Abstract
BACKGROUND AND PURPOSE:
Metachromatic leukodystrophy (MLD) is a devastating demyelinating disease for which novel therapies are being tested. We hypothesized that MR imaging of brain lesion involvement in MLD could be quantified along a scale.
MATERIALS AND METHODS:
Thirty-four brain MR images in 28 patients with proved biochemical and genetic defects for MLD were reviewed: 10 patients with late infantile, 16 patients with juvenile, and 2 patients with adult MLD. All MR images were reviewed by experienced neuroradiologists and neurologists (2 readers in Germany, 2 readers in the United States) for global disease burden, as seen on the T2 and fluid-attenuated inversion recovery images. A visual scoring method was based on a point system (range, 0–34) derived from the location of white matter involvement and the presence of global atrophy, analogous to the scoring system developed for adrenoleukodystrophy. The readers were blinded to the neurologic findings.
RESULTS:
Thirty-three of 34 MR images showed confluent T2 hyperintensities of white matter. The inter-rater reliability coefficient was 0.988. Scores between readers were within 2 points of each other. Serial MR imaging studies in 6 patients showed significant progressive disease in 3 patients (initial score average, 4; mean follow-up, 24.3) and no change or 1 point progression in 3 patients (initial score average, 12; mean follow-up, 12.66). Projection fibers and the cerebellum tended to be involved only in advanced stages of disease.
CONCLUSIONS:
The MLD MR severity scoring method can be used to provide a measure of brain MR imaging involvement in MLD patients.
Metachromatic leukodystrophy (MLD) is an autosomal recessive inherited disorder in which defective desulfation of glycolipids results in demyelination within the central and peripheral nervous system. Brain involvement in MLD can be detected by MR imaging.1,2 To date, there is no adequate quantitative measure of MR imaging brain involvement in patients with MLD to correlate with ongoing diagnostic and therapeutic research. Volume measurements are impeded by the fact that standard clinical MR images are acquired in a 2D dataset with skip areas, and the measurements are complicated by the frequent presence of cerebral atrophy. A scoring system developed for X-linked adrenoleukodystrophy (X-ALD) has proved to be a useful tool in defining the natural history of ALD and monitoring disease response to therapies.3 Similar work needs to be done in regard to MLD. The purpose of our study was to adapt the previously developed ALD scoring system to reliably measure MR imaging brain involvement in patients with MLD. We hypothesized that the range of MR imaging of brain lesion involvement in MLD could be quantified along a scale.
Materials and Methods
Subjects
The patients in this study group were derived from a pool of families with MLD seen at 1 US and 3 European institutions. Thirty-four MR images in 28 patients with proved biochemical defects for MLD were reviewed. All patients had deficiencies of arylsulfatase A (ARSA) in plasma and elevated urine sulfatides or mutations in the ARSA gene, confirming the diagnosis of MLD.4 The patients were subdivided into 3 clinical subtypes according to age of symptom onset: late-infantile (onset at younger than 2 years), juvenile (onset between 2 and 16 years), and adult (onset at older than 16 years). MR imaging of the brain was performed on a variety of scanners around the world. All scanning included a minimum of sagittal T1-weighted (500–600/15–25 ms [TR/TE]), axial T2-weighted (2500–3500/20–30 ms [TR/TE]), and axial fluid-attenuated inversion recovery (FLAIR) images (900–10,000/80–150/1800–2200 ms [TR/TE/TI]). The average section thickness was 5 mm. Although all images were evaluated, the images used for scoring were the axial FLAIR and axial T2 pulse sequences.
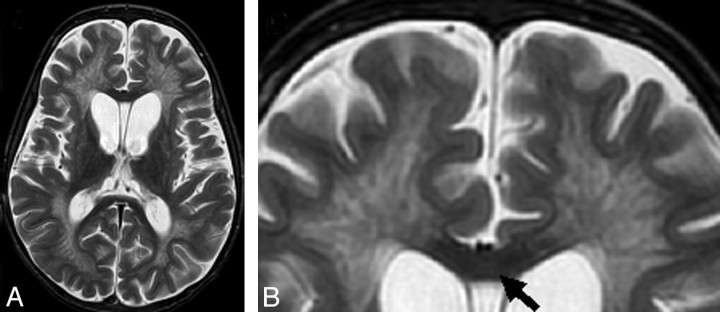
A severity score (0–34) was calculated for each MR image on the basis of a point system derived from the location and extent of involvement and the presence of central atrophy (Table 1). The MLD severity score point system was designed analogous to the well-established MR imaging scoring system for ALD,3 with several important exceptions: 1) There was no redundant scoring of the visual and auditory pathways, 2) local atrophy was not scored, and 3) signal-intensity hyperintensities were graded as faint (1 point) or dense (1 points) (Fig 1).
Table 1:
Brain MR imaging scoring system for MLD
| Brain Areas | Score* | Maximum per Area | ||
|---|---|---|---|---|
| Frontal WM | 6† | |||
| Periventricular | 0 | 1 | 2 | |
| Central | 0 | 1 | 2 | |
| U-fibers | 0 | 1 | 2 | |
| Parieto-occipital WM | 6† | |||
| Periventricular | 0 | 1 | 2 | |
| Central | 0 | 1 | 2 | |
| U-fibers | 0 | 1 | 2 | |
| Temporal WM | 6† | |||
| Periventricular | 0 | 1 | 2 | |
| Central | 0 | 1 | 2 | |
| U-fibers | 0 | 1 | 2 | |
| Corpus callosum | 4† | |||
| Genu | 0 | 1 | 2 | |
| Splenium | 0 | 1 | 2 | |
| Projection fibers | 6† | |||
| Internal capsule posterior limb | 0 | 1 | 2 | |
| Internal capsule anterior limb | 0 | 1 | 2 | |
| Midline pons | 0 | 1 | 2 | |
| Cerebral atrophy | 0 | 1 | 2 | 2† |
| Thalamus | 0 | 1 | 1† | |
| Basal ganglia | 0 | 1 | 1† | |
| Cerebellum | 2† | |||
| WM | 0 | 1 | ||
| Atrophy | 0 | 1 | ||
| Total | 34† | |||
Note:—MLD indicates metachromatic leukodystrophy; WM, white matter.
0 indicates normal; 1, faint hyperintensity; 2, dense hyperintensity.
XXX.
Fig 1.
Scoring details of the metachromatic leukodystrophy (MLD) MR imaging severity score. Differences between scoring faint (1 point) versus dense appearance (2 points) are shown. A, Periventricular and central involvement in the frontal white matter is categorized as faint (1 point, thin arrow), whereas myelination is preserved in the subcortical U-fibers (0 points, thick arrow). B, Periventricular and central involvement in the frontal white matter is categorized as dense (2 points, thin arrow), and there are areas that lack subcortical U-fiber myelination (2 points, thick arrow). C, Inner atrophy as measured in the third ventricle (arrow).
The scoring method was initially developed and tested on review of 10 MR images of patients with MLD in a German center (I.K.-M., W.G). Then 34 separate scans from the 4 centers were reviewed and scored by a neuroradiologist (D.J.L.) and a neurologist (F.E.) experienced in brain MR imaging observations in leukodystrophies. The correlation coefficient between scores of the 2 readers was used to determine inter-rater reliability. Differences of opinion were resolved by consensus for determining final scores. All institutions had institutional review board approval for retrospective evaluation of clinical brain MR images.
The major locations assessed were the supratentorial white matter, frontopontine corticospinal projection fibers, and deep gray nuclei. The supratentorial white matter was subcategorized into frontal, anterior temporal, and parieto-occipital regions. The posterior temporal white matter, defined as being posterior to the anterior margin of the midbrain, was considered to be within the parieto-occipital region, because early MLD involvement tends to be at the junction of these 3 lobes. Each supratentorial white matter region was further subdivided into periventricular, central, and subcortical white matter locations (Fig 1). The periventricular and subcortical white matter regions were defined as approximately equal in thickness to cortical gray matter, with the interposed parenchyma designated as central white matter. The projection fiber system was evaluated at the level of the internal capsule for the anterior and posterior limb separately (1 point for faint and 2 points for dense with respect to the surrounding tissue) and for the brain stem at the midline level of the pons, where hyperintensity was graded as 1 (faint) or 2 (dense). In addition to these categories, the basal ganglia and thalamus were also evaluated and scored. These T2 signal-intensity abnormalities in the basal ganglia and thalamus were decreased signal intensities.
Unlike ALD, focal atrophy was not assessed due to the diffuse nature of MLD brain involvement. A global atrophy measure distinguished between ventricular enlargement or inner widening (1 point) and inner and outer CSF space widening (2 points).
MR imaging severity scores were plotted with respect to the patients' ages at the time of the examinations. Patients with serial MR imaging studies were analyzed for changes with time.
Results
The age range of the late-infantile-aged patients was from 2 to 9.66 years (mean, 3.7 years), that of juvenile patients was from 3 to 27 years (mean, 8.88 years), and 2 adults were 34½ and 46 years of age (Table 2).
Table 2:
Subtypes and initial MR imaging severity scores of patients with MLD
| Patient No. | Phenotype* | Age at MRI | Deep Gray Nuclei | Projection Fibers | Cerebellum | Atrophy | MRI Severity Score |
|---|---|---|---|---|---|---|---|
| 1 | Late infantile | 1.92 | No | No | No | No | 1 |
| 2 | Late infantile | 2.08 | Yes | No | No | No | 17 |
| 3 | Late infantile | 2.5 | No | No | No | No | 4 |
| 4 | Late infantile | 2.5 | No | No | No | No | 4 |
| 5 | Late infantile | 2.5 | No | No | No | No | 4 |
| 6 | Late infantile | 3 | No | No | No | No | 6 |
| 7 | Late infantile | 2.08 | No | No | No | No | 0 |
| 8 | Late infantile | 3.66 | Yes | No | No | No | 21 |
| 9 | Late infantile | 7.16 | Yes | Yes | Yes | Yes | 30 |
| 10 | Late infantile | 9.6 | Yes | Yes | Yes | Yes | 32 |
| 11 | Juvenile | 4.84 | No | No | No | No | 12 |
| 12 | Juvenile | 4.84 | No | No | No | No | 12 |
| 13 | Juvenile | 8.08 | Yes | No | No | No | 13 |
| 14 | Juvenile | 10.25 | No | No | No | No | 3 |
| 15 | Juvenile | 3 | No | No | No | No | 2 |
| 16 | Juvenile | 4.17 | No | No | No | No | 6 |
| 17 | Juvenile | 4.17 | No | No | No | No | 10 |
| 18 | Juvenile | 5.66 | Yes | Yes | No | Yes | 26 |
| 19 | Juvenile | 6.25 | Yes | Yes | No | Yes | 27 |
| 20 | Juvenile | 7 | No | No | No | No | 18 |
| 21 | Juvenile | 7.75 | No | No | No | Yes | 19 |
| 22 | Juvenile | 8.25 | Yes | Yes | Yes | Yes | 30 |
| 23 | Juvenile | 9.4 | No | No | No | No | 12 |
| 24 | Juvenile | 13.08 | Yes | Yes | No | Yes | 26 |
| 25 | Juvenile | 18.42 | Yes | Yes | Yes | Yes | 28 |
| 26 | Juvenile | 27 | No | Yes | No | Yes | 24 |
| 27 | Adult | 34.5 | Yes | Yes | No | Yes | 22 |
| 28 | Adult | 46 | Yes | Yes | No | Yes | 26 |
Note:—MRI indicates MR imaging.
The individual phenotypes, age as well as the MR imaging severity score with its subscores at initial MR imaging are provided.
With the exception of 1 late-infantile-aged patient, all initial scans showed lesions consistent with MLD. Seventy-five percent of patients had confluent T2 hyperintensities in the central white matter. Of the 7 patients who showed no central white matter abnormalities on initial MR imaging, 3 had follow-up MR images, and all showed progression into the central white matter. The subcortical white matter tracts of the anterior temporal region were less frequently affected (involvement on 5 scans versus involvement of the frontal and parieto-occipital region on 17 and 15 scans, respectively).
Of the 27 patients with initial positive findings on scans, 25 had both frontal and parietal involvement. Of the 25 patients with both frontal and parietal involvement, 21 had involvement of both periventricular and central white matter in both locations and 12 had involvement of the subcortical white matter in addition to the periventricular and central white matter. Seven patients had only faint supratentorial white matter involvement on initial scans. Three of these 7 patients had serial examinations (identical triplets), and all showed progression to dense involvement. A tigroid pattern was seen in 13 of the 27 scans with positive findings; all had a score of ≥17.
Cerebellar involvement was seen in only 7 patients. All patients with cerebellar involvement had an MR imaging severity score of >20 points. There were 12 patients with projection fiber involvement. All of these patients had a severity score of ≥19 and showed involvement of the posterior limb of the internal capsule. Only 2 patients had involvement of the anterior limb in addition. There were 4 cases that, at an advanced stage, showed a decrease of signal intensity in the midline compared with adjacent regions of the corpus callosum (Fig 2).
Fig 2.
Midline of the corpus callosum in advanced stages of MLD. T2 lesion hyperintensities in the midline are reduced compared with the adjacent supratentorial white matter lesion signal intensity (arrow).
Serial brain scans were available in 6 patients, 3 late-infantile-aged patients and 3 juvenile patients. Serial MR imaging studies showed significant progressive disease in the 3 infantile patients (initial score average, 4; mean follow-up, 24.3; average follow-up interval, 2 years) and no change or 1 point progression in the 3 juvenile patients (initial score average, 12; mean follow-up, 12.66; average follow-up interval, 7 months). For each of these patients, only 1 follow-up image was available. The interval between the initial MR imaging and the follow-up study ranged from 5 months to 2 years (Fig 3).
Fig 3.
Correlation of age and MR imaging severity score in patients with MLD. The greatest variability in MR imaging lesion severity is seen in the first decade of life. Thereafter, most patients have a severity score of >20. Patients with serial scans have scores with connecting lines.
Inter-rater variability improved during development of the scoring. A first assessment of 10 patients in Germany (I.K.-M., W.G.) had a correlation coefficient of 0.955. The correlation coefficient during assessment of patients in our study (F.E., D.J.L.) had a correlation coefficient of 0.988. Areas of discrepancy (subcortical regions, thalamus) were resolved by consensus to arrive at a final score.
Discussion
Using international expert collaboration, we have developed an MLD MR severity scoring system, similar but not identical to the scoring system used in X-ALD. It is designed to be applicable to the standard MR imaging examination and reproducible by any neuroradiologist or other individual familiar with neuroanatomy and MR imaging of the pediatric brain. It should be more useful than a descriptive summary of abnormal brain involvement in these patients. It was developed to address the need for quantitative biomarkers of disease severity that can be used to assess the efficacy of novel therapeutics.
On the basis of our imaging results, we found it useful to categorize positive brain MR scores into 3 groups: mild disease (score, 1–6), moderate disease (score, 7–15), and severe disease (score, 16–34). On initial scans, 8 patients had mild disease (range, 1.92–10.25 years; mean, 3.73 years), 5 patients had moderate disease (range, 4.17–9.4 years; mean, 6.27 years), and 14 patients had severe disease (range, 2.08–46 years; mean, 13.52 years). Three of the patients with follow-up MR images moved from mild to severe disease within 2 years. It is yet to be established whether this MR imaging severity scale correlates with clinical disease severity, and prospective comparisons with existing clinical scales may help address this question.5
We found that MLD has a fairly characteristic pattern that does not appear to differ among the late infantile, juvenile, and adult-onset groups. A sheet-like area of abnormal T2 signal-intensity hyperintensity initially envelops the frontal and parietal periventricular and central white matter regions, faint in mild disease and denser in moderate-to-severe disease. As severe disease develops, the sheet of white matter signal-intensity abnormality also involves the inner half of the subcortical white matter and a tigroid pattern then emerges. The corpus callosum is involved in mild disease, but not as prominently as in ALD or globoid cell leukodystrophy (GLD). Projection fiber involvement, cerebellar white matter involvement, and basal ganglia/thalamic involvement are often present in severe disease.
The tigroid pattern, a pattern of radiating stripes with bands of normal signal intensity within the sheet-like white matter abnormality, is quite characteristic of severe disease. In a comparison of in vivo and postmortem MR imaging with histopathologic findings, van der Voorn et al6 showed that radial stripes were related to relative sparing of myelin in the perivenular regions but also in lipid-containing glial cells. These regions of myelin sparing are also seen in other neurodegenerative processes, such as other lysosomal disorders and Pelizaeus-Merzbacher disease. The occurrence of spared myelin may provide important insight into the pathophysiology of MLD. In our series, 13 of 17 patients with scores of >15 had this pattern, whereas no patients with scores of <15 had this pattern, further supporting the concept that the tigroid pattern is indicative of advanced disease. It was seen as early as 2 years of age. Several of these patients also had a tigroid pattern in the corpus callosum. Two of the 13 patients with a tigroid pattern demonstrated a cystic transformation.
Involvement of the corpus callosum was seen early, though it was not as striking as that seen in ALD or GLD. In our study population, 4 patients with advanced-stage disease showed reduction in T2 lesion hyperintensity within the midline of the corpus callosum compared with the remaining supratentorial white matter (Fig 2). This is different from ALD, where this sparing is not seen except in the context of thinning due to atrophy. Although in our 4 patients with MLD, central atrophy was present, the corpus callosum did not appear excessively thin to explain the signal-intensity change. Recently, it was reported that patients with the onset of multiple sclerosis before puberty have a reduction in the number of T2 bright lesions on follow-up scans.7 Perhaps developmental changes account for the unusual findings. Alternative explanations include remyelination or a deposition of by-products secondary to the disease.
Projection fiber and cerebellar white matter involvement were present only in severe disease. Similar to ALD, the projection fiber involvement was primarily in older patients (Table 2). However, the projection fiber involvement was not nearly as striking as that seen in both ALD8 and GLD.9 We did not see the sharply delineated tract involvement into the brain stem or the supratentorial white matter that is often seen in ALD and GLD. Additionally, cerebellar involvement was not a prominent feature of early-onset disease as seen in GLD.9
Decreased signal intensity in the basal ganglia and/or thalamus was seen on T2-weighted images in patients with moderate disease (2 of 6 patients) and severe disease (14 of 17 patients). This finding is also frequently seen in advanced stages of other leukodystrophies and probably is the result of abnormal accumulation of metal or other breakdown products in the brain.
In contrast to ALD, the outer subarachnoid spaces were less enlarged in MLD, even in severe brain disease. Perhaps this is due to sparing of the outer half of the subcortical white matter. During the development of the scoring system, local atrophy scoring was very difficult and hence was eliminated. Scoring global atrophy by inner widening or inner and outer widening made for a more reliable score than the method used in ALD.
A major difference to the ALD scoring system is the introduction of the terms “faint” versus “dense” lesions (Fig 1). This classification aims to capture the differences between early inhomogeneous poorly circumscribed lesions and the more advanced homogeneous lesion, which has a well-defined margin.
Another difference between the ALD and MLD scoring systems is that the auditory and visual pathways were not scored for MLD. Although visual manifestations occur early and auditory manifestations, late in MLD, they appear to be due to the sheet-like supratentorial white matter signal-intensity hyperintensity rather than selective tract involvement. MLD did not involve the auditory pathway in the brain stem in any patient in this series. To avoid redundancy in the scoring system and improve future inter-rater reliability, we chose not to score these pathways in patients with MLD.
One limitation of this study is that there are not enough data points to adequately judge the natural MR imaging history of MLD. Of the 6 patients with serial scans, 3 were late-infantile-onset identical triplets, who advanced from mild to severe disease in a 2-year period; 3 were juvenile (2 of them siblings), who had moderate disease without significant change during 5 months (1 sibling was stable, the other became 1 point worse); and the other patient had moderate disease, which progressed only 1 point during 7 months.
Limitations of the scoring system are mainly in the evaluation of mild disease in infants, in which the terminal myelination zones seen in normal development overlap early faint pathologic lesions. In addition, correlation of the MR imaging scoring system clinical rating scales may be hampered by spinal cord and peripheral nerve involvement that may equally contribute to disability10–12 and will not be reflected in a brain-only scoring system. However, the severity score will be the necessary basis for assessing potentially more sensitive advanced MR imaging techniques in MLD, such as diffusion-weighted imaging and MR spectroscopic measures.13
Conclusions
We hypothesized that the range of MR imaging of brain lesion involvement would allow quantification along a scale. To this end, we adapted a previously developed ALD scoring system to describe the spectrum of neuroanatomic lesion involvement and the presence or absence of brain atrophy in MLD. We demonstrated that the MLD MR imaging severity scoring method can be used to provide a measure of brain MR imaging involvement across different age groups and subtypes of MLD. When used in conjunction with clinical parameters, the MLD MR imaging severity score may help to better define the natural history of MLD and may be useful in monitoring the response to developing therapies.
Acknowledgments
We thank Ourania Giannikopoulos for her administrative assistance.
Footnotes
This work was supported by grant the National Institutes of Health grant 1K08NS52550.
References
- 1. Kim TS, Kim IO, Kim WS, et al. MR of childhood metachromatic leukodystrophy. AJNR Am J Neuroradiol 1997;18:733–38 [PMC free article] [PubMed] [Google Scholar]
- 2. Faerber EN, Melvin J, Smergel EM. MRI appearances of metachromatic leukodystrophy. Pediatr Radiol 1999;29:669–72 [DOI] [PubMed] [Google Scholar]
- 3. Loes DJ, Hite S, Moser H, et al. Adrenoleukodystrophy: a scoring method for brain MR observations. AJNR Am J Neuroradiol 1994;15:1761–66 [PMC free article] [PubMed] [Google Scholar]
- 4. von Figura K, Giselmann V, Jaeken J. Metachromatic leukodystrophy. In: Scriver CR, Sly WS, Childs A. eds. The Metabolic and Molecular Basis of Inherited Disease. New York: McGraw Hill; 2000:3695–3724 [Google Scholar]
- 5. Biffi A, Cesani M, Fumagalli F, et al. Metachromatic leukodystrophy: mutation analysis provides further evidence of genotype-phenotype correlation. Clin Genet 2008;74:349–57. Epub 2008 Sep 11 [DOI] [PubMed] [Google Scholar]
- 6. van der Voorn JP, Pouwels PJ, Kamphort W, et al. Histopathologic correlates of radial stripes on MR images in lysosomal storage disorders. AJNR Am J Neuroradiol 2005;26:442–46 [PMC free article] [PubMed] [Google Scholar]
- 7. Chabas D, Castillo-Trivino T, Mowry EM, et al. Vanishing MS T2-bright lesions before puberty: a distinct MRI phenotype? Neurology 2008;71;1090–03 [DOI] [PubMed] [Google Scholar]
- 8. Eichler F, Mahmood A, Loes D, et al. Magnetic resonance imaging detection of lesion progression in adult patients with X-linked adrenoleukodystrophy. Arch Neurol 2007;64:659–64 [DOI] [PubMed] [Google Scholar]
- 9. Loes DJ, Peters C, Krivit W. Globoid cell leukodystrophy: distinguishing early-onset from late-onset disease using a brain MR imaging scoring method. AJNR Am J Neuroradiol 1999;20:316–23 [PMC free article] [PubMed] [Google Scholar]
- 10. Toldo I, Carollo C, Battistella PA, et al. Spinal cord and cauda equina MRI findings in metachromatic leukodystrophy: case report. Neuroradiology, 2005;47:572–5. Epub 2005 Jul 15 [DOI] [PubMed] [Google Scholar]
- 11. Haberlandt E, Scholl-Burgi S, Meuberger J, et al. Peripheral neuropathy as the sole initial finding in three children with infantile metachromatic leukodystrophy. Eur J Paediatr Neurol 2009;13257–60. Epub 2008 Jun 20 [DOI] [PubMed] [Google Scholar]
- 12. Comabella M, Wayne JJ, Raquer N, et al. Late-onset metachromatic leukodystrophy clinically presenting as isolated peripheral neuropathy: compound heterozygosity for the IVS2+1G–>A mutation and a newly identified missense mutation (Thr408Ile) in a Spanish family. Ann Neurol 2001;50:108–12 [PubMed] [Google Scholar]
- 13. Kruse B, Hanefeld F, Christen HJ, et al. Alterations of brain metabolites in metachromatic leukodystrophy as detected by localized proton magnetic resonance spectroscopy in vivo. J Neurol 1993;241:68–74 [DOI] [PubMed] [Google Scholar]