Abstract
BACKGROUND AND PURPOSE:
To date, few studies have focused specifically on imaging findings in Aicardi-Goutières syndrome (AGS). We set out to evaluate retrospectively neuroradiologic data from a large sample of patients with AGS, focusing on the pattern of white matter abnormalities and the temporal evolution of the cerebral involvement to establish the radiologic natural history of the disease.
MATERIALS AND METHODS:
Thirty-six patients, 18 girls and 18 boys, were included. All had a clinical diagnosis of AGS, genetically confirmed in 31 of them. For every subject, we reviewed at least 1 CT and 1 MR imaging study; 19 (52.7%) had multiple examinations. In all, we reviewed 109 examinations. Clinical-neuroradiologic comparisons were analyzed by using the χ2 test.
RESULTS:
Calcifications were found in all subjects, mainly in the basal ganglia, lobar white matter, and dentate nuclei. Abnormal white matter was present in all the subjects, showing 2 patterns of distribution: diffuse in 18 (50%) and an anteroposterior gradient in 18 (50%). Cystic areas were observed in the temporal and/or frontal lobes in 12/36 patients (33.3%). A correlation was found between early age at onset and severity of the leukoencephalopathy in the frontal (P = .024) and temporal (P = .034) regions. A significant degree of cerebral atrophy was found in 31/36 subjects (86.1%). The neuroradiologic presentation remained substantially stable with time.
CONCLUSIONS:
The different neuroradiologic presentations of AGS are here outlined for the first time in a large sample of patients. These findings may facilitate more precise and earlier diagnosis of this rare but probably underdiagnosed syndrome.
Aicardi-Goutières syndrome (AGS, Online Mendelian Inheritance in Man, 225750; http://www.ncbi.nlm.nih.gov/omim), first described by Aicardi and Goutières in 1984,1 is a rare autosomal recessive disorder characterized by acquired microcephaly, chronic CSF lymphocytosis, and raised levels of CSF interferon-α (IFN-α), in association with radiologic evidence of intracranial calcifications, cerebral white matter abnormalities, and cerebral atrophy. Toxoplasmosis, rubella, cytomegalovirus, and herpes virus (TORCH) studies are negative, though the disease mimics a congenital infection.1–3
AGS typically has onset in the first year of life; the first symptoms are feeding difficulties, irritability, unexplained low-grade fever, and a loss of motor and social skills. It rapidly progresses, with patients displaying the onset of trunk hypotonia, severe pyramidal and extrapyramidal signs, and abnormal eye movements.1,2 The clinical presentation tends to stabilize, usually by the end of the second year of life.3 Extraneurologic signs are frequent in AGS; and the skin, affected by chilblain-like lesions, is the organ most often involved.2,4–7
In the past 3 years, Crow et al8 have identified mutations in 4 genes encoding the exonuclease TREX1 (AGS1) on chromosome 3 and all 3 subunits of the hetero-trimeric ribonuclease H2 (RNaseH2) endonuclease complex (AGS2 on chromosome 13, AGS3 on chromosome 11, AGS4 on chromosome 19)9 as responsible for the AGS phenotype. These nucleases seem to be involved in removing endogenous nucleic acid fragments produced as a result of normal cell cycles. It is hypothesized that a buildup of these nucleic acid fragments could trigger an innate IFN-α–mediated immune response. There is evidence that there must exist at least 1 other AGS-causing gene responsible for the syndrome in cases with negative screening of AGS1–4.5
Neuroradiologic findings, in particular punctuate calcifications, especially in the cerebral basal nuclei, white matter, and/or dentate nuclei, have been considered essential for diagnosing AGS since the very first descriptions of the syndrome.1,2 According to the literature, the neuroradiologic presentation is characterized by 3 features: cerebral calcifications, white matter abnormalities, and cerebral atrophy.3,4,6,7,10 However, to date, only a few studies (often just case reports) have focused specifically on imaging findings in the disease.11–15 Consequently, the neuroradiologic characteristics of AGS white matter abnormalities have not been clearly described or categorized, and the literature contains no unequivocal data on the evolution of the cerebral atrophy, which is reported as progressive, stable, or even regressive with time.7,13,15 It has, thus, become necessary to establish the radiologic natural history of AGS, especially in the light of recent developments of the pathogenetic hypotheses that could have therapeutic implications in the future.16,17
In 2000, the International Aicardi-Goutières Syndrome Association (IAGSA) was founded at our institution as a referral center for patients with AGS and their families. As a result, we have been ideally placed to gather a relatively large amount of data about the disease.3,7 The aim of the present study was to evaluate the neuroradiologic findings in a large sample of subjects with AGS, focusing on the pattern of white matter alterations and the temporal evolution of brain involvement.
Materials and Methods
Subjects
The informed consent requirement was waived in this study because of its retrospective nature.
Patients were selected from the IAGSA data base on the basis of the following inclusion criteria:
A genetic and/or clinical diagnosis of AGS in accordance with the literature1,2: neurologic signs of encephalopathy, cerebral calcifications, negative serologic investigations for TORCH and for congenital infections, and CSF lymphocytosis (>5 cell/mm3) and/or elevation of CSF IFN-α (>2 U/L or >10 pg/mL).
Availability of clinical data and information.
Availability of at least 1 brain CT scan and 1 brain MR image, both of diagnostic image quality.
On this basis, 36 patients, 18 (50%) girls and 18 (50%) boys, were identified and included in the study.
All the patients except 2 had undergone genetic analysis to confirm the clinical diagnosis, with specific genetic alterations being identified in 31 of them. Four subjects presented mutations in AGS1 (11.1% of the sample); 23 subjects, in AGS2 (63.8%); 2 subjects (siblings), in AGS3 (5.6%); and 1, in AGS4 (2.7%). One child had a single heterozygous mutation in AGS4 (2.7%). No mutations in the AGS1–4 genes were found in 3 subjects (8.3%).
Imaging
All the patients underwent neuroradiologic investigations as part of their diagnostic work-up.
The age of the patients at the time of the examinations ranged from 12 days to 17 years (mean, 29 ± 38.3 months). Nineteen patients (19/36, 52.7%) had multiple examinations, with a maximum interval of 11.1 years between the first and the last; the mean duration of follow-up was 39 ± 37.8 months. In all, we reviewed 109 neuroradiologic examinations (56 brain MR images and 53 brain CT scans).
Twenty-two patients (22/36; 61.1%) were examined at our institution. The clinical and neuroradiologic data for the other 14 came from several different specialist centers around the world, having been sent to us for our diagnostic opinion. Therefore, the studies we analyzed showed some variation in sequences and in image quality.
Four subjects (4/36; 11.1%) underwent scanning after intravenous administration of contrast medium: MR imaging in 3 patients at 2, 12, and 40 months of age, respectively, and CT in 1 patient at 2 weeks of age.
All the examinations were reviewed by a single neuroradiologist (C.U.) with >20 years' experience, who was initially blinded to the clinical data. The CT scans were analyzed for the presence, distribution, and severity of calcifications. The MR images were analyzed for changes in signal intensity in the supratentorial and infratentorial regions. Leukoencephalopathy was categorized by using a scoring list based on the one suggested by van der Knaap and Valk.18 The subcortical, periventricular, and lobar white matter (considering frontal, temporal, parietal, and occipital white matter as distinct entities); corpus callosum; internal and external capsules; optic radiations; cerebellar white matter; and brain stem were analyzed separately. We noted the following characteristics of the signal-intensity alterations: location, distribution (caudocranial, central-peripheral, or anteroposterior gradients), symmetry (symmetric/asymmetric), and homogeneity (confluent/isolated/multifocal involvement). The degree of white matter myelination (normal/delayed/no or hardly any myelin present) was also noted. The severity of leukoencephalopathy was graded from 0 to 4 on the basis of signal-intensity alteration (0 = no signal intensity alteration, 1 = mild signal intensity alteration, 2 = moderate signal intensity alteration, 3 = severe signal intensity alteration, 4 = severe signal intensity alteration and the presence of cystic degeneration).
Both the MR images and the CT scans were analyzed for patterns of brain atrophy. No volumetric studies were available, so we considered the following aspects instead: enlargement of the cortical sulci and dilation of the ventricular system. Atrophy was graded from 0 to 2 (0 = no atrophy, 1 = mild to moderate atrophy, 2 = severe atrophy).
Follow-up studies were compared with the original reference studies to look for increases in calcifications and to ascertain deterioration, stability, or improvement of the white matter abnormalities and of the brain atrophy.
Statistical Analysis
The direct comparisons of neuroradiologic findings (severity of calcifications/severity of leukoencephalopathy/degree of atrophy) with each other and with selected demographic/clinical variables (sex, age at onset, severity of neurologic involvement, systemic involvement, and presence of mutations in AGS2) were performed by using the χ2 test for discrete variables (P < .05 was considered significant). The size of the sample precluded more complex statistical analyses and multiple comparisons. The qualitative analysis of the data was performed by using the Statistical Package for Social Sciences software (Version 10.0 for Windows; SPSS, Chicago, Ill).
Results
On-line Tables 1–3 set out, in detail, the clinical features of the sample and the neuroradiologic findings (presence of calcifications and leukoencephalopathy). The numbering of the patients (progressively by age at onset) is exactly the same in all the tables, to facilitate comparison of data.
Clinical Features
In most of the patients (34/36 subjects, 94.4%), the clinical presentation was characterized by the presence of spastic-dystonic tetraplegia, usually (in 33 patients) associated with severe developmental delay or mental retardation; 1 patient showed mild developmental delay. All except 1 of these 34 subjects (33/36; 91.7%) had microcephaly. Two subjects (5.6%) had head circumferences in the normal range for their age and spastic diplegia, without developmental delay or mental retardation. The clinical data of the sample are set out in detail in on-line Table 1.
Neuroradiologic Findings
Cerebral Calcifications.
All patients (100%) showed cerebral calcifications on CT scans; in most cases, these were also evident on MR images as hypointense signals on T2-weighted images (Fig 1A). In the lentiform nucleus, the putamen and globus pallidus were equally affected by calcifications. The dentate nuclei were affected in 11 cases (30.5%). Cerebral calcifications in the white matter were located mainly in lobar zones and, in a small number of subjects, were also found in periventricular areas; otherwise, they were located as specified in on-line Table 2. The calcifications were typically small and punctuate, but in 3 patients (3/36; 8.3%), they were large and isolated (Fig 2). Calcific lesions were symmetrically distributed in 32 subjects (88.9%) and asymmetrically distributed in 4 (11.1%). Detailed data are reported in on-line Table 2.
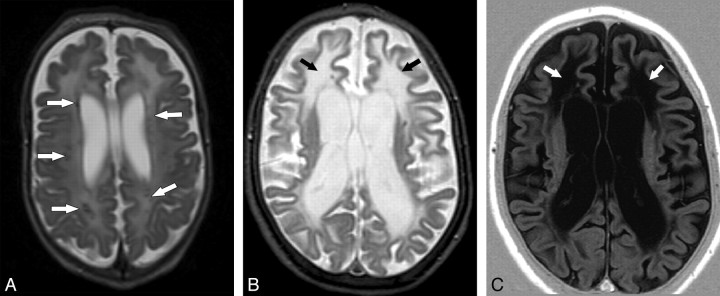
Fig 1.
Leukoencephalopathy. A, Case 19. Axial spin-echo T2-weighted (1.5T, TR = 2554 ms, TE = 100 ms) MR image shows a faint T2 hyperintensity involving the cerebral white matter in a diffuse pattern. Calcifications are seen in the lobar white matter as focal hypointensities (arrows). B and C, Axial T2- (1.5T, turbo spin-echo, TR = 5022 ms, TE = 100 ms) (B) and T1-weighted images (1.5T, inversion recovery, TR = 3430 ms, TE = 15 ms, TI = 400 ms) (C), with the same parameters in case 10, demonstrate extensive cerebral white matter abnormalities, showing an anteroposterior gradient. The T1-weighted images better highlight areas of cavities in the frontal lobes, whereas the signal-intensity properties of the involved white matter are almost identical to those of the liquoral spaces (arrows).
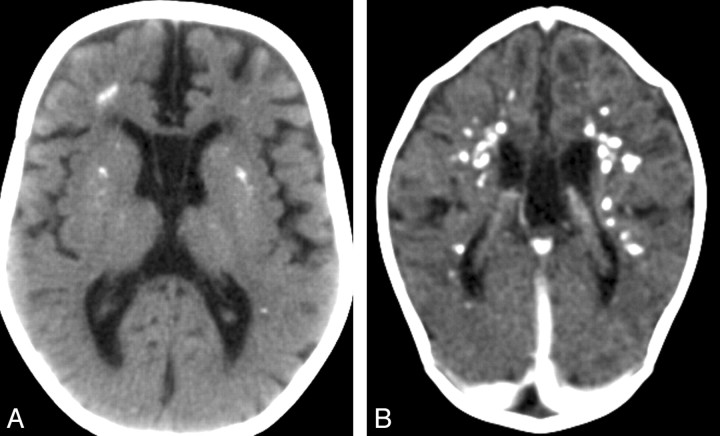
Fig 2.
Cerebral calcifications. A, Axial nonenhanced CT image of case 1 shows numerous punctuate calcifications within the basal ganglia and the cerebral white matter, a pattern typical in patients with AGS. B, Contrast-enhanced CT scan (case 19) shows large calcifications in the white matter. Although the CT examination was performed in the acute phase of the disease, no signs of contrast enhancement are seen. Atrophy, microcephaly, and areas of hypoattenuation in the periventricular white matter are also evident.
White Matter Abnormalities.
White matter abnormalities were found in all the subjects (100%). These could always be seen on T2-weighted images and, when particularly severe, also on T1-weighted images as hypointense signals. Four patients (11.1%) presented only mild alterations, which, also due to the age of these patients, could still be attributable to severe myelination delay. With the exception of 1 patient (2.7%) with leukoencephalopathy mainly involving the left hemisphere, all the images examined showed a symmetric distribution of the white matter alterations.
The signal-intensity alteration mainly involved the lobar white matter, whereas the white matter of the strictly periventricular area, of the corpus callosum, of the capsules, and of the optic radiations was relatively spared; conversely, the subcortical arcuate fibers frequently showed abnormalities (Fig 3). Signal intensity was homogeneous in 18 subjects (18/36, 50%). In the other 18 (18/36, 50%), it was inhomogeneous and often characterized by the presence of more intense signal-intensity alterations in frontopolar areas (Fig 1). In 11 subjects (30.6%), the altered signal intensity corresponded to cystic degeneration, which was located in both the frontal and the temporal lobes in 6 patients (16.7%), only in the frontal lobes in 2 patients (5.6%), and only in the temporal lobes in the other 3 patients (8.3%). More detailed data are reported in Table 3.
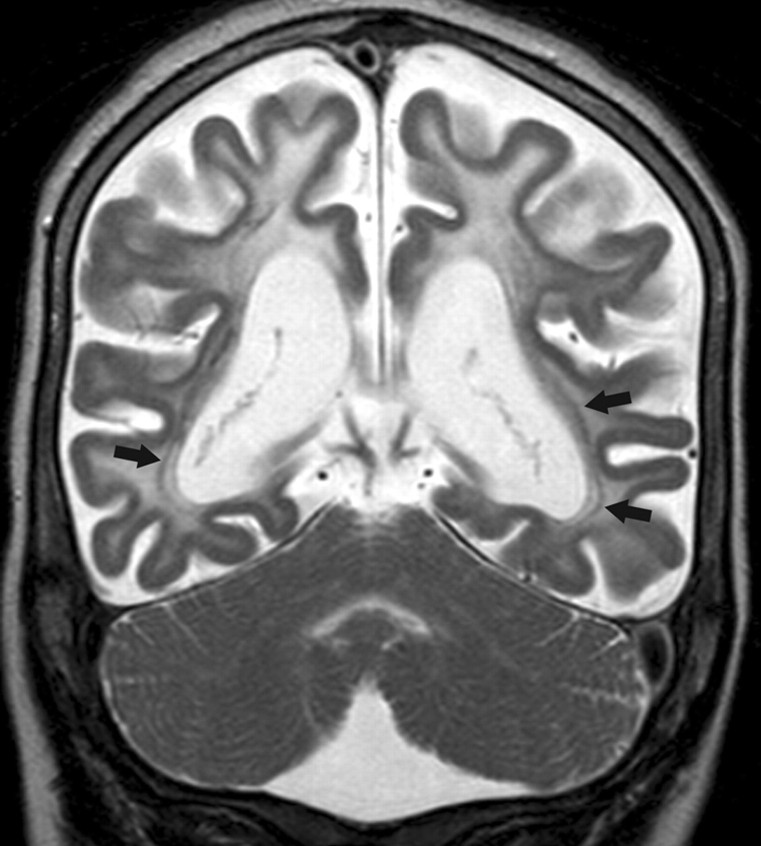
Fig 3.
Coronal fast spin-echo T2-weighted MR image (1.5T, TR = 5022 ms, TE = 100 ms) of case 10 shows a diffuse signal-intensity abnormality of the cerebral lobar white matter. Note that the cerebellar white matter and the optic radiations (arrows) are spared, whereas the subcortical U-fibers are involved. The cortex shows an even thickness. Severe cerebral atrophy is evident.
Cerebral Atrophy.
Some degree of cerebral atrophy was present in 31/36 subjects (86.1%). In 16/36 (44.4%), the atrophy, both superficial and deep, was severe (Figs 1 and 3). Moderate atrophy was present in 9 subjects (9/36, 25%). Six patients (6/36, 16.7%) had mild atrophy.
In 3 patients with microcephaly (3/36, 8.3%), there was no evidence of enlargement of the ventricular system or sulci. Two patients (2/36, 5.6%) showed neither microcephaly nor evidence of cerebral atrophy. The volume of the brain stem was reduced in 14/36 cases (14/36, 38.9%). No abnormalities of the cortex were found in any subject. Two siblings with mutations in the RNaseH2C gene (AGS3) showed enlargement of the cisterna magna and upward rotation of the vermis.
Contrast Enhancement.
No image enhancement was observed in any of the 4 subjects (4/36; 11.1%) scanned after intravenous administration of a contrast medium (Fig 2B).
Follow-Up.
Modifications of the calcifications, white matter abnormalities, and atrophy in the 19 subjects undergoing multiple examinations are detailed below and in on-line Table 4. The cerebral calcifications remained unchanged in 15/19 subjects (79%); in the other 4 (21%) (ie, cases 17, 23, 27, and 36), they increased in the first 2 years after diagnosis before becoming stable. The white matter abnormalities also remained stable in 15/19 subjects (79%), whereas in the other 4 (21%) (ie, cases 10, 13, 19, and 3), they became more marked in the first 2 years following the diagnosis, thereafter becoming stable. The degree of cerebral atrophy was unchanged in 12/19 subjects (63.2%), whereas it progressively increased in the other 7/19 subjects (36.8%).
Statistical Analysis
Comparisons of Neuroradiologic Findings.
Direct comparisons of neuroradiologic findings (severity of calcifications and severity of white matter abnormalities, severity of the calcifications and degree of cerebral atrophy, severity of white matter abnormalities and degree of cerebral atrophy) did not result in statistical significance.
Comparisons of Neuroradiologic Findings and Clinical Data.
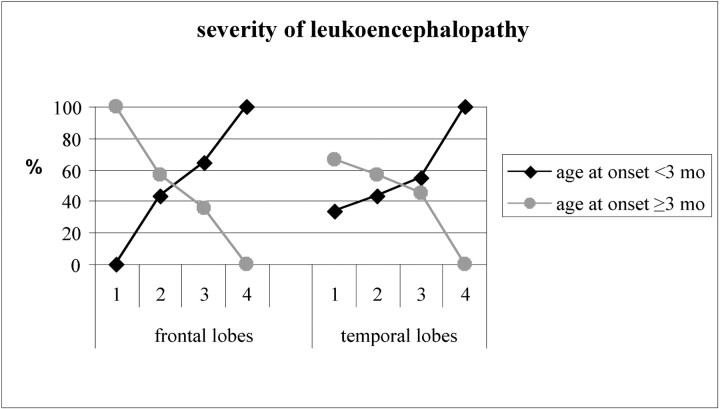
No correlations emerged between sex and neuroradiologic findings. A statistically significant correlation between the severity and site of white matter abnormalities and age at onset emerged when considering white matter abnormalities located in the frontal and temporal lobes (P = .024 and P = .034, respectively) (Fig 4).
Fig 4.
Correlation between age at onset and the severity of leukoencephalopathy involving the frontal and temporal lobes (leukoencephalopathy score: 1 = mild; 2 = moderate; 3 = severe; 4 = presence of cystic degeneration). Mths indicate months.
Of the subjects with severe white matter abnormalities in the frontal and temporal lobes, 64.3% and 54.5%, respectively, had an age at onset of younger than 3 months; of those with moderate white matter abnormalities in the frontal and temporal lobes, 42.9%, in both cases, had an age at onset of younger than 3 months. Cystic degeneration in the frontal and/or temporal lobes was always (12/12; 100%) associated with an age at onset of younger than 3 months.
Given the variables of severity of calcifications and age at onset, 8 subjects with an age at onset of younger than 3 months presented with severe cerebral calcifications, whereas a similar presentation was found in only 1 subject with an age at onset of older than 3 months. This difference failed to reach statistical significance.
In the 2 subjects with a mild neurologic presentation (pyramidal signs without developmental delay and normal head circumference), it was deemed opportune to look for qualitative differences in the neuroradiologic presentation compared with the rest of the sample. They showed mild signal-intensity alteration in the white matter and minimal cerebral atrophy. In the 8 subjects with systemic involvement other than skin lesions, no correlation was found between the clinical and neuroradiologic presentations.
Seven of the 8 subjects with mutations in genes other than AGS2 (87.5%) showed very severe cerebral calcifications. This degree of severity was found in only 2 (8.7%) of the 23 subjects with mutations in AGS2. This difference was highly statistically significant (P < .0001). Of the 7 subjects showing mutations in genes other than AGS2 and very severe cerebral calcifications, 6 also had severe white matter abnormalities. Despite this, the severity of white matter abnormalities was not found to correlate with genetic findings.
Discussion
This retrospective study of 36 subjects with AGS, which included the serial evaluation of 53 CT scans and 56 MR images, has allowed us to explore in detail the complex imaging findings associated with this disease and to describe the evolution of the neuroradiologic presentation with time.
The presence of cerebral calcifications is a classic criterion for a diagnosis of AGS.1,2 In this patient series, too, all the subjects had calcifications, which displayed the distribution and morphology typical of the syndrome. However, in view of the fact that in 1 of the subjects affected by late-onset AGS,19 calcifications were not detected on the first CT scan but appeared 3 months later, we believe that this classic diagnostic criterion should be reviewed and that a diagnosis of AGS cannot be excluded purely on the basis of absence of documented calcifications, certainly at the onset of the disease. This is, moreover, a view supported by literature reports of other cases, including 2 subjects described by Aicardi and Goutières1 and Goutières et al2 and a recent atypical case.16
Nevertheless, the presence of calcifications remains an extremely important finding in the diagnostic work-up of AGS, and in uncertain cases, we believe that an MR imaging investigation should always be associated with a CT scan, which is more sensitive in detecting them. Another pattern (not previously described) that emerged in our study, albeit without reaching statistical significance, was an apparent association between disease onset and severity of calcifications: Subjects with early onset of the disease have more numerous and more voluminous calcifications, whereas a late onset is often associated with a less severe presentation of calcifications. The distribution of the calcifications, which were found mainly in the basal ganglia and cerebral lobar white matter, may recall the finding of Barth et al20 and Barth21,22 of calcifications located on the walls of the arterioles.
The presence of white matter abnormalities, albeit poorly characterized, was another feature of the earliest descriptions of AGS.1,2 This feature has been documented as hypoattenuation on CT scans and as hyperintesity on T2-weighted MR images, mainly around the frontal horns.7,10 Our population allowed us to describe these alterations, present in all the patients, in more detail. The changes we observed tended to be symmetric and regular; in other words, they did not have the patchy and uneven appearance that can be expected in diseases of inflammatory microangiopathic origin. In 50% of the patients, the white matter abnormalities tended to show a symmetric distribution and homogeneous intensity in the different cerebral lobes, whereas in the other subjects, the signal-intensity alterations showed a clear anteroposterior gradient, with more marked involvement of the tips of the frontal and temporal lobes. In some cases, the white matter in the tips of the frontal and temporal lobes was highly rarefied, appearing as large cavities or cystlike formations of a kind never observed in other cerebral regions.
The key factor in both of these patterns was the age at onset of AGS: The prevalence of subjects with an age at onset of younger than 3 months was found to be proportional to the severity of the white matter alterations present in the frontotemporal regions, whereas all patients with cystic degeneration had an onset of AGS at birth or within the first 3 months of life. Conversely, the prevalence of subjects with an age at onset of older than 3 months was found to be inversely proportional to the severity of the white matter alterations in the frontotemporal regions. On the basis of these findings, it can be hypothesized that the less mature the white matter is when it is affected by the pathologic process, the more severe its involvement will be. This hypothesis is supported by the observation that the structures in which myelination occurs earlier (cerebellum, brain stem, optic radiations) tend to be spared, whereas the frontotemporal regions, in which myelination occurs later, are particularly affected.
The severity of white matter abnormalities and the severity of calcifications did not reach a statistical relationship. However, this could be due to the relatively small size of the sample.
The white matter MR imaging alterations were usually stable with time. In the few patients in whom they were progressive, this progression was noted only in the first 2 years following the clinical onset of the syndrome. This reflects the clinical pattern of AGS, which, after an initially acute period lasting ≤2 years, tends to show no signs of further progression. In these few patients with progressive white matter abnormalities, it was not changes in the distribution or size of the affected area that were observed but rather an increase in the signal intensity, culminating in the formation of areas of cystic degeneration in the already altered white matter.
Reduction in the quantity of cerebral white matter explained, in all the subjects displaying it, the presence of cerebral atrophy and microcephaly. The degree of cerebral atrophy also tended to be stable with time. Small changes (for better or for worse) in the width of the cerebral sulci were considered possible effects of changes in the general conditions of the patients and thus secondary to the hydration levels of the brain. Our findings suggest that the neuroradiologic presentation (like the clinical course) tends to remain stable with time, following an initial period in which it may be progressive.
Thinning of the brain stem, which is often considered a typical finding in AGS,10,23 was present in 38.9% of the patients in our series. Alternatively, brain stem hypotrophy, as well as thinning of the corpus callosum, could be linked to severe atrophy of the supratentorial structures, leading to thinning of the corticospinal tracts, and therefore might not constitute a definite sign of direct involvement of the brain stem. In fact, only 1 patient showed calcifications in both the pontine and the mesencephalic regions, and no specific morphologic or signal-intensity alterations were ever detected in the brain stem.
We did not observe any abnormalities of the cerebral cortex. From a neuroradiologic perspective, in accordance with literature data,20–22 AGS is, to all intents and purposes, a leukoencephalopathy, characterized by the presence of mixed features attributable to hypomyelination, dysmyelination, and gliosis. As the most recent immunohistochemical studies, too, have shown,24 astrocytes produce the INF-α, responsible for the cascade of events leading to the autoimmune inflammatory reaction, and the cerebral white matter is the “battlefield” where this reaction takes place.
The fact that there were no areas of contrast enhancement, even in the acute phases of this devastating inflammatory process, could be explained by the observation that a large proportion of subjects affected by AGS do not show a significant increase in the cytokine CCL2, which is responsible for breaking down the blood-brain barrier; conversely, raised CCL2 levels are found in congenital infections.24
An improved characterization of the neuroradiologic findings in AGS could help in the differential diagnosis of the syndrome versus the numerous forms of childhood leukoencephalopathy with calcifications,25 of which the main ones are the intrauterine TORCH infections, especially cytomegalovirus (CMV). The distribution of the calcifications could be useful in the differential diagnosis of AGS versus congenital CMV infection, in which they typically show a periventricular distribution.26 None of our subjects showed involvement of the cerebral cortex, which, instead, is a feature of the encephalopathy found in congenital CMV infection.26
Metabolic causes should also be considered in the differential diagnosis of AGS, in particular biotinidase deficiency27,28 and carbonic anhydrase II deficiency.27
In view of the relative sparing of the brain stem and cerebellum in AGS, the differential diagnosis of the syndrome versus diseases such as pontocerebellar hypoplasia23 does not seem to present a particular problem. Finally, the better trophism of the supratentorial compared with the cerebral structures could be a useful criterion in the differential diagnosis of AGS versus the neonatal form of Cockayne syndrome, in which cerebellar atrophy is prevalent.27 Cockayne syndrome, too, is linked to a deoxyribonucleic acid repair deficit.29
With regard to the different genetic forms of AGS, a statistically significant correlation emerged between the mutated gene and the severity of the presentation of cerebral calcifications: Compared with the subjects with AGS2, those with the AGS1, AGS3, and AGS4 forms had a much more severe presentation, in terms of the number, size, and extent of the lesions. These aspects could be useful in targeting the search for the genes responsible for the condition.
We were able to confirm, also from a neuroradiologic point of view, a finding produced by a previous genotype−clinical phenotype study,4,10 namely, that in AGS1 patients, the disease is particularly severe and has an earlier onset. In all the AGS1 subjects in our sample, the lesions were always characterized by an anteroposterior gradient and often by the presence of frontotemporal cavities. This pattern was also found in some of the patients with other genetic forms of AGS.
More precise correlations might emerge from similar comparisons of neuroradiologic and genetic data in larger samples of patients with AGS, which is a rare but probably underdiagnosed disease. Useful information on the involvement of the cerebral white matter might also be provided by MR imaging studies conducted by using advanced techniques, in particular diffusion tensor imaging and MR spectroscopy.
Conclusions
The brain imaging findings in AGS were leukoencephalopathy, either diffuse or with a frontal-to-posterior gradient, cysts in frontotemporal lobes, and calcifications. The severity of neuroradiologic findings, in particular white matter involvement, reflects the precocity of the disease onset.
Acknowledgments
We thank all physicians for kindly providing images and clinical data of patients for this study, especially Daniel R. Carvalho, Roberta Biancheri, Alice Pessagno, Andrea Rossi, Francesco Pisani, Antonella Squarcia, Francesca Ormitti, Cyril Goizet, Curtis Rogers, Sally Lynch, Mary King, Hans Jurgen Christen, Wilfried Kratzer, and Marianne Till. Our thanks go to Antonietta Citterio for helping with the statistical analysis and to Catherine Wrenn for her valuable help in translating the manuscript. Special thanks go to IAGSA, especially its president, Fiammetta Boni Longo, and to the affected children and their families for their support and encouragement in our work.
Footnotes
indicates article with supplemental on-line tables.
References
- 1. Aicardi J, Goutières F. A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol 1984;15:49–54 [DOI] [PubMed] [Google Scholar]
- 2. Goutières F, Aicardi J, Barth PG, et al. Aicardi–Goutières syndrome: an update and results of interferon-alpha studies. Ann Neurol 1998;44:900–07 [DOI] [PubMed] [Google Scholar]
- 3. Lanzi G, Fazzi E, D'Arrigo S, et al. The natural history of Aicardi–Goutières syndrome: follow-up of 11 Italian patients. Neurology 2005;64:1621–24 [DOI] [PubMed] [Google Scholar]
- 4. Goutières F. Aicardi-Goutières syndrome. Brain Dev 2005;27:201–06 [DOI] [PubMed] [Google Scholar]
- 5. Rice G, Patrick T, Parmar R, et al. Clinical and molecular phenotype of Aicardi-Goutières syndrome. Am J Hum Genet 2007;81:713–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Orcesi S, La Piana R, Fazzi E. Aicardi-Goutières syndrome. Br Med Bull 2009;89:183–201. Epub 2009 Jan 7 [DOI] [PubMed] [Google Scholar]
- 7. Lanzi G, Fazzi E, D'Arrigo S. Aicardi-Goutières syndrome: a description of 21 new cases and a comparison with the literature. Eur J Paediatr Neurol 2002;6(suppl A):A9–22, discussion A23–5, A77–86 [DOI] [PubMed] [Google Scholar]
- 8. Crow YJ, Hayward BE, Parmar R, et al. Mutations in the gene encoding the 3`-5` DNA exonuclease TREX1 cause Aicardi-Goutières syndrome at the AGS1 locus. Nat Genet 2006;38:917–20. Epub 2006 Jul 16 [DOI] [PubMed] [Google Scholar]
- 9. Crow YJ, Leitch A, Hayward B, et al. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutières syndrome and mimic congenital viral brain infection. Nat Genet 2006;38:910–16 [DOI] [PubMed] [Google Scholar]
- 10. Crow YJ, Livingston JH. Aicardi-Goutières syndrome: an important Mendelian mimic of congenital infection. Dev Med Child Neurol 2008;50:410–16. Epub 2008 Apr 14 [DOI] [PubMed] [Google Scholar]
- 11. Abdel-Salam GM, Zaki MS, Lebon P, et al. Aicardi-Goutières syndrome: clinical and neuroradiologic findings of 10 new cases. Acta Paediatr 2004;93:929–36 [DOI] [PubMed] [Google Scholar]
- 12. Robertson NJ, Stafler P, Battini R, et al. Brain lactic alkalosis in Aicardi-Goutières syndrome. Neuropediatrics 2004;35:20–26 [DOI] [PubMed] [Google Scholar]
- 13. Polizzi A, Pavone P, Parano E, et al. Lack of progression of brain atrophy in Aicardi-Goutières syndrome. Pediatr Neurol 2001;24:300–02 [DOI] [PubMed] [Google Scholar]
- 14. Kothare SV, Pungavkar SA, Patkar DP, et al. Regression of white matter hypodensities with age in Aicardi-Goutières syndrome: a case report. Childs Nerv Sys 2006;22:1503–06 [DOI] [PubMed] [Google Scholar]
- 15. østergaard JR, Christensen T. Aicardi-Goutières syndrome: neuroradiological findings after nine years of follow-up. Eur J Paediatr Neurol 2004;8:243–46 [DOI] [PubMed] [Google Scholar]
- 16. D'Arrigo S, Riva D, Bulgheroni S, et al. Aicardi-Goutières syndrome: description of a late onset case. Dev Med Child Neurol 2008;50:631–34 [DOI] [PubMed] [Google Scholar]
- 17. Rigby R, Leitch A, Jackson AP. Nucleic acid-mediated inflammatory diseases. Bioessays 2008;30:833–42 [DOI] [PubMed] [Google Scholar]
- 18. van der Knaap M, Valk J. Magnetic Resonance of Myelination and Myelin Disorders. 3rd ed Berlin, Germany: Springer-Verlag; 2005:12–19 [Google Scholar]
- 19. Orcesi S, Pessagno A, Biancheri R, et al. Aicardi-Goutières syndrome presenting atypically as a sub-acute leukoencephalopathy. Eur J Paediatr Neurol 2008;12:408–11 [DOI] [PubMed] [Google Scholar]
- 20. Barth PG, Walter A, van Gelderen I. Aicardi-Goutières syndrome: a genetic microangiopathy? Acta Neuropathol 1999;98:212–16 [DOI] [PubMed] [Google Scholar]
- 21. Barth PG. Inherited progressive disorders of the fetal brain: a field in need of recognition. In: Fukuyama Y, Suzuki Y, Kamoshita S, et al., eds. Fetal and Perinatal Neurology. Basel, Switzerland: Karger; 1992: 299–313 [Google Scholar]
- 22. Barth PG. The neuropathology of Aicardi-Goutières syndrome. Eur J Paediatr Neurol 2002;6(suppl A):A27–A31 [DOI] [PubMed] [Google Scholar]
- 23. Crow YJ, Massey RF, Innes JR, et al. Congenital glaucoma and brain stem atrophy as features of Aicardi-Goutières syndrome. Am J Med Genet A 2004;129A:303–07 [DOI] [PubMed] [Google Scholar]
- 24. van Heteren JT, Rozenberg F, Aronica E, et al. Astrocytes produce interferon-alpha and CXCL10, but not IL-6 or CXCL8, in Aicardi-Goutières syndrome. Glia 2008;56:568–78 [DOI] [PubMed] [Google Scholar]
- 25. Schiffmann R, van der Knaap M. Invited article: an MRI-based approach to the diagnosis of white matter disorders. Neurology 2009;72:750–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tortori-Donati P, Rossi A, Biancheri R. Infectious diseses. In: Pediatric Neuroradiology Brain. Berlin: Springer-Verlag; 468–535. [Google Scholar]
- 27. Patay Z. Metabolic diseases. In: Pediatric Neuroradiology Brain. Berlin: Springer-Verlag; 543–721. [Google Scholar]
- 28. van der Knaap M, Valk J. Magnetic Resonance of Myelinatin and Myelin disorders. 3rd ed Berlin: Springer-Verlag; 2005:248–51 [Google Scholar]
- 29. Brooks PJ, Cheng TF, Cooper L. Do all neurologic diseases in patients with DNA repair gene mutations result from the accumulation of DNA damage? DNA repair (Amst) 2008;7:834–48. [DOI] [PMC free article] [PubMed] [Google Scholar]