Abstract
BACKGROUND AND PURPOSE:
The posterior circulation Acute Stroke Prognosis Early CT Score (pc-ASPECTS) is a 10-point grading system to quantify ischemic changes in the posterior circulation. We analyzed whether pc-ASPECTS on CT angiography (CTA) source images (CTASI) predicted the final infarct extent and hemorrhagic transformation (HT) rate in patients with basilar artery occlusion.
MATERIALS AND METHODS:
A pc-ASPECTS score of 10 indicates absence of visible ischemic changes in the posterior circulation, and pc-ASPECTS score of 0 indicates ischemic changes in the midbrain, pons, and bilateral thalami, posterior circulation territories, and cerebellar hemispheres. We retrospectively studied patients with basilar artery occlusion on CTA within 24 hours from symptom onset. We applied pc-ASPECTS to noncontrast CT (NCCT), CTASI, and follow-up images by 3-reader-consensus and assessed HT on follow-up images. We calculated Spearman correlation coefficients and performed linear regression analysis. Final infarct extent and HT rates were compared across dichotomized CTASI pc-ASPECTS groups (≥ 8 vs < 8).
RESULTS:
Among 43 patients, median (range) onset to CTA time was 5.0 hours (range, 0.7–24 hours). Pc-ASPECTS on CTASI (r = 0.75; P < .001) but not NCCT (r = 0.29; P = .063) correlated with pc-ASPECTS on follow-up scans. Linear regression demonstrated a significant positive relationship between pc-ASPECTS on CTASI and follow-up scans (R2 = 0.58; P < 01). Median follow-up pc-ASPECTS was lower in patients with a CTASI pc-ASPECTS < 8 compared with patients with a CTASI pc-ASPECTS of 8 or more, respectively (P < .001). HT rates were 27.3% vs 9.5%, respectively (P = .24). None of 8 patients without thrombolysis had HT on follow-up scans.
CONCLUSIONS:
The extent of hypoattenuation on CTASI predicts the final infarct extent in patients with basilar artery occlusion.
Without reperfusion therapy, almost 80% of patients with acute basilar artery occlusion either die or are severely disabled.1–3 Recanalization of the basilar artery is essential for improved functional outcome.1,3–5 Consequently, intravenous (IV) and/or intra-arterial (IA) thrombolysis are frequently initiated.1,3,5 Factors predicting the final infarct size and the risk for hemorrhagic transformation (HT) have not been reported. In particular, an association of the extent of early ischemic changes (EIC) with the final infarct size and HT rate has not been demonstrated.
Among patients with ischemic stroke in the anterior circulation, the extent of EIC on baseline noncontrast CT (NCCT) correlates with the infarct size on follow-up scans.6 Moreover, the extent of EIC on baseline NCCT predicts the HT risk with and without thrombolysis.6–9 Compared with NCCT, CT angiography (CTA) source images (CTASI) have improved the prediction of final infarct size and clinical outcome in anterior circulation stroke.10–12 The degree of hypoattenuation on CTASI is a risk factor for HT in patients receiving IA reperfusion therapies.13
We have recently developed a 10-point grading system, the posterior circulation Acute Stroke Prognosis Early CT Score (pc-ASPECTS), to quantify ischemic changes in the posterior circulation.14 We have shown that CTASI improve the overall sensitivity to detect ischemic changes in the posterior circulation compared with NCCT. Furthermore, the extent of hypoattenuation on CTASI identified patients with basilar artery occlusion who were unlikely to achieve an independent functional outcome despite recanalization of the basilar artery.14
We sought to determine whether the extent of hypoattenuation on CTASI, quantified with the pc-ASPECTS score, predicted the final infarct extent and HT rate in patients with basilar artery occlusion.
Materials and Methods
Patients
Participants in this study are a subgroup of patients with basilar artery occlusion analyzed in the pc-ASPECTS study.14 The pc-ASPECTS study retrospectively (May 2001 to August 2006) analyzed patients with acute symptomatic basilar artery occlusion who presented to Foothills Medical Centre in Calgary or Dresden University Stroke Center, had a NCCT-to-CTA time of 2 hours or less, and underwent CTA within 24 hours from symptom onset. Diagnosis of basilar artery occlusion was based on acute onset of symptoms referable to the posterior circulation and CTA showing complete occlusion at any level of the basilar artery. For the current study, we analyzed patients who had NCCT, CTASI, and follow-up images by MR imaging or NCCT performed between 1 and 7 days after initial CTA available for review. Functional outcome was recorded prospectively at 3 months in a stroke follow-up clinic in Calgary. Missing 3-month outcomes were imputed from the discharge modified Rankin scale (mRS) score by use of the last-score-carried-forward principle. For patients with basilar artery occlusion in Dresden, 3-month functional outcomes were derived from telephone interview with the patients or their relatives. The local institutional ethics committee in Calgary approved this study. Patients with basilar artery occlusion in Dresden were treated under an institutional protocol approved by the local ethics committee.
Imaging
We performed standard nonhelical NCCT on a multisection CT scanner (GE Healthcare, Milwaukee, Wis, or Siemens, Erlangen, Germany) using 120 kV, 170 mAs with 5-mm section thickness. Coverage was from the skull base to the vertex with continuous axial sections parallel to the orbitomeatal line. NCCT was followed by CTA with a helical scan technique on the same scanner. Minimal coverage was from the foramen magnum to the centrum semiovale. Source images were reconstructed at 1.25- to 4.0-mm thickness in axial planes at half-thickness intervals. Follow-up NCCT or MR imaging was performed as indicated by the treating stroke neurologist. MR imaging routinely included diffusion-weighted imaging (DWI), gradient-recalled echo, T1, T2, and fluid-attenuated inversion recovery sequences adopting routinely used acquisition parameters. Because of the study design, different MR imaging systems were used during the study period (3 MR imaging systems [1.5T or 3T] in Calgary and 3 MR imaging systems [1T or 1.5T] in Dresden).
Image Analysis
The posterior circulation pc-ASPECTS is a semiquantitative grading system to quantify ischemic changes in the posterior circulation.14 From 10 points, 1 point each is subtracted for EIC on NCCT or hypoattenuation on CTASI in the left or right thalamus, cerebellar hemisphere, or posterior cerebral artery territory, respectively, and 2 points each for EIC (NCCT) or hypoattenuation (CTASI) in any part of the midbrain or pons. A pc-ASPECTS score of 10 indicates absence of visible posterior circulation ischemia; a score of 0 indicates visible ischemic changes in all pc-ASPECTS territories.
We independently applied pc-ASPECTS to NCCT, CTASI, and follow-up scans using a 3-reader consensus (2/3 reader consensus required). Images were reviewed on a high-resolution monitor with a DICOM viewer (OsiriX). Reading panels consisted of 1 experienced stroke neurologist (A.M.D. or S.B.C.) and 2 stroke fellows (P.N.S., I.D., or V.P.) with at least 1 year experience in reading NCCT and CTASI studies. Scans were anonymized and reviewed in a randomized order with at least 2 weeks' time between reading different imaging modalities. Raters were blinded to clinical information except basilar artery occlusion on CTA. NCCT images were evaluated for focal hypoattenuation or loss of gray-white differentiation. On CTASI, regions of relatively diminished contrast enhancement were scored as abnormal. Thin, raw CTA images and axial reformats with 1.25-, 2.5-, or 4.0-mm thickness were available for review. On follow-up NCCT, we applied pc-ASPECTS to regions of subacute brain infarction; on follow-up MR imaging, we scored hyperintense regions on DWI sequences.15 If patients had both follow-up NCCT and MR imaging, we used the lower pc-ASPECTS score for analysis.
Hemorrhagic transformation (HT) on follow-up images was classified as parenchymal hematoma (PH) or hemorrhagic infarction (HI) according to European Cooperative Acute Stroke Study criteria.16 These criteria have been successfully applied to assess PH and HI on CT and MR imaging.17 In patients with basilar artery occlusion who had digital subtraction angiography completed within 24 hours from symptom onset, we rated perfusion of the entire basilar artery with delayed flow or full perfusion with normal basilar artery flow as stated in the neuroradiology report (according to Thrombolysis In Myocardial Infarction trial 2–3 flow grades) as recanalization.18
Statistical Analysis
Data are reported with use of standard descriptive statistics. We calculated the sensitivity and specificity of NCCT and CTASI to detect subsequent infarction in each individual pc-ASPECTS territory where follow-up images represented the criterion standard. We performed Spearman rank correlation and simple linear regression analysis to assess the association of pc-ASPECTS on NCCT and CTASI with pc-ASPECTS on follow-up images. Median pc-ASPECTS values on baseline NCCT and CTASI were compared for trichotomized follow-up pc-ASPECTS categories (0–3, 4–7, and 8–10). We compared the pc-ASPECTS on follow-up images and the HT rates across dichotomized CTASI pc-ASPECTS groups (categorized as ≥ 8 vs < 8). This threshold has previously been shown to discriminate patients with basilar artery occlusion who have a favorable outcome from those who have an unfavorable functional outcome.14
Results
Patients
Of 46 patients with acute symptomatic basilar artery occlusion in the pc-ASPECTS study, no follow-up imaging was performed in 3 patients because of early fatal clinical course. Therefore, our study reports 43 patients. Overall, 29 patients (67%) were men, median age was 67 years (range, 40–90 years), baseline National Institutes of Health Stroke Scale (NIHSS) score was 22 (range, 0–40), baseline Glasgow Coma Scale (GCS) score was 9 (range, 3–15), and onset-to-CTA time was 5 hours (range, 0.7–24 hours).
We treated 6 patients with IV alteplase (rtPA); 7 patients with thrombolytic and/or mechanical IA therapy; and 22 patients with various combinations of IV (rtPA [n = 11] or abciximab [n = 11]) and IA therapies, respectively. The overall median time from symptom onset to initiation of thrombolysis was 4.7 hours (range, 3.2–7.6 hours). Recanalization of the basilar artery was achieved in 21 of 30 patients who had diagnostic and/or therapeutic angiography completed within 24 hours from symptom onset. We achieved no recanalization of the basilar artery within 24 hours from symptom onset in 9 patients. Acute recanalization was not assessed in 13 patients.
At 3 months, 13 patients (30%) had a favorable functional outcome (mRS scores 0–3), 12 patients (28%) had an unfavorable functional outcome (mRS scores 4, 5), and 18 patients (42%) had died. Eight (38%) of 21 patients with recanalization compared with 2 (22%) of 9 patients without recanalization and 3 (23%) of 13 patients in whom the acute recanalization status was not assessed had a favorable functional outcome (Fisher exact test, P = .48).
Final Infarct Extent
Twenty-two patients underwent follow-up NCCT, 10 patients had follow-up MR imaging, and 11 patients underwent both. The overall median (interquartile ratio) pc-ASPECTS on follow-up images was 5.3–7 The median pc-ASPECTS on follow-up MR imaging was lower compared with follow-up NCCT (6[3–6] vs 7[4–9]; P = .039). In patients who had both imaging modalities as follow-up, MR imaging revealed more ischemic changes (lower pc-ASPECTS) than follow-up NCCT in 9 patients, including 2 patients who had a follow-up NCCT score of 10 (ie, no detection of ischemic changes).
Association of Baseline Imaging with Final Infarct Extent
As seen in Table 1, the overall sensitivity to identify subsequent infarction in each individual pc-ASPECTS location was improved with CTASI compared with NCCT. Specificities were similar for both modalities. Regarding individual pc-ASPECTS regions, CTASI improved the sensitivity to identify subsequent infarction in the brain stem (pons and midbrain) and thalami, whereas the sensitivity to identify subsequent infarction in the cerebellum and posterior cerebral artery territory was similar for both modalities.
Table 1:
Sensitivity and specificity of CTASI compared with NCCT to identify subsequent infarction on follow-up images in individual pc-ASPECTS regions
| Pc-ASPECTS Region | CTASI |
NCCT |
||
|---|---|---|---|---|
| Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | |
| All pc-ASPECTS regions | 35 (29–43)* | 98 (95–99) | 18 (13–25) | 94 (89–96) |
| Categorization by recanalization status | ||||
| Recanalization ≤ 24 hours | 34 (25–44) | 96 (90–99) | 18 (12–28) | 93 (86–97) |
| No recanalization ≤ 24 hours | 58 (39–74)* | 100 (91–100) | 15 (6–33) | 86 (76–93) |
| Recanalization status not assessed | 25 (14–40) | 99 (91–100) | 21 (11–35) | 91 (84–95) |
| Individual regions | ||||
| Midbrain | 59 (41–76) | 96 (85–99) | 26 (14–45) | 98 (88–100) |
| Pons | 38 (23–56)* | 96 (84–99) | 6 (1–22) | 98 (87–100) |
| Thalamus | 37 (21–56) | 98 (88–100) | 17 (6–35) | 88 (75–95) |
| Cerebellum | 32 (20–47) | 100 (86–100) | 26 (15–41) | 100 (85–100) |
| PCA | 17 (7–35) | 100 (90–100) | 18 (7–35) | 87 (73–95) |
Note:—Pc-ASPECTS indicates posterior circulation Acute Stroke Prognosis Early CT Score; CTASI, CT angiography source images; NCCT; noncontrast CT; PCA, posterior cerebral artery.
Indicates results where 95% confidence intervals for CTASI and NCCT do not overlap.
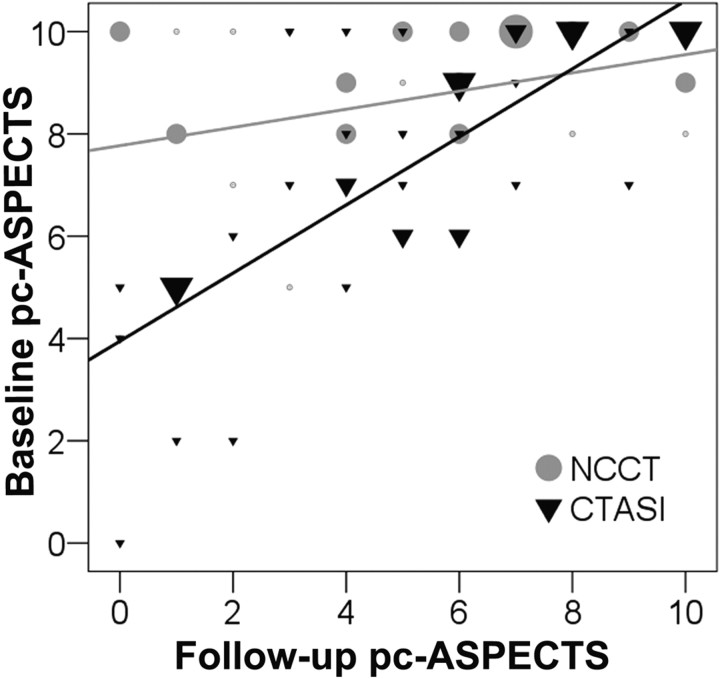
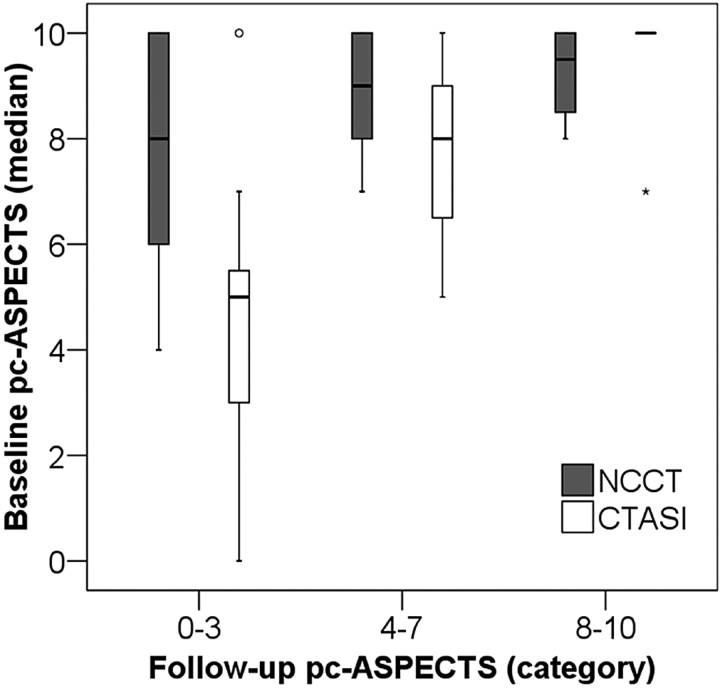
In univariable analysis, pc-ASPECTS on CTASI (Spearman r = 0.75; P < .001) but not NCCT (Spearman r = 0.29; P = .063) correlated with pc-ASPECTS on follow-up images. Linear regression demonstrated a significant positive relationship between pc-ASPECTS scores on CTASI and follow-up scans (R2 = 0.58, P < 01; slope of regression line = 0.68; Fig 1). The association of pc-ASPECTS scores on NCCT and follow-up scans was less robust (R2 = 0.13; slope of regression line = 0.18; P = .02). Figure 2 summarizes the differences between pc-ASPECTS scores on CTASI and NCCT at different follow-up pc-ASPECTS categories. The median pc-ASPECTS on CTASI was lower and closer to the midpoint of the follow-up pc-ASPECTS category in patients with large (follow-up pc-ASPECTS 0–3; P = .014) and moderate-sized final infarctions (follow-up pc-ASPECTS 4–7; P = .048) but not in patients with little or no visible ischemic changes on follow-up images (follow-up pc-ASPECTS 8–10; P = .41).
Fig 1.
Scatterplot of pc-ASPECTS scores on CTASI and NCCT compared with the pc-ASPECTS score on follow-up images. Larger symbols represent a larger number of patients at each data point. The estimated lines are derived from simple linear regression equations. CTASI (R2 = 0.58, p < 0.001; slope = 0.68) results in lower variance around the point estimate of the slope of the line compared with NCCT. (R2 = 0.13, p = 0.02; slope = 0.18).
Fig 2.
Comparison of median baseline pc-ASPECTS scores on NCCT and CTASI according to categorized follow-up pc-ASPECTS scores (box-whisker plot). Follow-up pc-ASPECTS category 0 to 3 indicates large, 4 to 7 medium-sized, and 8 to 10 small final infarct size.
We observed a nonsignificant trend toward higher percentages of patients with lower follow-up pc-ASPECTS scores compared with the pc-ASPECTS score on CTASI (ie, infarct extension) in patients who were recanalized within 24 hours from symptom onset compared with patients who did not undergo recanalization within 24 hours from symptom onset and patients in whom the acute recanalization status was not assessed (Table 2). This trend was similar for individual pc-ASPECTS regions (Table 2). Three of 11 patients with a CTASI pc-ASPECTS score of less than 8 had no final infarct extension with recanalization. In a consistent manner, the improved sensitivity of CTASI compared with NCCT was most prominent in patients who did not recanalize within 24 hours from symptom onset compared with patients with recanalization and patients with unknown recanalization status (Table 1).
Table 2:
Percentages of patients with infarct extension (ie, lower pc-ASPECTS score on follow-up images compared with the pc-ASPECTS score on CTASI) categorized by the recanalization status
| Parameter | Recanalization ≤ 24 Hours |
P | ||
|---|---|---|---|---|
| No | Yes | Not Assessed | ||
| Number of patients, n | 9 | 21 | 13 | − |
| Patients with infarct extension, n (%) | 6 (67) | 18 (86) | 10 (77) | .43 |
| Infarct extension in individual pc-ASPECTS regions* | ||||
| Pons, n (%) | 2 (22) | 7 (33) | 4 (21) | .91 |
| Midbrain, n (%) | 0 (0) | 6 (29) | 3 (23) | .29 |
| Thalamus, n/N (%) | 2/18 (11) | 10/42 | 7/26 (27) | .47 |
| PCA, n/N (%) | 3/18 (17) | 16/42 (38) | 8/26 (31) | .29 |
| Cerebellum, n/N (%) | 3/18 (17) | 23/42 (55) | 7/26 (27) | .008 |
Infarct extension in individual pc-ASPECTS regions indicates infarction on follow-up images that was not diagnosed on CTASI.
Twenty-one patients had a baseline CTASI pc-ASPECTS of 8 or more, and 22 patients had a baseline CTASI pc-ASPECTS of less than 8, respectively. As seen in Table 3, the final infarct extent was larger in patients with CTASI pc-ASPECTS of less than 8 compared with patients who had CTASI pc-ASPECTS of 8 or more, respectively (P < .001).
Table 3:
Pc-ASPECTS on follow-up images and HT rates for categorized CTASI pc-ASPECTS groups
| Outcome | CTASI pc-ASPECTS |
Risk Ratio (95% CI) | P value | |
|---|---|---|---|---|
| 8–10 | 0–7 | |||
| All patients, N | 21 | 22 | ||
| F/U pc-ASPECTS score, median (range) | 7 (3–10) | 3.5 (0–9) | − | < .001 |
| HT, % (n) | 9.5 (2) | 27.3 (6) | 0.4 (0.1–1.5) | .24 |
| PH, % (n) | 4.8 (1) | 18.2 (4) | 0.3 (0.03–2.2) | .34 |
| HI, % (n) | 4.8 (1) | 9.1 (2) | 0.5 (0.1–5.4) | 1.00 |
| Patients with thrombolysis, N | 16 | 19 | ||
| F/U pc-ASPECTS score, median (range) | 7 (3–10) | 3 (0–7) | − | < .001 |
| HT, % (n) | 12.5 (2) | 31.6 (6) | 0.4 (0.1–1.7) | .24 |
| PH, % (n) | 6.3 (1) | 21.1 (4) | 0.3 (0.04–2.4) | .35 |
| HI, % (n) | 6.3 (1) | 10.6 (2) | 0.6 (0.1–6.0) | 1.00 |
Note:—F/U denotes follow-up; HT, hemorrhagic transformation; PH, parenchymal hematoma; HI, hemorrhagic infarction.
Hemorrhagic Transformation
Overall, 8 (18.6%) of 43 patients had HT on follow-up images, with PH in 5 patients (11.6%) and HI in 3 patients (7.0%). Clinical and imaging characteristics associated with HT in univariable analysis are summarized in Table 4. Baseline clinical stroke severity (NIHSS score and GCS score), IV thrombolysis and combined IV-IA therapy, the final infarct extent (follow-up pc-ASPECTS), and the pc-ASPECTS value on CTASI were associated with HT (the latter with borderline statistical significance). The same trend, however not statistically significant, was notable for PH on follow-up images (data not shown).
Table 4:
Clinical and imaging characteristics associated with hemorrhagic transformation
| Characteristic | HT on Follow-up Image |
P value | |
|---|---|---|---|
| Yes | No | ||
| Number, N | 8 | 35 | |
| Age (yr), median (range) | 61 (42–78) | 69 (40–90) | .25 |
| History of diabetes, n (%) | 2 (25) | 7 (20) | 1.00 |
| Baseline NIHSS score, median (range) | 31 (11–40) | 18 (0–40) | .005* |
| GCS, median (range) | 4 (3–15) | 11 (3–15) | .012* |
| Onset-to-CTA (hrs), median (range) | 6.2 (1–24) | 4.6 (0.7–24) | .85 |
| Any thrombolysis, n (%) | 8 (100) | 27 (77) | .32 |
| IV lysis, n (%) | 8 (100) | 20 (57) | .036* |
| IA therapy, n (%) | 7 (88) | 22 (63) | .24 |
| Combined IV/IA therapy, n (%) | 7 (88) | 15 (43) | .046* |
| Onset-to-treatment (hrs), median (range) | 5.5 (1.9–24) | 4.2 (1.9–18.2) | .21 |
| Recanalization ≤ 24 hrs, n/N (%) | 4/7 (57) | 17/23 (74) | .33 |
| Pc-ASPECTS score on NCCT, median (range) | 8 (5–10) | 9 (5–10) | .11 |
| Pc-ASPECTS score on CTASI, median (range) | 5 (4–10) | 8 (0–10) | .051** |
| Pc-ASPECTS score on F/U image, median (range) | 1.5 (0–7) | 6 (0–10) | .015* |
Note:—NIHSS indicates National Institutes of Health Stroke Scale score; GCS, Glasgow Coma Scale score; CTA, CT angiography; IV, intravenous; IA, intra-arterial.
Indicates results that are statistically significant.
Indicates results with borderline statistical significance.
We observed a nonsignificant trend toward a lower HT rate, particularly a lower PH rate, in patients with CTASI pc-ASPECTS scores of 8 or more compared with patients with CTASI pc-ASPECTS scores of less than 8, respectively (Table 3; Fig 3). Patients who did not undergo recanalization therapy did not show HT on follow-up images.
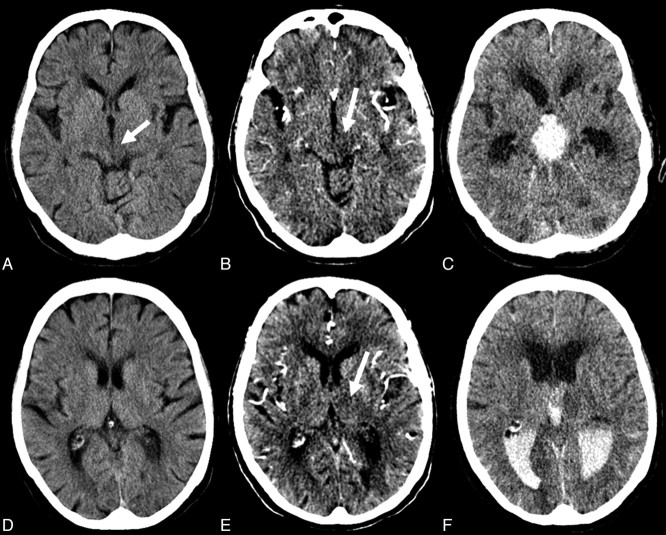
Fig 3.
The pc-ASPECTS score on NCCT (A,D) was rated as 8 (early ischemic changes left mesencephalon; arrow). CTASI (B,E) demonstrate additional hypoattenuation in the left thalamus (arrows). The pc-ASPECTS score was rated as 7. On follow-up NCCT (C,F), the patient had PH with intraventricular hemorrhage. The follow-up pc-ASPECTS score was rated as 7 (mesencephalon and left thalamus).
Subgroup of Patients Who Underwent Thromboembolization
Among 35 patients who received IV and/or IA recanalizing therapies, the final infarct extent was larger in patients with a CTASI pc-ASPECTS score of less than 8 compared with patients with a score of 8 or more, respectively (P < .001; Table 1). Patients with a CTASI pc-ASPECTS score of less than 8 had HT in 32.6% (6/19) compared with 12.5% (2/16) of patients with a CTASI pc-ASPECTS score of 8 or more. PH rates were 21.1% (4/19) compared with 6.3% (1/16), respectively (P values nonsignificant for both; Table 2).
Discussion
Our results suggest that more extensive hypoattenuation on CTASI, quantified with the pc-ASPECTS score, is associated with a larger final infarct extent in patients with basilar artery occlusion. Moreover, CTASI improves the sensitivity to identify the posterior circulation territories that will ultimately infarct compared with NCCT. The added value of CTASI is most pronounced in patients with large infarction compared with patients with less extensive infarction on follow-up scans. More extensive hypoattenuation on CTASI is also associated with a trend toward a higher HT rate with thrombolysis, though the precision of the estimated difference is low.
Schaefer et al19 have recently demonstrated that the extent of hypoattenuation on CTASI in the midbrain and pons predicts the functional outcome of patients with vertebrobasilar occlusive disease after IA thrombolysis. Supporting Schaefer et al's results, we have demonstrated that the number of posterior circulation territories with hypoattenuation on CTASI, quantified with the pc-ASPECTS score, predicts the functional outcome of patients with basilar artery occlusion, regardless of the recanalization status.14 The current study demonstrates that semiquantification of areas with diminished contrast enhancement on CTASI also predicts the final infarct extent in this population. Similar to findings in the anterior circulation, the added value of CTASI compared with NCCT was most notable in patients who had extensive final infarctions.10 In anterior circulation stroke, the final infarct size has been used to compare the accuracy of imaging modalities12,20,21 and as a surrogate of clinical outcome in therapeutic stroke trials.22,23 Our data suggest that pc-ASPECTS could be used as a tool to quantify the final infarct size in patients with posterior circulation stroke.
In patients with clinically suspected acute posterior circulation ischemia, the overall sensitivity to detect any ischemic changes in the posterior circulation is improved with CTASI compared with NCCT.14 Our study demonstrates that CTASI also improves the sensitivity to identify subsequent infarction in each individual pc-ASPECTS location in patients with basilar artery occlusion. The increased sensitivity was most pronounced to identify subsequent infarction in the brain stem (pons and midbrain) and the thalami. The study by Schaefer et al19 did not report sensitivities and specificities of CTASI to identify subsequent infarction in the posterior circulation. However, their results confirm that 1) hypoattenuation on CTASI can identify areas with ischemic changes in the posterior circulation territory including the brain stem, and 2) the identification of CTASI hypoattenuation in the brain stem has prognostic relevance in patients with basilar artery occlusion. Although spiral data acquisition can result in substantial artifacts in the posterior occipital lobes that mimic ischemic hypoattenuation, newer-generation multisection CT scanners may further improve the diagnostic accuracy of CT in the posterior circulation.24
In the theoretic sense, one would expect that the sensitivity for detection of final infarction should increase with adequate and timely reperfusion, as there would be no infarct extension. In patients with anterior circulation stroke as a result of middle cerebral artery occlusion, the relationship between infarct volumes on CTASI and the final lesion volume was especially strong in patients with complete recanalization (R2 = 0.94; P < .001; slope of regression line, 0.92), whereas patients without recanalization exhibited progression of the lesion volume (R2 = 0.50; P = .01; slope of regression line, 1.61).11 It was unexpected that the proportion of patients with infarct extension tended to be highest in patients with recanalization in our study. Consequently, the sensitivity to identify subsequent infarction was most prominent in patients with basilar artery occlusion who did not undergo recanalization within 24 hours from symptom onset.
Because the pc-ASPECTS score does not quantify lesion volumes but the number of different lesions in the posterior circulation weighted by functional importance, volumetric infarct extension does not necessarily translate into a lower pc-ASPECTS score on follow-up images. Correlation of CTASI and follow-up lesion volumes in the posterior circulation, categorized by the recanalization status, may be the subject of future studies. Moreover, the information derived from CTASI may vary. In early time windows, CTASI may represent potentially reversible hypoattenuation with recanalization.11 In later time windows, CTASI may be highly predictive of the final infarct volume. The lack of association of infarct extension with recanalization in our study may therefore indicate that reperfusion occurred too late to reverse hypoattenuation on CTASI.
We note that the sensitivity of CTASI remains diagnostically limited. MR imaging will remain the test of choice whenever the diagnosis is in doubt. However, the specificity of CTASI is very high; this means that when such signs are present, the extent of final infarct can be predicted with high prognostic certainty.
Overall symptomatic hemorrhagic complication rates in patients with basilar artery occlusion who have undergone thrombolysis are approximately 8% with IA thrombolysis and 11% with IV thrombolysis.1,3,25 Our overall PH rate (11.6%) is consistent with these results. Whereas the extent of EIC on pretreatment NCCT and the degree of hypoattenuation on CTASI predict the PH risk in patients with middle cerebral artery stroke,6–9,13 predictors of increased hemorrhagic complication risk in patients with basilar artery occlusion have not been reported. Our results suggest that the extent of hypoattenuation on CTASI modifies the HT risk in patients with basilar artery occlusion. Because no patients without thrombolytic therapy went on to have HT, this finding seems particularly important in patients who underwent thrombolysis. A similar trend was observed in patients with acute posterior circulation stroke treated with IV heparin, where lesion volumes on DWI images were a risk factor for symptomatic intracerebral hematomas.26
Our study was limited by its retrospective design and the small number of patients studied. All results require confirmation on the basis of prospective studies. The observation of increased HT and PH risk with lower pc-ASPECTS scores on CTASI was not statistically significant. Our data did not justify withholding thrombolytic therapy because of increased hemorrhagic risk in patients with acute symptomatic basilar artery occlusion. Moreover, the varying section thickness of CTASI, different imaging modalities (CT and MR imaging) applied for follow-up imaging, varying timing of follow-up imaging, and the possibility for recall bias may have influenced our results in the prediction of final infarct extent and classification of HT. Moreover, MR imaging might have tended to overestimate the degree of HT compared with CT.17 Because of various treatment regimens, the influence of treatment technique (IV vs IA) on HT risk could not be accurately assessed.
Conclusions
CTASI predict the final infarct extent in patients with basilar artery occlusion. Moreover, CTASI may indicate the HT risk with recanalizing therapies. Our results need validation in a larger dataset. Pc-ASPECTS could be useful for patient stratification in stroke trials and quantification of final infarct extent in patients with posterior circulation stroke.
Footnotes
V.P. was supported by grants from the German Federal Ministry of Education and Research (NBL-3, grant 01 ZZ 0502). M.D.H. was funded by the Alberta Heritage Foundation for Medical Research and the Heart & Stroke Foundation of Alberta, NWT and Nunavut. A.M.D. received salary support from Alberta Heritage Foundation for Medical Research. The study was supported from funding from the Heart and Stroke Foundation of Alberta, NWT and Nunavut.
References
- 1. Lindsberg PJ, Mattle HP. Therapy of basilar artery occlusion: a systematic analysis comparing intra-arterial and intravenous thrombolysis. Stroke 2006;37:922–28 [DOI] [PubMed] [Google Scholar]
- 2. Schonewille WJ, Wijman CAC, Michal P, et al. The basilar artery international cooperation study (BASICS). Int J Stroke 2007;2:220–23 [DOI] [PubMed] [Google Scholar]
- 3. Hacke W, Zeumer H, Ferbert A, et al. Intra-arterial thrombolytic therapy improves outcome in patients with acute vertebrobasilar occlusive disease. Stroke 1988;19:1216–22 [DOI] [PubMed] [Google Scholar]
- 4. Arnold M, Nedeltchev K, Schroth G, et al. Clinical and radiological predictors of recanalisation and outcome of 40 patients with acute basilar artery occlusion treated with intra-arterial thrombolysis. J Neurol Neurosurg Psychiatry 2004;75:857–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brandt T, von Kummer R, Muller-Kuppers M, et al. Thrombolytic therapy of acute basilar artery occlusion. Variables affecting recanalization and outcome. Stroke 1996;27:875–81 [DOI] [PubMed] [Google Scholar]
- 6. Dzialowski I, Hill MD, Coutts SB, et al. Extent of early ischemic changes on computed tomography (CT) before thrombolysis: prognostic value of the Alberta Stroke Program Early CT Score in ECASS II. Stroke 2006;37:973–78 [DOI] [PubMed] [Google Scholar]
- 7. Demchuk AM, Hill MD, Barber PA, et al. Importance of early ischemic computed tomography changes using ASPECTS in NINDS rtPA Stroke Study. Stroke 2005;36:2110–15 [DOI] [PubMed] [Google Scholar]
- 8. Larrue V, von Kummer RR, Muller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 2001;32:438–41 [DOI] [PubMed] [Google Scholar]
- 9. Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke 2008;39:2249–56 [DOI] [PubMed] [Google Scholar]
- 10. Coutts SB, Lev MH, Eliasziw M, et al. ASPECTS on CTA source images versus unenhanced CT: added value in predicting final infarct extent and clinical outcome. Stroke 2004;35:2472–76 [DOI] [PubMed] [Google Scholar]
- 11. Lev MH, Segal AZ, Farkas J, et al. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: prediction of final infarct volume and clinical outcome. Stroke 2001;32:2021–28 [DOI] [PubMed] [Google Scholar]
- 12. Schramm P, Schellinger PD, Klotz E, et al. Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion-weighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours' duration. Stroke 2004;35:1652–58 [DOI] [PubMed] [Google Scholar]
- 13. Schwamm LH, Rosenthal ES, Swap CJ, et al. Hypoattenuation on CT angiographic source images predicts risk of intracerebral hemorrhage and outcome after intra-arterial reperfusion therapy. AJNR Am J Neuroradiol 2005;26:1798–803 [PMC free article] [PubMed] [Google Scholar]
- 14. Puetz V, Sylaja PN, Coutts SB, et al. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke 2008;39:2485–90 [DOI] [PubMed] [Google Scholar]
- 15. Barber PA, Hill MD, Eliasziw M, et al. Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry 2005;76:1528–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 1999;30:2280–84 [DOI] [PubMed] [Google Scholar]
- 17. Thomalla G, Sobesky J, Kohrmann M, et al. Two tales: hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia: MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 hours. Stroke 2007;38:313–18 [DOI] [PubMed] [Google Scholar]
- 18. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med 1985;312:932–36 [DOI] [PubMed] [Google Scholar]
- 19. Schaefer PW, Yoo AJ, Bell D, et al. CT angiography-source image hypoattenuation predicts clinical outcome in posterior circulation strokes treated with intra-arterial therapy. Stroke 2008;39:3107–09 [DOI] [PubMed] [Google Scholar]
- 20. Aviv RI, Mandelcorn J, Chakraborty S, et al. Alberta Stroke Program Early CT Scoring of CT perfusion in early stroke visualization and assessment. AJNR Am J Neuroradiol 2007;28:1975–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parsons MW, Pepper EM, Chan V, et al. Perfusion computed tomography: prediction of final infarct extent and stroke outcome. Ann Neurol 2005;58:672–79 [DOI] [PubMed] [Google Scholar]
- 22. Hacke W, Albers G, Al-Rawi Y, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 2005;36:66–73 [DOI] [PubMed] [Google Scholar]
- 23. Warach S, Kaufman D, Chiu D, et al. Effect of the glycine antagonist gavestinel on cerebral infarcts in acute stroke patients, a randomized placebo-controlled trial: The GAIN MRI Substudy. Cerebrovasc Dis 2006;21:106–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schulte-Altedorneburg G, Bruckmann H. [Imaging techniques in diagnosis of brainstem infarction]. Nervenarzt 2006;77:731–43; quiz 744 [DOI] [PubMed] [Google Scholar]
- 25. Lisboa RC, Jovanovic BD, Alberts MJ. Analysis of the safety and efficacy of intra-arterial thrombolytic therapy in ischemic stroke. Stroke 2002;33:2866–71 [DOI] [PubMed] [Google Scholar]
- 26. Kang K, Yoon BW. Symptomatic intracerebral hematomas in posterior circulation stroke patients anticoagulated with heparin. J Thromb Thrombolysis 2006;21:249–55 [DOI] [PubMed] [Google Scholar]