the differences between the [semantic − syntactic] task across the whole group of healthy subjects (temporofrontal region of interest; FDR, P < .05, T = 2.710) overlaid on SPM5 standard brain in MNI space (compare Table 6).
Table 6:
Active Brodmann areas of the semantic − syntactic task in healthy subjects*
| Brodmann Area | No. voxels | % Total Activation |
|---|---|---|
| Temporofrontal ROI | 5528 | 100.0 |
| Left brain | 1217 | 22.02 |
| Brodmann area 21 (L) | 231 | 4.18 |
| Brodmann area 47 (L) | 230 | 4.16 |
| Brodmann area 13 (L) | 167 | 3.02 |
| Brodmann area 22 (L) | 138 | 2.50 |
| Brodmann area 45 (L) | 126 | 2.28 |
| Brodmann area 38 (L) | 100 | 1.81 |
| Brodmann area 9 (L) | 51 | 0.92 |
| Brodmann area 44 (L) | 49 | 0.89 |
| Brodmann area 37 (L) | 36 | 0.65 |
| Brodmann area 19 (L) | 20 | 0.36 |
| Brodmann area 39 (L) | 16 | 0.29 |
| Brodmann area 29 (L) | 13 | 0.24 |
| Brodmann area 46 (L) | 12 | 0.22 |
| Brodmann area 20 (L) | 10 | 0.18 |
| Brodmann area 41 (L) | 9 | 0.16 |
| Brodmann area 40 (L) | 6 | 0.11 |
| Brodmann area 34 (L) | 1 | 0.02 |
| Brodmann area 18 (L) | 1 | 0.02 |
| Brodmann area 11 (L) | 1 | 0.02 |
| Right brain | 367 | 6.64 |
| Brodmann area 47 (R) | 146 | 2.6 |
| Brodmann area 21 (R) | 58 | 1.05 |
| Brodmann area 13 (R) | 48 | 0.87 |
| Brodmann area 45 (R) | 44 | 0.80 |
| Brodmann area 38 (R) | 42 | 0.76 |
| Brodmann area 46 (R) | 14 | 0.25 |
| Brodmann area 22 (R) | 14 | 0.25 |
| Brodmann area 41 (R) | 1 | 0.02 |
| Other locations | 3944 | 71.35 |
| Unidentified | 3944 | 71.35 |
Active Brodmann areas of the [semantic − syntactic] task in a temporofrontal ROI with false discovery rate correction (P < .05) and cluster criterion of 5 voxels ordered by the number of active voxels in healthy subjects, showing the Brodmann areas with the highest number of active voxels on top (see “Materials and Methods” section for details of calculation).
Number of Participants Showing Activity in Specific BAs.
With MSU, 29/30 (97%) patients with TLE activated BA22 (Wernicke: left, 29/30; right, 28/30), whereas 26/30 (87%) showed activity in BA44 (Broca: left, 25/30; right, 20/30). Neighboring areas to BA44 like BA47 were active in 29/30 (97%; left, 28/30; right, 25/30) and BA6 (premotor cortex: left, 27/30; right, 29/30), even in 30/30 (100%) patients with TLE. Therefore, when extending the inferior frontal focus to a combination of BA6 + BA44 + BA47 (BA44 shows largely variable posterior extensions, which may well reach standard BA6 delineations),26 activity was found in 100% of the patients with TLE. In 23/23 (100%) healthy subjects, activity could be found in BA22 (left, 23/23; right, 23/23) and BA44 (left, 22/23; right, 18/23). In addition, BA6 was active in 23/23 (100%; left, 23/23; right, 23/23) and BA47 in 22/23 (96%) healthy subjects (left, 22; right, 21).
LIs
A tendency toward dominance of the left hemisphere (ie, positive LI) was detected, but we found atypical lateralization (according to international conventions, LI ≤ 0.20) of language as well.27 The 30 patients with TLE showed an atypical LI in 12/30 (40%) cases in the anterior region of interest and in 8/30 (27%) cases in the posterior region of interest (compare Table 1). The 23 healthy subjects showed 8/23 (35%) cases of atypical LI in the anterior region of interest and 4/23 (17%) cases in the posterior region of interest (compare with Table 2). Concerning only right-hemisphere dominance (LI ≤ 0.20), we found 2/30 (7%) in patients with TLE (both are patients with left TLE) and 1/23 (4%) in healthy subjects. Right dominance was only found in the Broca region. Bilateral lateralization (−0.20 ≤ LI ≤ 0.20) of either Broca or Wernicke areas could be seen in 15/30 (50%) patients with TLE and 6/23 (26%) healthy subjects. In summary, 16/30 (53%) patients with TLE and 9/23 (39%) healthy subjects showed some kind of atypical language lateralization. Therefore, the number of atypical lateralizations in patients with TLE is moderately larger than that in healthy subjects. (These atypical language lateralizations cannot be seen in Figs 3 and 4 because they are based on individual statistical calculations and the figures show group results.)
Discussion
The optimal design of a robust and feasible clinical fMRI language task that reliably detects essential language areas is still an unsolved issue. Currently, silent word generation,23,28 picture naming,23 semantic-decision,6,23,24 rhyme detection,24 word stem completion,29 and silent reading tasks30 are most frequently used. Most of these studies applied covert speech. Although some researchers found only minimal differences between overt and covert speech,31 most concluded that the 2 modalities produce similar but nevertheless unequal activations29,32,33 and that the use of spoken output in language localization studies is desirable. Others recently suggested overt speech fMRI techniques34,35 and applied single-word tasks within an event-related design lead to lower total contrast-to-noise ratios compared with blocked designs36 and reduce the sensitivity and consistency of word tasks compared with sentence tasks.37
In this work, we tested an extended paradigm in a typical clinical population (excluding patients with extended morphologic brain abnormalities) referred for presurgical language mapping performed with a reliability-based data analysis technique.38,39 For testing under realistic clinical conditions, only patients with relevant brain distortions were excluded. We used an optimized plaster cast helmet for comfortable and safe head fixation. Although this device does not provide total fixation, it considerably reduces head-motion components compared with head fixation with conventional restraining straps, according to Edward et al.10 To optimize functional data quality, we used a long total measurement time of 50 minutes altogether (pure scanning time, not including rest periods between individual runs) per patient/subject. Taking initial technical settings, anatomic MR imaging, and rest periods into account, participants had to stay ≤90 minutes inside the scanner. Due to this rather long time, it would have been difficult to include any other previously published language tasks for the purpose of direct comparison. Comparisons of the fMRI results with the Wada test (or other modalities) could be not done due to the small number of patients who had undergone Wada testing.
Global Results
The SPM5 group analysis showed a comprehensive and robust language map, closely corresponding to the results in Foki et al (Fig 3).7 No significant differences were found between patients with left and right TLE. The left IFG, including Broca language area—a clinical key region for language production—was strongly activated (compare with “General Activation Patterns in Patients and in Healthy Subjects” in the Results section).
Within our experimental block design, which combined several components of language processing, a strict assignment of specific language processes or subprocesses to different cortical regions was neither intended nor possible. However, we were able to show the ability of the paradigms to robustly activate cortical regions essential for everyday language, with a high sensitivity in a rather heterogeneous patient population regularly referred for presurgical language mapping and in healthy controls.
Task Design
Indications exist that word tasks have a lower detection rate for essential language areas than sentence tasks.40 In addition, the magnitude of activation is usually greater in tasks requiring overt speech.33 Aldenkamp et al41 concluded that “active semantic language processing or comprehensive procedures with multiple language tasks have the highest guarantee for individual activation lateralization.” Moreover, Binder et al42 obtained optimal results by contrasting an active semantic-decision task with a tone-decision task. They especially emphasized the need for an active nonlinguistic task as a baseline to identify brain regions involved in semantic processing.
We combined several components of language processing (semantic, syntactic, orthographic, and phonologic) into 1 single reading task and contrasted it with an active nonlinguistic task involving the production of nonspeech sounds.
Semantic/Syntactic Differences
Despite the fact that the syntactic and/or semantic error is only a small part of both tasks, some significant differences could be found (Tables 5 and 6 and Fig 4). Primarily the left insula (BA13) and the left IFG (BA47) were more active during the semantic task, which argues for concentration on this task type for clinical applications. The finding is in good accordance with the literature about semantic processing,43 and we were able to replicate these differences in patients and healthy subjects (compare Tables 5 and 6).
Lateralization
For diagnostic purposes, it is also important to be able to determine language lateralization in patients reliably. Therefore, we created standardized regions of interest of the Broca and Wernicke regions and calculated the LIs according to Weber et al6 and Fernandez et al,14 because their methods are independent of individual task performances and avoid the problem of arbitrarily chosen thresholds.
We found an increase of atypical LIs (according to international conventions, LI ≤ 0.20) in patients compared with healthy subjects. This result may be explained by reorganization processes in the brain due to pathology. Although Springer et al27 reported 22% of atypical language dominance (right-sided and bilateral) in right-handed patients with TLE and 6% in healthy subjects, we found much higher rates of 53% in patients with TLE and 39% in healthy subjects (mostly due to bilateral language lateralization). However, these results cannot be directly compared because Springer et al27 used different tasks, and most important, they applied extended single regions of interest comprising the lateral two thirds of a hemisphere. In contrast, Thivard et al44 defined atypical language lateralization as LI ≤ 0.20 (ie, only right-sided) and reported atypical language representation rates as high as 19% in their patients with TLE. Therefore, LIs may vary greatly from study to study and should be interpreted with caution.45 It might also be that in our case a stricter definition of atypical lateralization would be preferable to retain the literal meaning of “atypical.” For clinical safety reasons, Wellmer et al46 suggested calculating LIs from >1 region of interest, taking only the least lateralized region of interest into account and using high thresholds for the LIs, to be able to identify correctly typical language dominance in patients by using fMRI.
Remarkably, one of the patients with left TLE (L1) showed crossed lateralization (right Broca, left Wernicke). Nevertheless, according to Kurthen et al,47 such an interhemispheric dissociation of language functions is possible in patients with epilepsy.
In another patient with left TLE (L11), we found right Broca and bilateral Wernicke lateralization. In addition, 1 healthy subject (S19) also showed right Broca and bilateral Wernicke lateralization.
Concerning patients with left and right TLE, there was no relevant difference. This indicates that the underlying pathology in our patient cohort was not sufficient for major language-related laterality changes.
Percentage of Subjects Activating Normally Expected Language Sites
Multitask approaches report high sensitivity rates like 100% for Broca and 83% for Wernicke regions48 or 98% for Broca and Wernicke regions in patients.49 Another patient study even reported 100% sensitivity for both Broca and Wernicke regions.50 However, all these results were achieved with rather low statistical thresholds. Compared with these earlier publications, a high sensitivity of our sentence-based language paradigm could be confirmed in patients with left and right TLE and in controls. In 100% of the 23 healthy subjects, BA22 (Wernicke region) and BA44 (Broca region) were found active. With 97% of our patients, activity was found in BA22 (Wernicke area) and BA44 and BA47. Inclusion of inferior premotor areas increased left inferofrontal sensitivity to 100% in our patients. This indicates relevant improvements compared with earlier language designs when considering the strict statistical criteria we applied. Therefore, the application of an overtly spoken sentence task that imitates everyday language seems to be advantageous, especially in a clinical setting in which high-signal-intensity quality and sensitivity may be crucial due to pathologically altered brains. However, direct and systematic comparisons of our fMRI results with postoperative surgical outcome are yet required.
Study Limitations
One major limitation of this study is that no comparisons of the results with independent methodology (intraoperative cortical mapping, Wada test) were possible. Due to our efforts to achieve reliable fMRI maps (requiring long measurement times), other fMRI language tasks could not be directly compared. Therefore, increased sensitivity for language areas in our study relates to previously published results achieved with other task designs. Systematic comparisons of our fMRI results with other tasks or other modalities would be of great value and should be the focus of follow-up studies. Furthermore, no detailed data concerning subject performance were recorded. However, we designed our language task to be also easily solvable for impaired patients, and no patient showed high error rates during practice or monitoring in the scanner. Of course, it is possible that more difficult tasks would correlate with even more brain activity. Nevertheless, in favor of patient cooperation, we opted for relatively easy tasks. An additional point concerns exclusion of patients with extended morphologic brain abnormalities. Our results are not predictive for such a patient group.
Practical Application
Due to their relative simplicity, the proposed tasks can be applied to a wide range of patients/patients' states. For cognitively impaired patients and children, the task difficulty may be easily reduced by slowing down the language-processing requirements.
Conclusions
In summary, we developed a clinical language fMRI task that integrates various everyday language aspects. According to our experience with other patient groups (children, elderly, etc) not included in this study, the task can be easily applied to a wide range of patients. It provides robust localization of essential brain areas according to widely used statistical criteria. In addition, the data provide indications that an integration of semantic processing requirements might be beneficial for clinical language fMRI.
Footnotes
References
- 1. Petrella JR, Shah LM, Harris KM, et al. Preoperative functional MR imaging localization of language and motor areas: effect on therapeutic decision making in patients with potentially resectable brain tumors. Radiology 2006;240:793–802 [DOI] [PubMed] [Google Scholar]
- 2. Pelletier I, Sauerwein HC, Lepore F, et al. Non-invasive alternatives to the Wada test in the presurgical evaluation of language and memory functions in epilepsy patients. Epileptic Disord 2007;9:111–26 [DOI] [PubMed] [Google Scholar]
- 3. Loring DW, Meador KJ. Pre-surgical evaluation for epilepsy surgery. Saudi Med J 2000;21:609–16 [PubMed] [Google Scholar]
- 4. Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance: 1960. J Neurosurg 2007;106:1117–33 [DOI] [PubMed] [Google Scholar]
- 5. Szaflarski JP, Holland SK, Jacola LM, et al. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav 2008;12:74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weber B, Wellmer J, Schur S, et al. Presurgical language fMRI in patients with drug-resistant epilepsy: effects of task performance. Epilepsia 2006;47:880–86 [DOI] [PubMed] [Google Scholar]
- 7. Foki T, Gartus A, Geissler A, et al. Probing overtly spoken language at sentential level: a comprehensive high-field BOLD-fMRI protocol reflecting everyday language demands. Neuroimage 2008;39:1613–24. Epub 2007 Oct 26 [DOI] [PubMed] [Google Scholar]
- 8. Swanson SJ, Sabsevitz DS, Hammeke TA, et al. Functional magnetic resonance imaging of language in epilepsy. Neuropsychol Rev 2007;17:491–504 [DOI] [PubMed] [Google Scholar]
- 9. Gracco VL, Tremblay P, Pike B. Imaging speech production using fMRI. Neuroimage 2005;26:294–301 [DOI] [PubMed] [Google Scholar]
- 10. Edward V, Windischberger C, Cunnington R, et al. Quantification of fMRI artifact reduction by a novel plaster cast head holder. Hum Brain Mapp 2000;11:207–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brammer MJ. Head motion and its correction. In: Jezzard P, Matthews PM, Smith SM. eds. Functional MRI: An Introduction to Methods. Oxford, UK: Oxford University Press; 2001:243–50 [Google Scholar]
- 12. Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 2002;15:870–78 [DOI] [PubMed] [Google Scholar]
- 13. Walter B, Blecker C, Kirsch P, et al. MARINA: an easy to use tool for the creation of MAsks for Region of INterest Analyses. In: Proceedings of the 9th International Conference on Functional Mapping of the Human Brain, New York. June 19–22, 2003 [Google Scholar]
- 14. Fernandez G, de Greiff A, von Oertzen J, et al. Language mapping in less than 15 minutes: real-time functional MRI during routine clinical investigation. Neuroimage 2001;14:585–94 [DOI] [PubMed] [Google Scholar]
- 15. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273–89 [DOI] [PubMed] [Google Scholar]
- 16. Papathanassiou D, Etard O, Mellet E, et al. A common language network for comprehension and production: a contribution to the definition of language epicenters with PET. Neuroimage 2000;11:347–57 [DOI] [PubMed] [Google Scholar]
- 17. Humphries C, Binder JR, Medler DA, et al. Time course of semantic processes during sentence comprehension: an fMRI study. Neuroimage 2007;36:924–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haller S, Radue EW, Erb M, et al. Overt sentence production in event-related fMRI. Neuropsychologia 2005;43:807–14 [DOI] [PubMed] [Google Scholar]
- 19. Price CJ, McCrory E, Noppeney U, et al. How reading differs from object naming at the neuronal level. Neuroimage 2006;29:643–48 [DOI] [PubMed] [Google Scholar]
- 20. Price CJ, Wise RJ, Warburton EA, et al. Hearing and saying: the functional neuro-anatomy of auditory word processing. Brain 1996;119:919–31 [DOI] [PubMed] [Google Scholar]
- 21. Demonet JF, Chollet F, Ramsay S, et al. The anatomy of phonological and semantic processing in normal subjects. Brain 1992;115:1753–68 [DOI] [PubMed] [Google Scholar]
- 22. Petersen SE, Fox PT, Posner MI, et al. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature 1988;331:585–89 [DOI] [PubMed] [Google Scholar]
- 23. Deblaere K, Backes WH, Hofman P, et al. Developing a comprehensive presurgical functional MRI protocol for patients with intractable temporal lobe epilepsy: a pilot study. Neuroradiology 2002;44:667–73 [DOI] [PubMed] [Google Scholar]
- 24. Seghier ML, Lazeyras F, Pegna AJ, et al. Variability of fMRI activation during a phonological and semantic language task in healthy subjects. Hum Brain Mapp 2004;23:140–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakai KL, Hashimoto R, Homae F. Sentence processing in the cerebral cortex. Neurosci Res 2001;39:1–10 [DOI] [PubMed] [Google Scholar]
- 26. Amunts K, Schleicher A, Burgel U, et al. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 1999;412:319–41 [DOI] [PubMed] [Google Scholar]
- 27. Springer JA, Binder JR, Hammeke TA, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain 1999;122:2033–46 [DOI] [PubMed] [Google Scholar]
- 28. Vikingstad EM, George KP, Johnson AF, et al. Cortical language lateralization in right-handed normal subjects using functional magnetic resonance imaging. J Neurol Sci 2000;175:17–27 [DOI] [PubMed] [Google Scholar]
- 29. Palmer ED, Rosen HJ, Ojemann JG, et al. An event-related fMRI study of overt and covert word stem completion. Neuroimage 2001;14:182–93 [DOI] [PubMed] [Google Scholar]
- 30. Gaillard WD, Balsamo L, Xu B, et al. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology 2002;59:256–65 [DOI] [PubMed] [Google Scholar]
- 31. Forn C, Ventura-Campos N, Belenguer A, et al. A comparison of brain activation patterns during covert and overt paced auditory serial addition test tasks. Hum Brain Mapp 2008;29:644–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barch DM, Sabb FW, Carter CS, et al. Overt verbal responding during fMRI scanning: empirical investigations of problems and potential solutions. Neuroimage 1999;10:642–57 [DOI] [PubMed] [Google Scholar]
- 33. Shuster LI, Lemieux SK. An fMRI investigation of covertly and overtly produced mono- and multisyllabic words. Brain Lang 2005;93:20–31 [DOI] [PubMed] [Google Scholar]
- 34. Grabowski TJ, Bauer MD, Foreman D, et al. Adaptive pacing of visual stimulation for fMRI studies involving overt speech. Neuroimage 2006;29:1023–30 [DOI] [PubMed] [Google Scholar]
- 35. Huang J, Francis AP, Carr TH. Studying overt word reading and speech production with event-related fMRI: a method for detecting, assessing, and correcting articulation-induced signal changes and for measuring onset time and duration of articulation. Brain Lang 2008;104:10–23 [DOI] [PubMed] [Google Scholar]
- 36. Friston KJ. Experimental design and statistical parametric mapping. In: Frackowiak RS, Friston KJ, Frith CD, et al., eds. Human Brain Function. 2nd ed London: Elsevier Academic Press; 2004:599–632 [Google Scholar]
- 37. Rutten GJ, Ramsey NF, van Rijen PC, et al. Reproducibility of fMRI-determined language lateralization in individual subjects. Brain Lang 2002;80:421–37 [DOI] [PubMed] [Google Scholar]
- 38. Beisteiner R, Lanzenberger R, Novak K, et al. Improvement of presurgical patient evaluation by generation of functional magnetic resonance risk maps. Neurosci Lett 2000;290:13–16 [DOI] [PubMed] [Google Scholar]
- 39. Roessler K, Donat M, Lanzenberger R, et al. Evaluation of preoperative high magnetic field motor functional MRI (3 Tesla) in glioma patients by navigated electrocortical stimulation and postoperative outcome. J Neurol Neurosurg Psychiatry 2005;76:1152–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haberg A, Kvistad KA, Unsgard G, et al. Preoperative blood oxygen level-dependent functional magnetic resonance imaging in patients with primary brain tumors: clinical application and outcome. Neurosurgery 54:902–14, 2004, discussion 14–15 [DOI] [PubMed] [Google Scholar]
- 41. Aldenkamp AP, Boon PA, Deblaere K, et al. Usefulness of language and memory testing during intracarotid amobarbital testing: observations from an fMRI study. Acta Neurol Scand 2003;108:147–52 [DOI] [PubMed] [Google Scholar]
- 42. Binder JR, Swanson SJ, Hammeke TA, et al. A comparison of five fMRI protocols for mapping speech comprehension systems. Epilepsia 2008;49:1980–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci 2002;6:78–84 [DOI] [PubMed] [Google Scholar]
- 44. Thivard L, Hombrouck J, du Montcel ST, et al. Productive and perceptive language reorganization in temporal lobe epilepsy. Neuroimage 2005;24:841–51 [DOI] [PubMed] [Google Scholar]
- 45. Seghier ML. Laterality index in functional MRI: methodological issues. Magn Reson Imaging 2008;26:594–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wellmer J, Weber B, Weis S, et al. Strongly lateralized activation in language fMRI of atypical dominant patients: implications for presurgical work-up. Epilepsy Res 2008;80:67–76 [DOI] [PubMed] [Google Scholar]
- 47. Kurthen M, Helmstaedter C, Linke DB, et al. Interhemispheric dissociation of expressive and receptive language functions in patients with complex-partial seizures: an amobarbital study. Brain Lang 1992;43:694–712 [DOI] [PubMed] [Google Scholar]
- 48. Rutten GJ, Ramsey NF, van Rijen PC, et al. FMRI-determined language lateralization in patients with unilateral or mixed language dominance according to the Wada test. Neuroimage 2002;17:447–60 [DOI] [PubMed] [Google Scholar]
- 49. Stippich C, Rapps N, Dreyhaupt J, et al. Localizing and lateralizing language in patients with brain tumors: feasibility of routine preoperative functional MR imaging in 81 consecutive patients. Radiology 2007;243:828–36 [DOI] [PubMed] [Google Scholar]
- 50. Lehericy S, Cohen L, Bazin B, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology 2000;54:1625–33 [DOI] [PubMed] [Google Scholar]


