Abstract
BACKGROUND AND PURPOSE: White matter (WM) injury in carbon monoxide (CO) intoxication is thought to be related to delayed cognitive sequelae. To determine if microstructural changes in WM are responsible for the delayed onset of cognitive symptoms, we performed diffusion tensor imaging (DTI) in patients with CO intoxication.
MATERIALS AND METHODS: DTI was performed in 14 patients with delayed sequelae after CO intoxication and in 16 sex- and age-matched healthy volunteers. The fractional anisotropy (FA) and apparent diffusion coefficient (ADC) of several WM regions were measured. We also determined the correlation between FA of the selected WM and neuropsychological rating scores for the CO intoxication group.
RESULTS: FA of patients with CO intoxication decreased in the corpus callosum, orbitofrontal WM, high frontal WM, parietal WM, and temporal lobes in comparison with the corresponding regions of healthy controls. FA of the WM in the occipital lobe and internal capsule of patients was not significantly different from that in controls. ADCs of all measured WM increased significantly in patients exposed to CO. High correlations were found between the FA of all selected WM and the Mini-Mental State Examination score (γ = 0.631, P = .016) and the digit span backward task (γ = 0.759, P = .001).
CONCLUSIONS: CO intoxication may cause FA decline in associated cortical areas. This observation indicates microstructural WM pathology in CO intoxication, which is related to delayed cognitive encephalopathy.
Many individuals with acute carbon monoxide (CO) intoxication die or survive with permanent neurologic sequelae despite adequate treatment. One third of the patients are lucid for 2–40 days and then develop encephalopathy gradually.1,2 Clinical manifestations include cognitive impairment, akinetic mutism, sphincteric incontinence, gait ataxia, and extrapyramidal syndrome (eg, chorea, dystonia, and parkinsonism).2 Cognitive sequelae include impairments in memory, executive function, attention, visual spatial skills, mental processing speed, and apraxia and dementia.3 Formal neuropsychological tests have confirmed these impairments.3,4
After acute CO intoxication, patients may develop progressive white matter (WM) demyelination.5 This damage is variable and ranges from discrete perivascular foci in the corpus callosum to extensive periventricular demyelination and axonal destruction.6 The damage can disconnect the cortex and lead to functional disintegration of neurocognitive networks,7 a process very likely related to delayed CO encephalopathy.8,9 With conventional MR imaging, WM lesions are identified in 12%–37% of patients, but the findings are not related to the severity of intoxication.10,11 Imaging appears to be relatively insensitive for assessing the clinical consequences of neurotoxicity in these patients.
It has been proposed that new advanced MR imaging techniques improve diagnostic accuracy and deepen our understanding of the pathophysiology of CO intoxication.8,12-15 Among these techniques, diffusion tensor imaging (DTI) is a quantitative and noninvasive method that permits clinicians to delineate the anatomy of WM pathways by measuring molecular diffusion-driven displacement in restricted organized tissues. The directionality of water diffusivity can be quantified by measuring fractional anisotropy (FA), whereas the apparent diffusion coefficient (ADC) indicates the degree of molecular mean diffusivity.16 These indices have been applied in many WM diseases such as neural degeneration disease, demyelination, and loss of neural packing attenuation.17,18 Decreased FA among older individuals shows a high correlation with the decline of cognitive abilities such as working memory and verbal fluency.19,20
Accordingly, we performed DTI in 14 patients with delayed encephalopathy after CO intoxication and 16 healthy adults. Our goal was to investigate possible microstructural changes in WM following CO intoxication. Correlations between DTI indices and neuropsychological results were also analyzed for associations between WM lesions and cognitive impairment.
Materials and Methods
Patients
Fourteen patients with delayed sequelae after CO intoxication (8 men; mean age, 41.6 ± 11.7 years; range, 24–62 years) and 16 age-, sex-, and education-matched healthy subjects (8 men; mean age, 40.2 ± 10.8 years; range, 26–59 years) were recruited and underwent MR imaging examinations. All these patients and controls were right-handed. Patients were eligible if they had CO intoxication caused by burning of charcoal or gas intoxication in an enclosed space and/or if they had a carboxyhemoglobin level ≥10%. Delayed encephalopathy was defined as a combination of the following events: an initial change in consciousness due to CO exposure, recovery from the acute stage, a lack of symptoms for days to weeks, and then exacerbation associated with neurologic and/or psychiatric symptoms.1
This study was approved by the ethics committee of Chang Gung Memorial Hospital. All subjects provided written informed consent before MR imaging, and all underwent detailed clinical and neurologic examinations. No subject had a history of neurologic or psychiatric illness, none were using medications for unrelated conditions, and none had head injury.
MR Imaging Acquisition
MR imaging was performed by using a 3T unit (Signa Excite HD; GE Healthcare, Milwaukee, Wis). All sections were acquired along the anterior commissure–posterior commissure plane, which was ensured by a multiplanar T1-weighted localizer image before each study. Both axial T1-weighted images (TR/TE = 500 ms/minimum, matrix = 224 × 224, section thickness = 5 mm, intersection gap = 2 mm, FOV = 24 × 24 cm) and axial T2-weighted images (TR/TE = 4200/102 ms, matrix = 224 × 224, section thickness = 5 mm, intersection gap = 2 mm, FOV = 24 × 24 cm) were obtained.
DTI datasets were acquired by using single-shot diffusion spin-echo echo-planar imaging with a TR/TE of 7000/72 ms, a section thickness of 5 mm, a matrix of 128 × 128, and an FOV of 24 × 24 cm, yielding an in-plane resolution of 1.875 mm. DTI encoding entailed 25 noncollinear directions with b = 1000 s/mm2 and a nondiffusion T2-weighted image.
Twenty-eight contiguous sections (n = 28) were obtained without an intersection gap to achieve total cerebral coverage. Images were transferred to an off-line workstation equipped with FuncTool DTI (GE Healthcare) to obtain FA and ADC maps.
Neuropsychological Testing
All neuropsychological tests were performed by a clinical psychologist who was blinded to the patient's CO exposure status. The MR imaging results were interpreted by an experienced neurologist who was also blinded. The Mini-Mental State Examination (MMSE) was used as a screen.21 Executive functions were evaluated by using digit span forward and backward studies to reflect the attention (immediate registration) and the working memory (ie, the ability to hold information in short-term memory while simultaneously manipulating the information). Raw scores were used for further statistical analysis.22 To evaluate verbal fluency, subjects were asked to name as many items as possible from semantic categories (animals and vegetables).23
DTI Data Analysis
Regions of interest, 50–100 mm2 depending on the anatomic region, were measured by 1 radiologist and confirmed by another radiologist to avoid malpositioning (Fig 1). These measurements were performed according to the method described by Bozzali et al.24 Regions of interest were placed on the genu and the splenium of the corpus callosum, the anterior and the posterior limbs of the internal capsule, and the WM of the subcortico-orbitofrontal, high frontal, parietal, occipital, and temporal regions. For temporal lobe WM, we sampled 2 contiguous sections. Regions of interest were placed lateral to the temporal horn of the lateral ventricle (Fig 1A). The anterior internal capsule (bounded by the head of the caudate nucleus and the globus pallidus) and the posterior internal capsules (defined by the globus pallidus and the thalamus) were measured on 2 contiguous sections. In the orbitofrontal WM, regions of interest were bilaterally placed on the most caudal section of the lateral ventricles. Occipital lobe regions of interest were placed in the optic radiations of 2 contiguous sections, starting from the most caudal section on which the occipital horn of the lateral ventricle was imaged (Fig 1B). Regions of interest at the genu and splenium of the corpus callosum were placed on 3 consecutive sections on which they were fully volumed (Fig 1C). Regions of interest at the high frontal and parietal lobes were positioned in the WM, anterior and posterior to the central sulcus on the most caudal section on which they were visible and on the subsequent cranial section (Fig 1D).
Fig 1.
Location of the WM regions of interest in a patient with CO intoxication. A, WM of temporal lobes. B, WM of orbitofrontal, occipital, anterior, and posterior limbs of the internal capsule. C, Genu and splenium of the corpus callosum. D, WM of the frontal and parietal lobes.
Cerebral structures were carefully identified on T1- and T2-weighted images to avoid partial volume averaging due to CSF. Regions of interest were drawn on a null image of DTI and were automatically transferred onto FA and ADC maps for each subject. Average FA and ADC of 16 regions of interest were calculated. The raters were blinded to the subject's details.
Statistical Analysis
Two separate statistical analyses were performed. FA and ADC values for the patients with CO intoxication and the control group were logarithmically transformed to improve normality, and comparisons between the 2 groups were made by using the Student t test. Because both the FA of the measured WM and the mean scores for neuropsychological findings were not normally distributed, Spearman rank correlation analysis rather than the Pearson correlation was used to explore the relationship between the 2 for the CO-intoxication group. P < .05 was deemed as showing statistical significance. Statistical analyses were performed by using the Statistical Package for the Social Sciences software, Version 10.0 (SPSS, Chicago, Ill).
Results
Patients
Table 1 shows the patients’ demographic data. Patients 6, 9, 11, 12, and 13 had elevated carboxyhemoglobin levels of 25%, 24.5%, 23.6%, 26.9%, and 25.6%, respectively, in the emergency department. Nine patients with a history of CO intoxication were recruited due to delayed sequelae, whereas their initial carboxyhemoglobin levels were not available. Causes were gas intoxication in patient 6 and charcoal burning in the other 13 patients. All patients were initially comatose or had disturbed consciousness and regained full consciousness within hours to days after their acute exposure. Ten patients accepted hyperbaric oxygen treatment within days, whereas patients 2, 3, 8, and 11 were treated with normobaric oxygenation by face mask. The mean lucid interval from intoxication to the onset of delayed sequelae was 16.9 days (range, 6–42 days). Eleven patients had MR imaging and neuropsychological testing in the subacute phase; 3 patients were in the chronic phase (>1 year). MR imaging was performed between 3 and 442 days (median, 43 days; mean, 123.1 days) after the onset of delayed encephalopathy, and neuropsychological testing was performed between 2 and 423 days (median, 23 days; mean, 113.1 days).
Table 1:
Demographic data of patients with delayed encephalopathy after CO intoxication
| Patient/Age (yr)/Sex | Neurologic Features | WM Involved in Delayed Encephalopathy on MRI | Time (days) |
||
|---|---|---|---|---|---|
| Lucid | To MRI | To Testing | |||
| 1/57/M | Acute: coma, conscious in 2 days; delayed: impaired memory, personality change, parkinsonism, general weakness | Globus pallidus, frontal, parietal, temporal, occipital | 12 | 12 | 22 |
| 2/44/M | Acute: coma, conscious in ≤1 day; delayed: impaired cognition and memory | Globus pallidus, frontal, parietal | 42 | 83 | 44 |
| 3/26/F | Acute: coma, conscious in a few days; delayed: impaired memory, parkinsonism, amnesia, bad temper | Frontal, parietal | 13 | 120 | 115 |
| 4/35/M | Acute: coma; delayed: impaired memory, inattention, personality change, amnesia | Parietal, occipital | 20 | 31 | 24 |
| 5/34/M | Acute: coma, conscious in 1 week; delayed: ataxia, impaired memory, ideomotor apraxia | Globus pallidus, frontal, parietal | 7 | 393 | 383 |
| 6/62/M | Acute: coma, conscious in ≤1 day; delayed: confusion, impaired cognition, apraxia, amnesia, parkinsonism | Globus pallidus, frontal, parietal, occipital | 25 | 4 | 3 |
| 7/41/F | Acute: coma; delayed: parkinsonism, dysmetria, dyssynergia, urine retention, difficulty defecating | Globus pallidus, frontal, parietal | 7 | 4 | 15 |
| 8/24/F | Acute: coma; delayed: ataxia, impaired cognition | Globus pallidus, frontal, parietal | 20 | 107 | 106 |
| 9/41/F | Acute: coma; delayed: disorientation, incoherent speech, dysexecution, behavioral changes, acute dystonia | Globus pallidus, frontal, parietal | 19 | 26 | 8 |
| 10/43/M | Acute: coma, conscious in ≤1 day; delayed: impaired memory and cognition, parkinsonism | Globus pallidus, frontal, parietal | 17 | 30 | 18 |
| 11/48/M | Acute: disturbed consciousness in 1 day; delayed: depression, parkinsonism | Globus pallidus | 10 | 3 | 2 |
| 12/36/F | Acute: disturbed consciousness in 1 day; delayed: amnesia, apraxia | Globus pallidus, parietal, occipital | 23 | 414 | 414 |
| 13/37/F | Acute: coma; delayed: impaired memory and cognition | Frontal, parietal, occipital | 6 | 55 | 7 |
| 14/41/M | Acute: coma; delayed: ataxia, disorientation, urinary incontinence | No obvious lesion | 15 | 442 | 423 |
Note:—MRI indicates MR imaging.
FA and ADC Values of DTI
Among the patients with CO exposure, FA was significantly reduced relative to that in controls in the genu and the splenium of the corpus callosum and in the bilateral orbitofrontal, high frontal, parietal, and temporal WM (Fig 2). FA of the entire measured WM was significantly decreased as well (Table 2). In patients with CO exposure, FA was also reduced in regions with abundant WM, such as the anterior and the posterior limbs of the internal capsule and in the occipital WM, though differences were not significant. ADC increased significantly in patients in all measured WM (Table 3).
Fig 2.
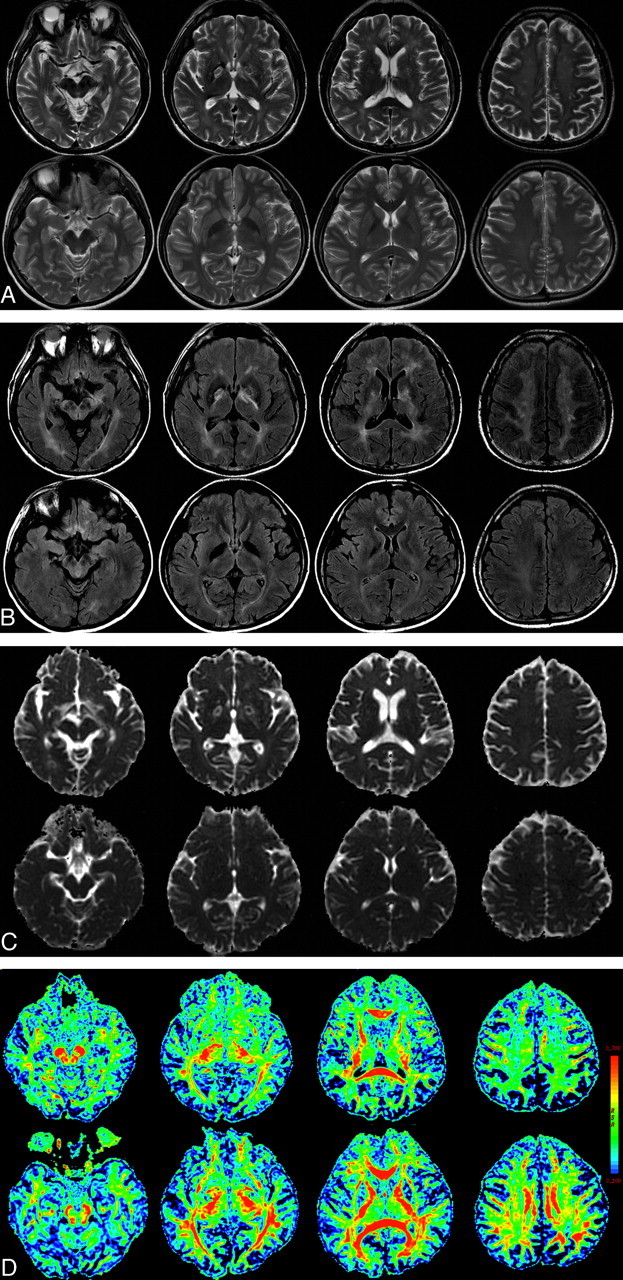
A 57-year-old man with delayed neuropsychiatric syndrome after CO intoxication (upper) and a healthy control (lower). A and B, Axial T2-weighted images (A) and FLAIR MR images (B) show symmetric necrosis of the bilateral globus pallidus and extensive hyperintensity of the subcortical WM. C, ADC map. D, The FA map of DTI reveals clearly reduced fiber integrity of the corresponding WM. The color bar represents the FA values.
Table 2:
Mean FA values of the selected WM areas from CO intoxication in patients and controls
| Region | Mean ± SD, × 10−3 |
P* | |
|---|---|---|---|
| Patients (n = 14) | Control Subjects (n = 16) | ||
| Corpus callosum, genu | 614.3 ± 97.5 | 750.6 ± 40.2 | <.001* |
| Corpus callosum, splenium | 725.7 ± 10.8 | 791.3 ± 58.0 | .044* |
| Anterior limb internal capsule, L | 536.4 ± 12.6 | 563.8 ± 54.8 | .461 |
| Anterior limb internal capsule, R | 530.7 ± 82.5 | 573.8 ± 32.6 | .085 |
| Posterior limb internal capsule, L | 627.9 ± 46.4 | 655.0 ± 59.9 | .181 |
| Posterior limb internal capsule, R | 621.4 ± 46.9 | 651.3 ± 41.8 | .086 |
| Orbitofrontal, L | 343.6 ± 71.3 | 438.1 ± 43.1 | <.001* |
| Orbitofrontal, R | 357.9 ± 75.2 | 435.6 ± 36.7 | .002* |
| High frontal, L | 322.8 ± 60.0 | 411.3 ± 38.8 | <.001* |
| High frontal, R | 312.2 ± 46.5 | 408.1 ± 22.2 | <.001* |
| Parietal, L | 380.9 ± 80.8 | 456.9 ± 46.4 | <.001* |
| Parietal, R | 361.2 ± 66.8 | 465.0 ± 38.1 | <.001* |
| Occipital, L | 406.4 ± 66.6 | 442.5 ± 42.7 | .063 |
| Occipital, R | 409.3 ± 63.3 | 446.9 ± 61.3 | .110 |
| Temporal, L | 383.6 ± 63.6 | 432.5 ± 52.1 | .028* |
| Temporal, R | 346.4 ± 67.1 | 431.3 ± 44.4 | <.001* |
| All WM areas | 456.2 ± 39.7 | 522.1 ± 17.8 | <.001* |
Note:—L indicates left; R, right; FA, fractional anisotropy; WM, white matter; CO, carbon monoxide.
<.05 indicates significance.
Table 3:
Mean ADC values of the selected WM areas from CO intoxication in patients and controls
| Region | Mean ± SD, mm2s−1 × 10−12 |
P* | |
|---|---|---|---|
| Patients (n = 14) | Control Subjects (n = 16) | ||
| Corpus callosum, genu | 1006.0 ± 117.1 | 851.3 ± 59.9 | <.001* |
| Corpus callosum, splenium | 1336.0 ± 186.2 | 784.1 ± 79.3 | .023* |
| Anterior limb internal capsule, L | 837.7 ± 73.1 | 750.4 ± 47.9 | .001* |
| Anterior limb internal capsule, R | 839.4 ± 77.7 | 731.1 ± 46.6 | <.001* |
| Posterior limb internal capsule, L | 771.9 ± 72.6 | 686.3 ± 60.3 | .001* |
| Posterior limb internal capsule, R | 750.4 ± 33.6 | 687.3 ± 50.2 | <.001* |
| Orbitofrontal, L | 942.1 ± 75.4 | 838.9 ± 77.8 | .001* |
| Orbitofrontal, R | 949.3 ± 72.7 | 878.8 ± 52.8 | .005* |
| High frontal, L | 852.1 ± 95.4 | 731.3 ± 63.9 | <.001* |
| High frontal, R | 848.1 ± 105.0 | 745.6 ± 53.8 | .005* |
| Parietal, L | 765.7 ± 72.2 | 707.8 ± 54.3 | .018* |
| Parietal, R | 796.4 ± 74.2 | 708.4 ± 55.3 | <.001* |
| Occipital, L | 825.2 ± 50.6 | 750.4 ± 51.0 | <.001* |
| Occipital, R | 818.1 ± 57.9 | 748.6 ± 70.7 | .007* |
| Temporal, L | 859.1 ± 80.1 | 788.7 ± 49.1 | .006* |
| Temporal, R | 895.0 ± 44.8 | 792.1 ± 59.2 | <.001* |
| All WM areas | 880.8 ± 123.3 | 761.3 ± 38.6 | .001* |
Note:—ADC indicates apparent diffusion coefficient.
<.05 indicates significance.
Patients 1, 6, 7, and 11 (mean age, 52.0 ± 9.3 years) had MR imaging studies performed immediately after the appearance of sequelae. The average FA and ADC of the overall selected WM in these 4 patients were 415.0 ± 26.1 (×10−3) and 942.2 ± 23.8 (mm2s−1 × 10−12), respectively. The other 10 patients (mean age, 36.0 ± 6.8 years) had MR imaging studies performed an average (mean) of 170 days after the appearance of delayed sequelae. The average FA and ADC of the entire selected WM in these 10 patients were 472.7 ± 31.6 (×10−3) and 856.2 ± 28.0 (mm2s−1 × 10−12), respectively.
Neuropsychological Results in Relation to FA Values
Among the 14 subjects, 10 patients completed their neuropsychological tests either before or within 2 weeks after the MR imaging studies, whereas the other 4 patients completed their tests anywhere between 2 and 8 weeks before or after the MR imaging studies. Table 4 summarizes the correlations between FA and the neuropsychological results. Decreased FA in the left (γ = 0.755, P = .002) and right (γ = 0.683, P = .007) orbitofrontal WM was associated with decreased MMSE scores. Decreased digit span backward was associated with FA decreases in the splenium of the corpus callosum (γ = 0.577, P = .031), in the left (γ = 0.669, P = .009) and right (γ = 0.675, P = .008) orbitofrontal WM, in the left (γ = 0.697, P = .006) and right (γ = 0.588, P = .027) parietal WM, and in the left (γ = 0.543, P = .045) and right (γ = 0.598, P = .024) temporal WM. Digit forward span and verbal fluency (ability to name animals or vegetables) were not significantly correlated with FA in any region. High correlations were also found between the average FA of the overall measured WM and the MMSE score (Fig 3A; γ = 0.631, P = .016) and the digit span backward (Fig 3B; γ = 0.759, P = .001).
Table 4:
Spearman rank correlation coefficients for neuropsychological results and FA values
| Region | MMSE Score | Digit Span |
Verbal Fluency |
||
|---|---|---|---|---|---|
| Forward | Backward | Animals | Vegetables | ||
| Corpus callosum, genu | 0.462 | −0.192 | 0.272 | −0.099 | 0.327 |
| Corpus callosum, splenium | 0.291 | −0.136 | 0.577* | −0.164 | 0.112 |
| Anterior limb internal capsule, L | 0.482 | −0.199 | 0.250 | −0.044 | 0.234 |
| Anterior limb internal capsule, R | 0.501 | 0.031 | 0.384 | −0.011 | 0.223 |
| Posterior limb internal capsule, L | 0.407 | −0.343 | 0.046 | −0.060 | 0.348 |
| Posterior limb internal capsule, R | 0.532 | −0.269 | 0.295 | 0.135 | 0.201 |
| Orbitofrontal, L | 0.755* | −0.009 | 0.669* | −0.186 | 0.410 |
| Orbitofrontal, R | 0.683* | −0.027 | 0.675* | −0.156 | 0.323 |
| High frontal, L | −0.035 | −0.410 | 0.265 | 0.102 | 0.147 |
| High frontal, R | −0.077 | −0.476 | 0.198 | 0.082 | 0.235 |
| Parietal, L | 0.470 | −0.058 | 0.697* | −0.033 | 0.211 |
| Parietal, R | 0.402 | −0.175 | 0.588* | −0.153 | 0.273 |
| Occipital, L | 0.257 | 0.120 | 0.480 | −0.185 | 0.069 |
| Occipital, R | 0.448 | 0.040 | 0.484 | −0.022 | 0.301 |
| Temporal, L | 0.393 | −0.289 | 0.543* | −0.229 | −0.009 |
| Temporal, R | 0.172 | −0.335 | 0.598* | −0.290 | −0.022 |
| All WM areas | 0.631* | −0.179 | 0.795* | −0.079 | 0.453 |
Note:—MMSE indicates Mini-Mental State Examination.
<.05 indicates significance.
Fig 3.
Scatterplots show the FA value of average overall WM versus MMSE (A) and digit span backward results (B) in patients with CO intoxication.
Discussion
White matter abnormalities are the principal neuropathologic feature of the delayed encephalopathy that follows CO intoxication. However, this feature is not pathognomonic, and images are variable on conventional MR imaging after CO poisoning.25,26 The relationship between delayed sequelae and imaging findings was ambiguous. In previous studies, researchers focused only on changes in signal intensity or water diffusivity in lesions or in the periventricular WM.3,4,8,10 Without extensive investigation, less apparent or microstructural changes in WM may be overlooked. Because different cognitive functions are related to different subanatomies, the lobar WM, the corpus callosum, and the internal capsule were separately studied to explore the causal relationship between DTI indices and clinical presentations. WM integrity was quantified by measuring the degree of molecular diffusivity and directionality. Although WM hyperintensities were also variable in our patients, DTI was more sensitive in the detection of WM changes than conventional MR imaging. Our results, which showed an extensive increase in ADC values, suggested global brain injury after CO intoxication (Table 3). The selective reduction in FA, which has been thought to originate from architectural changes in the WM, was well correlated with subsequent cognitive impairment. This finding suggests that mapping WM changes after CO intoxication in vivo with DTI is feasible.
Mechanisms of brain injury after CO intoxication include hypoxia and reduced cellular oxygen metabolism in the acute stage and lipid peroxidation leading to oxidative injury, excitotoxicity, and, later, apoptosis.10 All of these may result in WM damage. Diffusion-weighted imaging and 1H-MR spectroscopy confirmed that tissue injury was associated with the onset of delayed sequelae.8 Reduced ADC has been observed both in the acute and delayed relapse phases in patients with CO intoxication and indicates cytotoxic edema.12,15 1H-MR spectroscopy has also shown high levels of choline-containing compounds and depletion of N-acetylaspartate in the delayed phase.8 These findings are thought to reflect WM demyelination and neuronal necrosis. Our FA values indicated a reduction in microstructural integrity of the WM. In addition, high ADC indicated a decrease in diffusion restriction.17 Hence, our findings may result from decreased cellular attenuation and fiber coherence in the WM of patients with CO intoxication. Ultrastructural damage indeed occurs in the brain tissue of patients with CO intoxication during the delayed neuropsychiatric phase.
Changes in WM may reflect different underlying mechanisms. First, WM changes may indicate anterograde wallerian degeneration, especially in regions near cortical areas with the greatest pathologic burden.24 Second, WM rarefaction may be present with axonal damage and gliosis.27 This change is diffuse, it does not follow the regional extension of pathologically involved gray matter, and it may be vascular or ischemic in origin. Third, breakdown of myelin may be an important component of the disease process.28 DTI has the ability to reveal these changes in WM—degeneration, axonal damage, demyelination, and neuronal necrosis—but still cannot reveal the underlying neuropathologic basis for loss of WM integrity. Wallerian degeneration secondary to neuronal injury has been proposed as a cause for diffuse WM injury after global brain anoxia or ischemia.29 It occurs only after the neuronal perikarya are destroyed.30 Nevertheless, some experimental evidence suggests that the severe damage to nerve fibers that occurs in the WM is the direct effect of hypoxic-ischemic injury and is probably independent of the injury to the neuronal perikarya.29 The variable clinical course noted in our study and references suggests different individual vulnerabilities to CO intoxication, directly or indirectly. It is difficult to conclude which process or which mixed etiologies caused the changes in DTI indices in our study. Our hypothesis was that early cytotoxic edema after CO intoxication launches demyelination and progresses to neuronal necrosis.
Serial diffusion imaging findings in the delayed encephalopathy of CO poisoning have been reported.8,15 The ADC value decreased progressively and persisted much longer than the few days after acute cerebral infarction. The values then slowly increased and were higher 6 months after exposure than at the moment after the appearance of sequelae. The DTI findings in our patients (mean, 123.1 days after the onset of delayed encephalopathy) were consistent with earlier reports. Although parameters of a diffusion image are sensitive, revealing cell status or WM integrity, the interpretation of the clinical evolution from 1 time point imaging study is complex. We noted that patients 1, 6, 7, and 11 (mean age, 52.0 ± 9.3 years) underwent MR imaging studies immediately after the appearance of sequelae and had lower FA and higher ADC than the other 10 patients (mean age, 36.0 ± 6.8 years), whose MR imaging studies were performed with a mean time of 170 days after delayed sequelae. The latter had better WM integrity than the former, which suggests the possibility of remyelination in WM after the delayed encephalopathy following CO intoxication. However, the higher mean age of the former patients, with possible atherosclerotic chronic-ischemic changes due to aging, may have predisposed them to more severe WM injury than the younger patients. The severity in CO intoxication varied among individuals and could lead to different chronologic changes in FA and ADC values.15 Prospective sequential DTI studies are necessary to explore the underlying mechanisms.
In our study, the decrease in FA associated with CO intoxication was not homogeneously distributed (Table 2). Rather, it involved select regions of brain WM, including the corpus callosum and the frontal, parietal, and temporal lobes. It somewhat spared the internal capsule (which subserves motor functions) or the optic radiations (which subserve visual functions). Nevertheless, lower FA values in these regions were also found in the patient group. The section thickness was 5 mm, and the lack of statistically significant results for the internal capsule may be attributable to intravoxel heterogeneity. We have tried to lessen the effect of partial volume effects on FA values by using a region of interest of adequate size. However, bias may still arise, due to anatomic limitations. Chu et al12 confirmed that damage in WM is more severe in the frontal areas than in the parieto-occipital areas by using diffusion-weighted MR imaging. Single-photon emission CT scans also revealed increased hypoperfusion in the frontal lobes.12
Compared with early-myelinating regions (primary cortical regions), late-myelinating regions (cortical association areas) have relatively few oligodendrocytes to maintain heightened numbers of axons.28 In cortical association areas, oxidative stress during the ischemic and reperfusion phases of CO intoxication may amplify pathologic processes due to the high metabolic demands of oligodendrocytes. Our result of a decrease in WM integrity in late-myelinating regions supports this hypothesis.
Cognitive sequelae of CO poisoning are evident.1-4,8-15 They include hindered executive function and slowed mental processing speed, which are often observed after frontal lobe damage, and altered visuospatial abilities, which are commonly observed after parietal lobe injury.10 Central executive function involves corticocortical connectivity,31 which depends mainly on complex WM networks.32 In our study, the high correlation between the MMSE score and the digit span backward with the FA of multiple WM regions supports the hypothesis that WM microstructural changes of CO intoxication may contribute to the decline in cognitive functions. Although not all patients agreed to MR imaging and neuropsychological tests immediately after the onset of delayed sequelae, high correlations of neuropsychological tests to quantitative FA values showed that DTI is very practical in the evaluation of WM integrity and neuropsychological performance.
In this study, the digit span backward test was correlated with FA in various foci in the frontal, parietal, and temporal WM. Relative to the forward test and the verbal fluency test, digit span backward is a more complex task requiring more sophisticated planning, rehearsal, and recall strategies involving executive function that may increase cognitive load.33 We also found that digit span backward has a higher correlation with WM damage than the MMSE did. It may be due to the fact that the MMSE is biased in evaluating cognitive functions associated with cortical lesions (eg, memory, language) but less sensitive in evaluating executive dysfunction associated with subcortical lesions.
There are several limitations in this study. This is an anecdotal case series. Both the duration and intensity of CO exposure may have affected the clinical presentation of patients with neuropsychiatric disorders and their DTI findings. We selected patients because of prominent leukoencephalopathy after insult and, therefore, did not enroll patients with subclinical CO intoxication. The findings of DTI may not generalize to all patients with CO intoxication. In addition, early DTI values were not available. Although DTI is very useful in revealing WM integrity, a causal relationship between an acute cytotoxic effect and subsequent WM change cannot be determined. Moreover, the neuropsychological tests and MR imaging were not performed simultaneously, which could have biased our study. Only limited neuropsychological tests were performed, possibly underestimating the cognitive sequelae. Nevertheless, further careful study of these effects is needed. Despite these limitations, we showed that WM injury and its correlations to neuropsychological scores can be evaluated by using DTI. This issue should be examined in a prospective study of a wide variety of patients with CO intoxication.
In conclusion, DTI appears to be a sensitive tool for identifying neuropathologic and neuropsychological changes associated with CO intoxication. Our study supplements previous MR imaging studies by adding a level of anatomic detail to the relationship between WM damage and cognitive dysfunction. We also demonstrated that WM injury is globally involved in the delayed phase of CO intoxication. Although our results are based on a relatively small cohort, the strength of the clinical MR imaging correlations underpin the importance of WM pathology in CO intoxication and suggest that quantifying tissue damage of WM may improve our ability to monitor the development of CO intoxication.
Footnotes
This work was partly supported by grants from the National Science Council (NSC 97-2752-H-010-004-PAE to C.-P. Lin) and Chang Gung Memorial Hospital (Chang Gung Medical Research Project; CMRPG870481 to W.-C. Lin). The authors also acknowledge support from MR imaging core facility in National Yang-Ming University.
References
- 1.Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol 1983;40:433–35 [DOI] [PubMed] [Google Scholar]
- 2.Garland H, Pearce J. Neurological complications of carbon monoxide poisoning. Q J Med 1967;36:445–55 [PubMed] [Google Scholar]
- 3.Gale SD, Hopkins RO, Weaver LK, et al. MRI, quantitative MRI, SPECT, and neuropsychological findings following carbon monoxide poisoning. Brain Inj 1999;13:229–43 [DOI] [PubMed] [Google Scholar]
- 4.Prockop LD. Carbon monoxide brain toxicity: clinical, magnetic resonance imaging, magnetic resonance spectroscopy, and neuropsychological effects in 9 people. J Neuroimaging 2005;15:144–49 [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita T, Sugihara S, Matsusue E, et al. Pallidoreticular damage in acute carbon monoxide poisoning: diffusion-weighted MR imaging findings. AJNR Am J Neuroradiol 2005;26:1845–48 [PMC free article] [PubMed] [Google Scholar]
- 6.Lapresle J, Fardeau M. The central nervous system and carbon monoxide poisoning. II. Anatomical study of brain lesions following intoxication with carbon monoxide (22 cases). Prog Brain Res 1967;24:31–74 [DOI] [PubMed] [Google Scholar]
- 7.Geschwind N. Disconnexion syndromes in animals and man. II. Brain 1965;88:237–94 [DOI] [PubMed] [Google Scholar]
- 8.Murata T, Kimura H, Kado H, et al. Neuronal damage in the interval form of CO poisoning determined by serial diffusion weighted magnetic resonance imaging plus 1H-magnetic resonance spectroscopy. J Neurol Neurosurg Psychiatry 2001;71:250–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JH, Chang KH, Song IC, et al. Delayed encephalopathy of acute carbon monoxide intoxication: diffusivity of cerebral white matter lesions. AJNR Am J Neuroradiol 2003;24:1592–97 [PMC free article] [PubMed] [Google Scholar]
- 10.Parkinson RB, Hopkins RO, Cleavinger HB, et al. White matter hyperintensities and neuropsychological outcome following carbon monoxide poisoning. Neurology 2002;58:1525–32 [DOI] [PubMed] [Google Scholar]
- 11.Pavese N, Napolitano A, De Iaco G, et al. Clinical outcome and magnetic resonance imaging of carbon monoxide intoxication: a long-term follow-up study. Ital J Neurol Sci 1999;20:171–78 [DOI] [PubMed] [Google Scholar]
- 12.Chu K, Jung KH, Kim HJ, et al. Diffusion-weighted MRI and 99mTc-HMPAO SPECT in delayed relapsing type of carbon monoxide poisoning: evidence of delayed cytotoxic edema. Eur Neurol 2004;51:98–103 [DOI] [PubMed] [Google Scholar]
- 13.Lo CP, Chen SY, Chou MC, et al. Diffusion-tensor MR imaging for evaluation of the efficacy of hyperbaric oxygen therapy in patients with delayed neuropsychiatric syndrome caused by carbon monoxide inhalation. Eur J Neurol 2007;14:777–82 [DOI] [PubMed] [Google Scholar]
- 14.Otubo S, Shirakawa Y, Aibiki M, et al. Magnetic resonance imaging could predict delayed encephalopathy after acute carbon monoxide intoxication [in Japanese]. Chudoku Kenkyu 2007;20:253–61 [PubMed] [Google Scholar]
- 15.Terajima K, Igarashi H, Hirose M, et al. Serial assessments of delayed encephalopathy after carbon monoxide poisoning using magnetic resonance spectroscopy and diffusion tensor imaging on 3.0T system. Eur Neurol 2008;59:55–61. Epub 2007 Oct 4 [DOI] [PubMed] [Google Scholar]
- 16.Assaf Y, Beit-Yannai E, Shohami E, et al. Diffusion- and T2-weighted MRI of closed-head injury in rats: a time course study and correlation with histology. Magn Reson Imaging 1997;15:77–85 [DOI] [PubMed] [Google Scholar]
- 17.Pierpaoli C, Barnett A, Pajevic S, et al. Water diffusion changes in wallerian degeneration and their dependence on white matter architecture. Neuroimage 2001;13 (6 Pt 1):1174–85 [DOI] [PubMed] [Google Scholar]
- 18.Song SK, Sun SW, Ju WK, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003;20:1714–22 [DOI] [PubMed] [Google Scholar]
- 19.Charlton RA, Barrick TR, McIntyre DJ, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology 2006;66:217–22 [DOI] [PubMed] [Google Scholar]
- 20.O'Sullivan M, Jones DK, Summers PE, et al. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology 2001;57:632–38 [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98 [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised. San Antonio, Tex: Psychological Corp;1981
- 23.Amieva H, Lafont S, Rouch-Leroyer I, et al. Evidencing inhibitory deficits in Alzheimer's disease through interference effects and shifting disabilities in the Stroop test. Arch Clin Neuropsychol 2004;19:791–803 [DOI] [PubMed] [Google Scholar]
- 24.Bozzali M, Falini A, Franceschi M, et al. White matter damage in Alzheimer's disease assessed in vivo using diffusion tensor magnetic resonance imaging. J Neurol Neurosurg Psychiatry 2002;72:742–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durak AC, Coskun A, Yikilmaz A, et al. Magnetic resonance imaging findings in chronic carbon monoxide intoxication. Acta Radiol 2005;46:322–27 [DOI] [PubMed] [Google Scholar]
- 26.Inagaki T, Ishino H, Seno H, et al. A long-term follow-up study of serial magnetic resonance images in patients with delayed encephalopathy after acute carbon monoxide poisoning. Psychiatry Clin Neurosci 1997;51:421–23 [DOI] [PubMed] [Google Scholar]
- 27.Englund E. Neuropathology of white matter changes in Alzheimer's disease and vascular dementia. Dement Geriatr Cogn Disord 1998;(9 suppl 1):6–12 [DOI] [PubMed]
- 28.Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging 2004;25:5–18, author reply 49–62 [DOI] [PubMed] [Google Scholar]
- 29.Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke 1996;27:1641–46, discussion 47 [DOI] [PubMed] [Google Scholar]
- 30.Daniel PM, Strich SJ. Histological observations on wallerian degeneration in the spinal cord of the baboon, Papio papio. Acta Neuropathol 1969;12:314–28 [DOI] [PubMed] [Google Scholar]
- 31.Andres P. Frontal cortex as the central executive of working memory: time to revise our view. Cortex 2003;39:871–95 [DOI] [PubMed] [Google Scholar]
- 32.Morris RG, Craik FI, Gick ML. Age differences in working memory tasks: the role of secondary memory and the central executive system. Q J Exp Psychol A 1990;42:67–86 [DOI] [PubMed] [Google Scholar]
- 33.Zinn S, Bosworth HB, Hoenig HM, et al. Executive function deficits in acute stroke. Arch Phys Med Rehabil 2007;88:173–80 [DOI] [PubMed] [Google Scholar]




