Abstract
BACKGROUND AND PURPOSE:
It is important to try to clarify the methodology of vertebroplasty such as amount of cement needed, how many needles to use and the significance of cement extravasation. This prospective study evaluated the potential of vertebroplasty to increase the likelihood of an adjacent vertebral compression fracture (VCF) 1 year or less after vertebroplasty, the correlation between the cement volumes injected and pain relief, and the consequences of cement extravasation.
MATERIALS AND METHODS:
Pain relief and the incidence of a subsequent fracture of adjacent vertebrae 1 year or less after vertebroplasty were evaluated in 357 patients (660 vertebrae) of mean age 77.5 years with osteoporotic VCFs. The correlation between cement volume and pain relief was assessed with a Pearson correlation coefficient; factors potentially predictive of subsequent adjacent VCFs were explored by multiple logistic regression analysis.
RESULTS:
Refracture of any vertebrae (adjacent or nonadjacent to the primary fracture) occurred in 18% of the patients 1 year or less after vertebroplasty. Refracture of adjacent vertebrae occurred 1 year or less after vertebroplasty in 12% of the patients. Neither cement volume nor extravasation of cement into the intravertebral disk was a significant predictor of adjacent VCFs. No correlation was found between cement volume and pain relief (r = −0.029). Extravasation of cement into the veins, soft tissue, or disk was observed in 33% of all of the treated VCFs and resulted in no complications.
CONCLUSIONS:
The incidence of an adjacent VCF 1 year or less after vertebroplasty was comparable with that expected for untreated osteoporotic VCFs. Neither the volume of cement injected nor extravasation of cement into the intravertebral disk affected the likelihood of subsequent adjacent VCFs. Cement volume did not correlate with pain relief.
Percutaneous vertebroplasty is a relatively noninvasive, low-risk procedure1,2 that provides immediate and durable pain relief3–5 and improved function6,7 to patients with painful vertebral compression fractures (VCF).
Although vertebroplasty has a low complication rate,1,2 it is not without risk. Among these is the potential for the procedure itself to increase the risk for new VCFs in untreated vertebral bodies at other levels.8–12 Such fractures have been reported within a year following vertebroplasty,10 with subsequent new VCFs adjacent to the primary VCF occurring earlier than those nonadjacent to the primary VCF.11 Although the cause is not understood, it has been proposed that the subsequent new VCFs adjacent to the primary VCF may be caused by the augmented stiffness of the treated vertebrae as a result of the amount of cement injected or as a result of cement leakage in the adjacent vertebral disk space.13,14 Alternatively, it has also been proposed that the new VCFs may be attributable to the natural progression of osteoporosis.2
Although percutaneous vertebroplasty has been performed for more than 20 years, no standard methodology for the procedure exists. The variability in the manner in which the procedure is performed is largely due to the fact that the optimal amount and distribution of cement needed for stabilization of VCFs are unknown, as well as to perceptions that injection of larger volumes of cement leads to better outcomes as a result of increased strength and stiffness and improved internal casting and immobilization through complete filling of the vertebral body. However, injection of larger volumes of cement may also result in increased risk to the patient as a result of extravasation of the cement into the soft tissue, veins, or disk.
We conducted a prospective observational study in successive patients who were undergoing vertebroplasty, primarily to treat osteoporotic VCFs, to evaluate: 1) the potential of vertebroplasty to increase the likelihood of a subsequent adjacent VCF within 1 year of the procedure; 2) the correlation between the amount of cement injected and pain relief; and 3) the consequences of cement extravasation into the soft tissue, veins, or disk.
Materials and Methods
Patients
Between April 30, 2002, and June 26, 2008, a total of 403 successive patients (298 women and 105 men) aged 35 to 98 years (mean age, 76.7 ± 10.3 years) underwent vertebroplasty at our institution to treat painful VCFs because of osteoporosis (357 patients), trauma (35 patients), or metastatic carcinoma (11 patients). All of the VCFs were confirmed by imaging studies, and all of the patients had moderate to severe back pain that was refractory to conventional medical therapy (bed rest, pain medication, and/or bracing). Written informed consent was obtained from all of the patients.
The institutional review board of Borgess Medical Center (Kalamazoo, Mich) approved this study.
Vertebroplasty Procedures
A total of 720 VCFs in 403 patients were treated by vertebroplasty between April 30, 2002, and June 26, 2008. Of the 720 VCFs, 544 of the VCFs were first-time fractures (referred to as primary fractures), and 176 of the VCFs (in 84/403 patients) were new fractures, adjacent or nonadjacent to, the primary fracture (referred to as secondary fractures).
Percutaneous vertebroplasty with polymethylmethacrylate (PMMA) was performed by 1 physician (F.A.-A.) in a consistent manner for all patients. The patient was placed in the prone position on an angiography table under sterile conditions, and intravenous fentanyl citrate and midazolam were administered to induce conscious sedation. The vertebral body was localized with fluoroscopic control, and the skin overlying this area was prepared. The skin over the pedicle and the periosteum of the pedicle were anesthetized with 0.25% bupivacaine, and an 11- or 13-gauge needle was placed and then advanced through the pedicle into the vertebral body under biplanar fluoroscopic guidance. The targeted placement of the needle tip was in the anterior third of the vertebral body near the midline.
The PMMA was prepared and injected by use of a vertebroplasty injection system (Spineplex; Stryker Interventional Pain, Kalamazoo, Mich). Injection of cement continued until the cement filled the known cavity within the vertebral body and reached the posterior third of the vertebral body. If significant leakage of cement occurred, the needle was pulled back a few millimeters. After an approximate 2-minute wait to allow the cement to harden, the injection was resumed.
A unipedicular approach was used in 97% (ie, for 701 of the total 720 VCFs that were treated by vertebroplasty) of the procedures, and a bipedicular approach was used in 3% (ie, for 19 of the total 720 VCFs that were treated by vertebroplasty) of the procedures. The bipedicular approach was only used if diffusion of cement was insufficient after treatment on 1 side.
Patients were required to remain supine for 30 minutes after the procedure had been completed and were discharged from the hospital once it had been determined that they were ambulatory and medically stable.
Clinical Outcome Measures
The patients assessed their pain intensity immediately before the vertebroplasty procedure on a visual analog scale (VAS) on which 0 corresponded to “no pain” and 10 corresponded to the “most severe pain that the patient had ever had.” Patients used the same VAS to assess their pain intensity at 1 day, 1 week, 1 month, and 1 year after the vertebroplasty procedure. The postprocedure pain assessments were obtained via telephone contacts between the study nurse and the patients.
During each of the postprocedure telephone contacts, the patients were asked to perform the maneuver (eg, bending over, coughing, twisting, walking) that had caused them their back pain before the vertebroplasty procedure and to rate their current level of pain during the maneuver as “gone,” “better,” or “worse.” The patients were also asked to call us immediately if they experienced any new back pain. If a patient reported new back pain, an MR image or bone scan (if the patient had a pacemaker) was obtained to confirm the presence of a new VCF.
Determination of Cement Volume
The volume of cement injected into each vertebral body was recorded. Radiographs that were taken during the vertebroplasty procedure were examined to determine the percentage of anterior fill and the percentage of posterior fill of the vertebrae, as well as to determine whether extravasation of the cement into the veins, soft tissue, or disk space had occurred. The percentage of anterior fill and the percentage of posterior fill were established by drawing a line on the lateral view of the vertebrae and approximating the percentages of the anterior and posterior vertebrae that had been filled with cement.
Statistical Analyses
We performed the statistical analyses using only data for patients with osteoporotic VCFs (as the cause of the VCFs was osteoporosis in 89% of our patients). Summary statistics were used to summarize continuous variables, and frequency distributions were used to summarize categoric variables. We examined correlations between continuous variables by computing Pearson correlation coefficients. Associations between categoric variables were investigated with χ2 analyses for contingency tables. Multiple logistic regression analysis was performed to identify potentially significant predictors of recurrent VCF of an adjacent vertebra. A complete model approach was followed up by forward selection and backward elimination methods for confirmation of results. A P value of < .05 was considered statistically significant.
Results
Patients
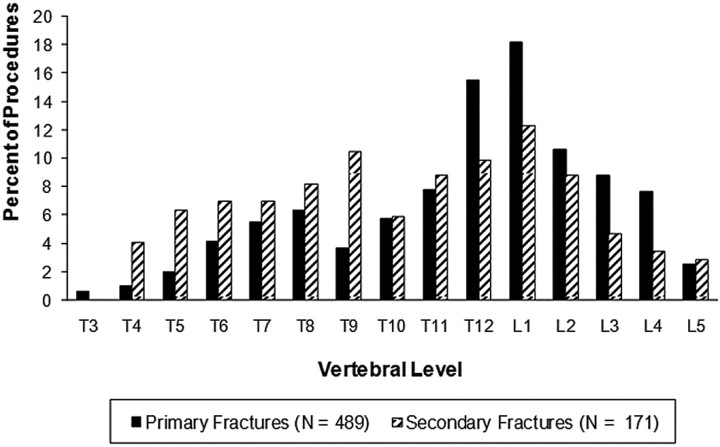
A total of 357 patients ranging in age from 40 to 98 years (mean age, 77.5 years) with osteoporotic VCFs underwent vertebroplasty at our institution between April 30, 2002, and June 28, 2008 (Table 1); most of the patients (74%) were women. In total, 660 VCFs were treated; 489 of the VCFs were primary fractures (ie, first-time fractures) and 171 were subsequent new fractures either adjacent or nonadjacent to the primary fracture (ie, secondary fractures). The most common fracture levels were T12, L1, and L2 (Fig).
Table 1:
Characteristics of patients with osteoporotic vertebral compression fractures (N = 357)
| Characteristic | |
|---|---|
| Mean age ± SD (range), years | 77.5 ± 9.5 (40–98) |
| No. (%) female | 263 (74) |
| Total number of VCFs | 660 |
| No. (%) primary VCFs* | 489 (74) |
| No. (%) secondary VCFs† | 171 (26) |
| Preprocedure VAS pain intensity—all fractures | |
| N | 652 |
| Mean ± SD | 7.9 ± 2.2 |
| Median | 8.0 |
| Range | 0–10 |
| Postprocedure VAS pain intensity—all fractures | |
| N | 560 |
| Mean ± SD | 1.6 ± 2.8 |
| Median | 0 |
| Range | 0–10 |
| Pain improvement—all fractures | |
| N | 560 |
| Mean ± SD | −6.2 ± 3.5 |
| Median | −7.0 |
| Range | −10.0–6.0 |
Note:—VAS indicates visual analog scale.
Primary vertebral compression fractures (VCFs) are first-time fractures for which vertebroplasty was performed.
Secondary VCFs are subsequent new fractures that are adjacent or nonadjacent to the primary fractures.
Figure.
Distribution of primary and secondary vertebral compression fractures in patients with osteoporotic vertebral compression fractures. Note: Primary fractures are first-time fractures for which vertebroplasty was performed. Secondary fractures are subsequent new fractures that are adjacent or nonadjacent to the primary fractures.
Volume and Distribution of Injected Cement
For all VCFs, the volume of cement injected ranged from 1.0 to 16.0 mL, for a mean of 5.1 mL (Table 2). The mean volume of cement injected was 3.5 mL for vertebral level T3-T8, 5.0 mL for vertebral levels T9-T12, and 6.0 mL for vertebral levels L1-L5. The mean anterior fill was 64%, and the mean posterior fill was 54% for all VCFs.
Table 2:
Volume of cement injected and distribution (osteoporotic vertebral compression fractures)
| All Fractures (N = 660) | |
|---|---|
| Amount of cement injected (cc)—all fractures | |
| N | 652 |
| Mean ± SD* | 5.1 ± 2.2 |
| Range | 1.0–16.0 |
| Amount of cement injected by spinal group (cc) | |
| T3–T8 | |
| N | 151 |
| Mean ± SD | 3.5 ± 1.2 |
| Range | 1.0–8.0 |
| T9–T12 | |
| N | 218 |
| Mean ± SD | 5.0 ± 2.0 |
| Range | 1.0–11.0 |
| L1–L5 | |
| N | 283 |
| Mean ± SD | 6.0 ± 2.3 |
| Range | 1.0–16.0 |
| Anterior cement fill (%) | |
| N | 649 |
| Mean ± SD | 63.8 ± 23.1 |
| Range | 0–100 |
| Posterior cement fill (%) | |
| N | 649 |
| Mean ± SD | 54.0 ± 23.9 |
| Range | 0–100 |
| Cement crossed midline | |
| N | 660 |
| Yes | 555 (84%) |
| Any extravasation | |
| N | 660 |
| Yes | 219 (33%) |
| Intravascular extravasation | |
| N | 660 |
| Yes | 61 (9%) |
| Extravascular extravasation | |
| N | 660 |
| Yes | 164 (25%) |
| Extravasation into disk | |
| N | 660 |
| Yes | 111 (17%) |
Extravasation of cement into the veins, soft tissue, or disk was observed in 33% of all of the treated VCFs (Table 2). Extravasation into the disk occurred in 17% of the treated vertebrae and accounted for 51% of all cases of extravasation. No complications occurred as a result of extravasation of cement into the veins, soft tissue, or disk.
Secondary VCFs within 1 Year of Vertebroplasty Procedure
Of the 357 patients, 66 (18%) underwent a second vertebroplasty procedure within 1 year of the first vertebroplasty to repair a subsequent new VCF that was either adjacent to or nonadjacent to the primary fracture. The secondary fracture was adjacent to the primary VCF in 44 (12%) of the 357 patients.
A multiple logistic regression analysis was performed to identify potentially significant predictors of recurrent fracture of an adjacent vertebra. A complete model approach was followed up by forward selection and backward elimination methods for confirmation of results. The dependent variable was a primary VCF with 1 or more associated adjacent secondary VCFs (yes or no), and the independent variables were age, sex, volume of cement injected, percentage of anterior fill, percentage of posterior fill, extravasation of cement (yes or no), vertebral level (T3-T8, T9-T12, L1-L5), cement crossed the midline (yes or no), fracture line filled (yes or no), and extravasation into the disk (yes or no).
In the complete model, the volume of cement injected and fracture level were identified as significant predictors (P = .0487 and P = .0021, respectively, χ2 test), and the percentage of posterior fill was identified as a marginally significant predictor (P = .0884, χ2 test) of a secondary adjacent VCF. A forward selection and backward elimination model was then used to confirm this result. In this latter model, only fracture level was identified as a significant predictor of a subsequent adjacent VCF (P = .007, χ2 test), with secondary adjacent VCFs tending to occur more frequently in patients whose primary VCFs occurred at levels T3-T8 than in those whose primary VCFs occurred at levels T9-T12 or at levels L1-L5.
Correlation between the Amount of Cement Injected and Pain Relief
The mean preprocedure and postprocedure pain scores for all treated vertebrae were 7.9 ± 2.2 and 1.6 ± 2.8, respectively, for a mean pain improvement score of −6.2 ± 3.5 (Table 1). The volume of cement injected ranged from 1.0 to 16.0 mL, for a mean of 5.1 ± 2.2 mL (Table 2). No correlation was observed between the volume of cement injected and pain improvement (r = −0.029, P = .5027).
Discussion
The objective of percutaneous vertebroplasty is the reduction of pain caused by a VCF. However, the mechanism by which percutaneous vertebroplasty results in the improvement or resolution of pain is not known. It is thought that the cement restores the biomechanical integrity of the fractured vertebra, which leads to a return of its rigidity and robustness15 and a reduction of the stress on the intraosseous and periosteal nerve endings accentuated by the movement at the site of fracture. The effectiveness of the procedure for providing immediate and durable pain relief has been well documented.
Multiple authors question whether vertebroplasty increases the likelihood of new VCF, so one of the objectives of our study was to evaluate the potential of vertebroplasty to increase the likelihood of a subsequent adjacent VCF. Of the 357 patients with osteoporotic VCFs who underwent vertebroplasty in our study, 66 (18%) subsequently underwent a second vertebroplasty procedure within 1 year of the first procedure to repair a new VCF. Of those patients with secondary fractures, 44 patients experienced secondary fractures adjacent to the primary fracture (12% of the 357 patients). These results (18% for any secondary fracture and 12% for adjacent compression fracture) are very similar to the 1-year incidence of secondary VCFs in patients with untreated fractures (19%)11,16 and suggest that vertebroplasty does not substantially increase the likelihood of a new VCF. Rather, these data demonstrate that the occurrence of subsequent adjacent VCFs may, as others have suggested, be the result of the natural course of the osteoporosis itself.2
It is interesting to note that the only significant predictor of a subsequent adjacent VCF in our study was the level of the primary VCF (P = .007, χ2 test). Secondary adjacent VCFs, however, tended to occur more frequently in patients whose primary VCFs occurred at levels T3 to T8 than in those at levels T9 to T12 or at levels L1 to L5. Our hypothesis for this spatial distribution (clustering of fractures) is that, all things being equal in patients with osteoporosis, it is expected that a primary VCF will occur in the area of maximal stress to the spine. Hence, it is expected that any subsequent VCF would occur in the same segment of the spine because it is the area of maximal stress. It is also important to note that the area of maximum stress on the spine will vary from patient to patient, depending on the patient's posture, degree of kyphosis, and type of activity. Our study confirms this premise because approximately 41% (70/171) of the new fractures in the patients in our study occurred adjacent to a new vertebroplasty (Fig). The higher incidence of secondary VCF at the midthoracic level is an interesting finding for which we have no clear explanation, but it most likely reflects a shift in the maximal area of stress on the spine because of kyphotic deformity and posture.
It has been proposed that subsequent secondary VCFs may be caused by the augmented stiffness of the treated vertebrae as a result of the amount of cement injected or cement leakage in the adjacent vertebral disk space.13,14 However, in our study, the volume of cement injected was not found to be a significant predictor of subsequent secondary adjacent VCFs, a finding that is in agreement with those of others.17,18 The results of a recent biochemical study,19 which investigated the effects of bone mineral attenuation on the mechanical strength and stiffness of vertebral bodies left untreated (intact) or 4%, 12%, or 24% filled with cement, showed that only the highest fill volume resulted in improved stiffness relative to the untreated fractures. However, the stiffness was not restored to prefracture levels, and improvements in stiffness and strength were found to depend significantly on bone attenuation, with highly osteoporotic vertebrae showing the least benefit. This study concluded that the mechanical benefits offered by additional cement may be limited by the bone mineral attenuation and that, therefore, the volume of cement injected should be limited to the amount needed for fracture reduction.
A second biomechanical study20 has shown that only a small amount of cement (14%) is needed to restore stiffness of a damaged vertebral body to the predamaged level and that large fill volumes may not be the most biomechanically optimal. Taken together, the results of these studies suggest that large volumes of cement should not be routinely injected, as such volumes offer no benefits to the patient in terms of fracture repair. Biochemical data also suggest that cement volumes on the order of 30% of the vertebral body volume are sufficient to achieve good outcomes21; depending on the vertebral level, this value corresponds to a cement volume between 4 and 8 mL.21 As there seems to be no correlation between the volume of cement injected and the occurrence of a new adjacent VCF, the degree of osteoporosis before intervention may be the determining factor of a new adjacent VCF.
The optimal amount of cement needed to achieve pain relief is not documented in the literature, so our study included an investigation of whether a correlation exists between the volume of cement and pain relief. Cement volumes as large as 15 mL22 and as small as 1 mL5 have been reported to result in pain relief and in satisfactory outcomes in patients with VCFs in other studies. The volume of cement injected for all vertebral levels ranged from 1 to 16 mL; for a mean of 5.1 mL, in our study. In agreement with others,23,24 our study found no correlation between the volume of cement injected and pain relief (r = −0.029, P = .5027). The volume of cement that was injected in the patients in our study was sufficient to fill the intravertebral cleft, as filling of the intravertebral cleft is known to be needed for long-term pain relief.25 Thus, the results of our study show that, as long as the intravertebral cleft is filled, the volume of cement is not a determining factor of the degree of pain relief.
Extravasation of cement into the veins, soft tissue, or disk was observed in 33% of treated VCFs in our study. Extravasation inside the disk was observed in 17% of patients with treated VCFs and accounted for 51% of all cases of extravasation. No complications of extravasation into the veins, soft tissue, or disk were observed. Other investigators have found a relationship between extravasation of cement into the disk and the occurrence of new VCFs18,26; however, our study did not identify extravasation of cement into the disk (which occurred in 17% of the treated vertebrae) as a significant predictor of subsequent secondary adjacent VCFs.
More than 95% of our procedures were performed by using a unipedicular approach. A bipedicular approach was used only if diffusion of cement was insufficient after treatment on 1 side. By this, we mean that when injecting the first needle, most of the cement did extravasate into the soft tissue because of fracture in the vertebral body wall, and very little cement stayed in the vertebrae. Some concern exists that use of only 1 site to introduce the cement may result in lateral placement of cement, which may pose the risk for vertebral collapse on the nonaugmented portion of the vertebral body24; however, our experience does not support this added risk. Use of the unipedicular approach was effective in providing pain relief, without risk for vertebral collapse, while minimizing the risk for infection and procedure time relative to a bipedicular approach.
Conclusions
The incidence of a new adjacent VCF within 1 year after vertebroplasty was comparable with that expected for untreated VCFs in patients with osteoporosis. Neither the volume of cement injected nor extravasation of cement into the intravertebral disk significantly affected the likelihood of subsequent adjacent VCFs. Cement volume did not correlate with pain relief.
Acknowledgments
We thank Tom Oliphant, PhD, for statistical analysis and Lillian Neff for medical writing (Innovative Analytics, Kalamazoo, Mich).
Footnotes
This study was supported by a grant from Stryker Corp., Kalamazoo, Mich.
References
- 1. Baumann C, Fuchs H, Kiwit J, et al. Complications in percutaneous vertebroplasty associated with puncture or cement leakage. Cardiovasc Intervent Radiol 2007;30:161–68 [DOI] [PubMed] [Google Scholar]
- 2. Ploeg WT, Veldhuizen AG, The B, et al. Percutaneous vertebroplasty as a treatment for osteoporotic vertebral compression fractures: a systematic review. Eur Spine J 2006;1:1749–58 [DOI] [PubMed] [Google Scholar]
- 3. Evans AJ, Jensen ME, Kip KE, et al. Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty retrospective report of 245 cases. Radiology 2003;226:366–72 [DOI] [PubMed] [Google Scholar]
- 4. McGraw JK, Lippert JA, Minkus KD, et al. Prospective evaluation of pain relief in 100 patients undergoing percutaneous vertebroplasty: results and follow-up. J Vasc Interv Radiol 2002;13:883–86 [DOI] [PubMed] [Google Scholar]
- 5. Diamond TH, Champion B, Clark WA. Management of acute osteoporotic vertebral fractures: a nonrandomized trial comparing percutaneous vertebroplasty with conservative therapy. Am J Med 2003;114:257–65 [DOI] [PubMed] [Google Scholar]
- 6. Zoarski GH, Snow P, Olan WJ, et al. Percutaneous vertebroplasty for osteoporotic compression fractures: quantitative prospective evaluation of long-term outcomes. J Vasc Interv Radiol 2002;13:139–48 [DOI] [PubMed] [Google Scholar]
- 7. Winking M, Stahl JP, Oertel M, et al. Treatment of pain from osteoporotic vertebral collapse by percutaneous PMMA vertebroplasty. Acta Neurochir (Wien) 2004;146:469–77 [DOI] [PubMed] [Google Scholar]
- 8. Kallmes DF, Jensen ME. Percutaneous vertebroplasy. Radiology 2003;229:27–36 [DOI] [PubMed] [Google Scholar]
- 9. Jensen ME, Kallmes DF. Does filling the crack break more of the back? AJNR Am J Neuroradiol 2004;25:166–67 [PMC free article] [PubMed] [Google Scholar]
- 10. Syed MI, Patel NA, Jan S, et al. New symptomatic vertebral compression fractures within a year following vertebroplasty in osteoporotic women. AJNR Am J Neuroradiol 2005;26:1601–04 [PMC free article] [PubMed] [Google Scholar]
- 11. Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. AJNR Am J Neuroradiol 2006;27:217–23 [PMC free article] [PubMed] [Google Scholar]
- 12. Trout AT, Kallmes DF. Does vertebroplasty cause incident vertebral fractures? A review of available data. AJNR Am J Neuroradiol 2006;27:1397–403 [PMC free article] [PubMed] [Google Scholar]
- 13. Grados F, Depriester C, Cayrolle G, et al. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford) 2000;39:1410–14 [DOI] [PubMed] [Google Scholar]
- 14. Uppin AA, Hirsch JA, Centenera LV, et al. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology 2003;226:119–24 [DOI] [PubMed] [Google Scholar]
- 15. Belkoff SM, Mathis JM, Jasper LE, et al. The biomechanics of vertebroplasty. The effect of cement volume on mechanical behavior. Spine 2001;26:1537–41 [DOI] [PubMed] [Google Scholar]
- 16. Fribourg D, Tang C, Sra P, et al. Incidence of subsequent vertebral fracture after kyphoplasty. Spine 2004;29:2270–76 [DOI] [PubMed] [Google Scholar]
- 17. Voormolen MH, Lohle PN, Juttmann JR, et al. The risk of new osteoporotic vertebral compression fractures in the year after percutaneous vertebroplasty. J Vasc Interv Radiol 2006;17:71–76. [DOI] [PubMed] [Google Scholar]
- 18. Komemushi A, Tanigawa N, Kariya S, et al. Percutaneous vertebroplasty for osteoporotic compression fracture: multivariate study of predictors of new vertebral body fracture. Cardiovasc Intervent Radiol 2006;29:580–85 [DOI] [PubMed] [Google Scholar]
- 19. Graham J, Ahn C, Hai N, et al. Effect of bone density on vertebral strength and stiffness after percutaneous vertebroplasty. Spine 2007;32:E505–11 [DOI] [PubMed] [Google Scholar]
- 20. Liebschner MA, Rosenberg WS, Keaveny TM. Effects of bone cement volume and distribution on vertebral stiffness after vertebroplasty. Spine 2001;26:1547–54 [DOI] [PubMed] [Google Scholar]
- 21. Molloy S, Mathis JM, Belkoff SM. The effect of vertebral body percentage fill on mechanical behavior during percutaneous vertebroplasty. Spine 2003;28:1549–54 [PubMed] [Google Scholar]
- 22. Cotten A, Deprez X, Migaud H, et al. Malignant acetabular osteolyses: percutaneous injection of acrylic bone cement. Radiology 1995;197:307–10 [DOI] [PubMed] [Google Scholar]
- 23. Kaufmann TJ, Trout AT, Kallmes DF. The effects of cement volume on clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol 2006;27:1933–37 [PMC free article] [PubMed] [Google Scholar]
- 24. Hodler J, Peck D, Gilula LA. Midterm outcome after vertebroplasty: predictive value of technical and patient-related factors. Radiology 2003;227:662–68 [DOI] [PubMed] [Google Scholar]
- 25. Mathis JM. Vertebroplasty for vertebral fractures with intravertebral clefts. AJNR Am J Neuroradiol 2002;23:1619–20 [PMC free article] [PubMed] [Google Scholar]
- 26. Lin EP, Ekholm S, Hiwatashi A, et al. Vertebroplasty: cement leakage into the disc increases the risk of new fracture of adjacent vertebral body. AJNR Am J Neuroradiol 2004;25:175–80 [PMC free article] [PubMed] [Google Scholar]