Abstract
BACKGROUND AND PURPOSE: The hippocampus and parahippocampal gyrus have a central role in the acquisition of new memories. Although functional MR imaging (fMRI) can provide information on the functional status of these brain regions, it has not reached widespread use in the presurgical assessment of patients undergoing temporal lobectomy. We aimed to evaluate whether simple memory-encoding paradigms could be used to elicit robust activations in the hippocampus and parahippocampal gyrus and to determine the lateralization of verbal and nonverbal memory. We also studied the relative contribution of the anterior and posterior portions of these structures.
MATERIALS AND METHODS: We conducted this study on 16 healthy subjects by performing event-related fMRI using 3 memory encoding tasks with words, objects, and faces. In addition to a second-level group analysis, region-of-interest (ROI)–based measurements of the signal intensity percent change and of the percentage of activated voxels, determined at 2 thresholds, were performed. ROIs were drawn on the hippocampus and parahippocampal gyrus, divided into anterior and posterior segments.
RESULTS: We found overall left-lateralized activation with words, bilateral activation with objects, and right-lateralized activation with faces. In particular, significant hippocampal activations were observed with all 3 categories of stimuli, and the head of the hippocampus was generally more engaged than its body and tail. Data on the signal intensity percent change and percentage of activated voxels are provided for each ROI and task.
CONCLUSIONS: The combination of these 3 undemanding memory tasks could be considered, following appropriate validation, as a tool to assess the functional status of the medial temporal lobe in clinical settings.
It is widely documented that the hippocampus and parahippocampal gyrus are critical for memory encoding. The importance of these structures has been demonstrated by neuropsychological data, in particular by the seminal case H.M.,1 and later by many neuroimaging studies.2
The medial temporal lobe (MTL) can be affected by a focal seizure disorder often associated with hippocampal sclerosis and refractory to drugs: temporal lobe epilepsy (TLE). Although TLE can be treated by surgical intervention, temporal lobectomy may lead to a postoperative memory deficit, usually in verbal memory after dominant temporal lobectomy and in nonverbal memory after nondominant temporal lobectomy.3,4 However, as memory lateralization in patients with TLE is often atypical due to the functional reorganization induced by epileptic activity,5 the material-specific memory deficit after temporal lobectomy is not always predictable. Another problem is the considerable interindividual variability in the clinical characteristics of patients (age at surgery, age at seizure onset, and IQ6); therefore, patients with TLE are very heterogeneous also in their memory performance: patients with left-sided TLE are impaired on verbal tests, whereas patients with right-sided TLE perform significantly worse on visual tasks.7
Functional MR imaging (fMRI) has become a tool widely used for preoperative planning. Its application to memory tasks may enable prediction of material-specific memory deficits after temporal lobectomy. Toward this purpose, there is a need to develop memory paradigms that are simple to administer and that reliably activate the hippocampus and related neocortical regions.
In agreement with neuropsychological studies, functional neuroimaging has shown that lateralization during memory encoding depends on the material to be encoded: activations are left lateralized for words, right lateralized for visuospatial material, and bilateral for objects.8–10 Activations of the hippocampus and parahippocampal gyrus are enhanced by stimulus novelty during encoding of single items9,11–13 and of pairs of stimuli.14–16 It is important to note that the hippocampus exhibits a successful memory effect, with stronger activation during encoding of items that will be subsequently remembered compared with items that will be forgotten.5,17,18
As demonstrated by recent work, in addition to its role in long-term memory, the MTL also subserves higher-order perceptual functions.19 In particular, the perirhinal cortex and the anterior part of the hippocampus have a role in visual discrimination and in processing the relationships between the features that constitute an object or a scene.20
Converging evidence has shown functional differentiation between the anterior and posterior portions of the hippocampus. Although fMRI studies show that both parts are involved in memory encoding,17,21,22 there is a tendency for the anterior part to be more strongly engaged independent of task type (single item or stimulus pair) and modality (visual or auditory).10,16,18,23–26 The hypothesis of a functional specialization along the longitudinal axis of the hippocampus is supported by neuroanatomic studies in animals, which highlight distinct patterns of connectivity for different portions of the hippocampus.27–29
Despite its central role in memory functions, the hippocampus is notoriously difficult to activate in neuroimaging studies. The main determinant of this problem is that the difference in intensity of neural activity between baseline and stimulation conditions is smaller for the hippocampus compared with neocortical areas; this seems to be primarily because the hippocampus is also active in resting conditions, putatively subserving memory consolidation processes.30,31 In addition, fMRI studies are affected by signal intensity loss and magnetic susceptibility artifacts, especially in the anterior part of the MTL, because of proximity with the nasal sinuses.22
There is good agreement among studies that event-related designs are more suitable than blocked designs to elicit reliable fMRI activations of the hippocampus. This advantage is partly related to better ecologic validity of the paradigm timing and partially related to the fact that event-related analysis enables categorization of the presented stimuli according to the subject's subsequent memory performance.32
To corroborate and extend the existing literature in this field, we present results obtained in a population of healthy subjects, comparing 3 types of material: words, objects, and faces. We performed a region-of-interest (ROI)–based analysis of activations in the hippocampus and parahippocampal gyrus, considering both intensity (percent signal intensity change) and extent (voxel count). We assessed both the lateralization of verbal and nonverbal memory and the relative contribution of the anterior and posterior regions. We used a simple and undemanding event-related fMRI paradigm intended to be applicable to patients with TLE in clinical settings.
Materials and Methods
Subjects
Sixteen native Italian-speaking healthy volunteers (9 women: mean age, 31 years; age range, 24–39 years) with no history of neurologic or psychiatric disease were recruited. All subjects were right-handed, had normal or corrected-to-normal vision, and normal hearing. At enrollment, the purpose of the experiment was explained. Written informed consent was obtained for all subjects. The study was conducted in accordance with institutional guidelines and regulations.
Stimuli
Stimuli were generated by a computer running the e-Prime software (Psychology Software Tools, Pittsburgh, Pa) and were presented visually, with use of a back-projector.
Three categories of visual stimuli (words, objects, and faces) were presented to the subjects in separate scans. Words were single Italian concrete nouns, objects were black-and-white line drawings,33 and faces were black-and-white photographs unfamiliar to the subjects. A total of 120 stimuli, 40 for each of the 3 categories, were presented for 3.5 s each; the interstimulus time was 10.3 ± 1.2 s (range, 8.4–12.2 s). The order of categories was counterbalanced across subjects. Subjects performed a deep encoding task, which involved making a judgment with the thumb or the index of 1 hand (the response side was counterbalanced across subjects) on whether each stimulus was pleasant or unpleasant,10 but the type of response was not used in any part of the fMRI analysis. Participants were explicitly instructed to remember the stimuli for a later test. The duration of each fMRI task was approximately 10 minutes.
After completion of the MR imaging scanning session, subjects performed a recognition test outside the scanner. For each category, subjects viewed the 40 previously presented stimuli, randomly mixed with 30 foils and presented in a manner identical to that used during scanning. Participants were asked to indicate whether each stimulus had been previously seen, and responses were collected by button push. The 120 stimuli that were presented during scanning were then classified as correctly recognized or not recognized.
Data Acquisition
Subjects underwent MR imaging with a Magnetom Avanto 1.5T scanner (Siemens, Erlangen, Germany), with use of an 8-channel phased-array head coil. Anatomic images were acquired with a magnetization-prepared gradient-echo volumetric T1-weighted sequence (1 mm3 isotropic voxels; TR, 1640 ms; TE, 2 ms). Functional images were acquired by means of a gradient-echo echo-planar sequence (TR, 2000 ms; TE, 50 ms). Twenty-one 5-mm oblique coronal sections with 2 × 2-mm in-plane voxel size, aligned perpendicularly to the long axis of the hippocampus and covering the temporal lobes, were acquired in interleaved order. The frequency-encoding direction was in the craniocaudal direction, and the phase-encoding direction was in the mediolateral direction. Before functional imaging, first- and second-level shimming were performed using the slice packet as volume-of-interest. The signal-to-noise ratio (defined as average intensity divided by the SD of noise in the background) in the ROIs (see below) ranged between approximately 45 and 70.
Data Analysis
fMRI data were analyzed by means of the SPM5 software (Wellcome Neuroimaging Department, London, UK) running under Matlab 7 (Mathworks, Natick, Mass). After motion and slicetiming correction, functional images were co-registered with the corresponding anatomic images and then transformed into Montreal Neurologic Institute space, and smoothing was performed with an isotropic Gaussian kernel with a width of 8 mm.
At the first level, encoding trials, classified as recognized or not recognized, were modeled using a canonical hemodynamic response function and its time and dispersion derivatives. Six movement parameters were also included in the model. Parameter estimates were calculated for remembered stimuli, and single-subject contrast maps were obtained.
For the second-level group analysis, each subject's contrast image was entered into a 1-sample t test to examine effects across the whole group. Significance values were false discovery rate-corrected (FDR, P < .0001), and clusters smaller than 5 voxels were discarded.
ROI Analysis
The ROI–based analysis was performed to study the activations within the hippocampus and the parahippocampal gyrus, both divided into anterior and posterior segments. The ROIs were defined by manually tracing these structures on a normalized T1-weighted image, by using a stereotactic atlas34 as anatomic reference. The ROIs were visualized in all 3 planes, landmarked in the sagittal and coronal planes, and checked for the anatomy of each subject. ROI tracing was performed by a senior neuroradiologist, blinded to the functional data. The ROIs were drawn only once and were subsequently checked by 2 other neuroradiologists, who confirmed adherence to the anatomic criteria. We also visually checked the ROIs for each subject, using a procedure similar to that outlined in Asano et al,35 to confirm that they did not include areas of signal intensity dropout because of susceptibility artifacts.
For the hippocampus, the anterior boundary was located by visualizing in the sagittal plane where the temporal horn turns around the hippocampus, leaving the amygdala positioned superiorly and the hippocampus inferiorly. The posterior boundary was set to the last coronal section where the hippocampus was visible.22 The hippocampus was divided into 2 portions: the head anteriorly, and the body and tail posteriorly.
For the parahippocampal gyrus, the anterior boundary was set to the most anterior coronal section where the uncus was visible. The posterior boundary was marked by the disappearance of the splenium. The parahippocampal gyrus was divided into anterior and posterior segments by identification of a virtual plane, parallel to the anterior boundary of the midbrain on the midline, and perpendicular to the major axis of the parahippocampal gyrus. Figure 1 depicts a typical tracing of the hippocampus and parahippocampal gyrus on the basis of above criteria.
Fig 1.
Sagittal view of ROIs for a representative subject. Hippocampus: head in red; body and tail in green. Parahippocampal gyrus: anterior half in violet; posterior half in blue.
For each ROI, 2 distinct measures of activation were obtained: the average blood oxygen level–dependent (BOLD) signal intensity percent change and the percentage of activated voxels, calculated both at a permissive threshold (P < .05) and at a more restrictive threshold (P < .025). For both thresholds, significance values were FDR-corrected at the level of the whole brain, and activation clusters smaller than 5 voxels were rejected. Although the measured magnitude of the BOLD percent change, averaged over the whole ROI, is not influenced by choice of a statistical significance threshold, analysis of the percentage of activated voxels was repeated at 2 thresholds to confirm that the results were not due to a particular cutoff. These values were analyzed by repeated-measure analyses of variance (ANOVAs) with Hemisphere (left or right) and Axis (anterior or posterior) as factors; main effects of Hemisphere and Axis as well as an interaction between the 2 factors were searched. Normality was verified by means of Kolmogorov-Smirnov tests. For the percentage of activated voxels, data were not normally distributed; as a consequence, the values were square-root transformed.
To assess the lateralization of the activations, a laterality index (LI) was calculated as LI = [(xleft–xright)/(xleft + xright)].
Results
Behavioral Data
Subjects successfully encoded the stimuli of each category: for words, the performance was 0.98 ± 0.02 (mean ± SD); for objects, it was 0.98 ± 0.03; and for faces, it was 0.79 ± 0.11.
fMRI Data
Results from the second-level group analysis are shown in Fig 2. For the word-encoding task, the overall activation pattern was left-lateralized. Strong engagement of the left inferior frontal gyrus, of the premotor and supplementary motor cortices, and of the posterior temporal-occipital regions was found. In the hippocampus, strong activation was observed in the left head only (Fig 2A); in the parahippocampal gyrus, activation was essentially bilateral and predominantly in the posterior regions.
Fig 2.
Results from the second-level group analysis for the encoding tasks of (A) words, (B) objects, and (C) faces. Activations of the hippocampus are indicated with white arrowheads, and activations of the parahippocampal gyrus are indicated with green arrowheads. The 3 leftmost sections show the head of the hippocampus and the anterior portion of the parahippocampal gyrus, whereas the 2 rightmost sections show the body and tail of the hippocampus and the posterior portion of the parahippocampal gyrus.
For the object-encoding task, the overall activation pattern was similar to that observed for word encoding; here, however, activations in the inferior frontal gyrus and in the posterior temporal-occipital regions were essentially symmetric. In the hippocampus, the head was activated bilaterally but more strongly on the left side; the body and tail were also activated bilaterally but more strongly on the right side (Fig 2B). In the parahippocampal gyrus, activation was essentially bilateral.
For the face-encoding task, the overall activation pattern was clearly right-lateralized, and strong activation of the right inferior frontal gyrus was found; activation in the temporal-occipital regions was less lateralized. In the hippocampus (Fig 2C), activation was stronger in the right head and less intense in the right body and tail.
ROI Analysis: Hippocampus
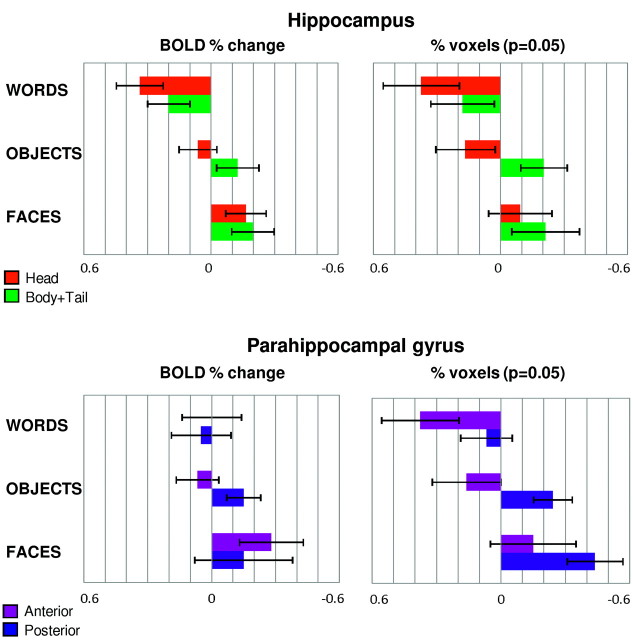
The results of the ANOVA with the measurements of the BOLD percent change and the percentage of activated voxels (at the permissive and more restrictive thresholds) are given in on-line Table 1, followed by corresponding bar charts (Fig 3) and by the results of the LI (Fig 4).
Fig 3.
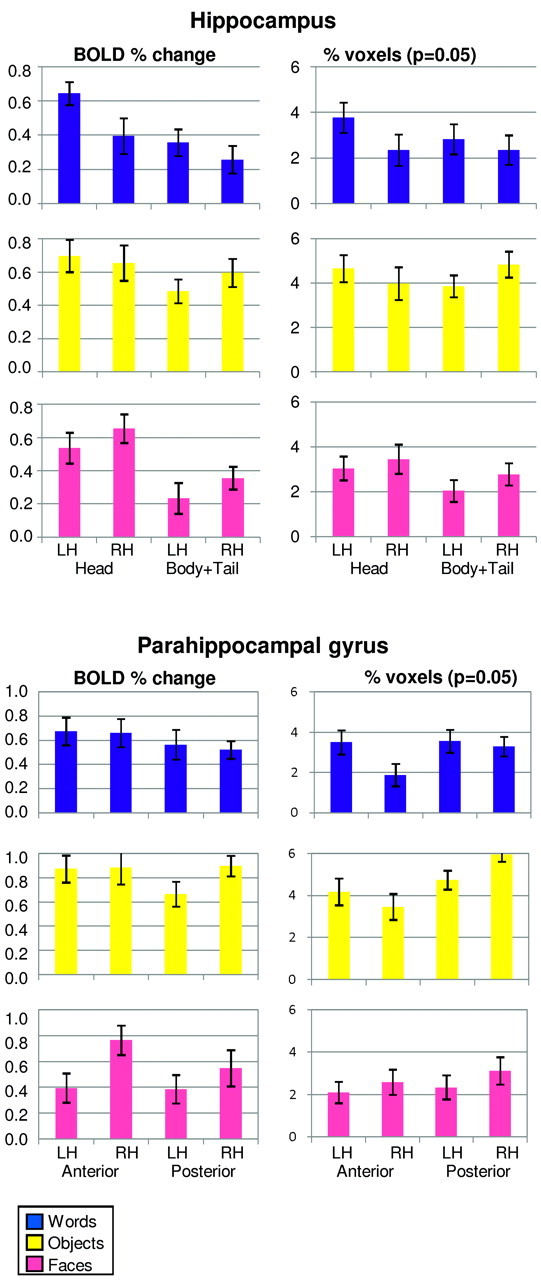
Results of the ROI-based analysis performed in the hippocampus and in the parahippocampal gyrus for the word-encoding, object-encoding, and face-encoding tasks. Measurements of the BOLD percent change and of the percentage of activated voxels (P = .05), square-root transformed, are shown. Mean and SE are reported.
Fig 4.
Laterality index (LI) for the hippocampal ROI divided into head and body plus tail, and for the parahippocampal ROI divided into 2 halves. LI (mean and SE), calculated with the BOLD percent change and with the percentage of activated voxels (P = .05), square-root transformed, is given. A positive LI indicates left-lateralized activations, and negative LI implies right-lateralized activation pattern.
For the word-encoding task, analysis of the BOLD percent change revealed a larger response in the head of the hippocampus (P = .005) and an overall larger response in the left hemisphere (P = .01). Analysis of the percentage of activated voxels confirmed the main effect of Hemisphere, which was significant (P = .03) for the permissive threshold and approached significance (P = .055) for the more restrictive threshold. The LI indicated left lateralization, which was stronger for the head than for the body and tail according to all 3 measures. At the permissive threshold, a significant activation cluster was found in the left hippocampus for 94% of subjects and in the right hippocampus for 75% of subjects.
For the object-encoding task, there were no main effects of Axis and Hemisphere. Only for the percentage of activated voxels, there was an Axis × Hemisphere interaction, which was significant (P = .03) for the permissive threshold and approached significance (P = .063) for the more restrictive threshold: there was greater activation in the left head and in the right body and tail of the hippocampus (Fig 3). The LI calculated according to all 3 measures indicated that activations were left-lateralized in the head but right-lateralized in the body and tail of the hippocampus; the lateralization was weaker for the BOLD percent change than for the percentage of activated voxels at the 2 thresholds. At the permissive threshold, a significant activation cluster was found in the left hippocampus for 94% of subjects and in the right hippocampus for 94% of subjects.
For the face-encoding task, analysis of the BOLD percent change revealed greater activation in the head of the hippocampus (P = .0001) and an overall larger response in the right hemisphere (P = .04). Analysis of the percentage of activated voxels revealed a trend for an effect of Axis at the permissive threshold (P = .063). The LI calculated according to all 3 measures indicated right lateralization, which was stronger for the body and tail than for the head of the hippocampus. At the permissive threshold, a significant activation cluster was found in the left hippocampus for 88% of subjects and in the right hippocampus for 94% of subjects.
ROI Analysis: Parahippocampal Gyrus
The results of the ANOVA are given in on-line Table 2, followed by the corresponding bar charts (Fig 3) and by the results of the LI (Fig 4). For the word-encoding task, analysis of the BOLD percent change revealed no significant effects. The percentage of activated voxels revealed greater activation in the left hemisphere (P = .03 for the permissive and P = .02 for the more restrictive threshold), and there was a trend for an effect of Axis with greater activation in the posterior than in the anterior segment (P = .07 for the permissive and P = .054 for the more restrictive threshold). The Axis and Hemisphere factors interacted significantly (P = .04 for both thresholds): there was greater difference between the 2 hemispheres for the anterior than for the posterior segment (Fig 3). The LI calculated from the BOLD percent change did not show any clear lateralization, but calculated with the percentage of activated voxels at the 2 thresholds, demonstrated left lateralization, which was stronger for the anterior than for the posterior segment. At the permissive threshold, a significant activation cluster was found in the left parahippocampal gyrus for 88% of subjects and in the right parahippocampal gyrus for 94% of subjects.
For the object-encoding task, analysis of the BOLD percent change revealed no significant effects. According to the percentage of activated voxels, there was greater activation in the posterior than in the anterior segment (P = .001 for both thresholds) and no main effect of Hemisphere. The Axis and Hemisphere factors interacted significantly (P = .03 for the permissive and P = .02 for the more restrictive threshold): there was greater activation in the left anterior and in the right posterior segments (Fig 3). The LI calculated according to all 3 measures demonstrated left lateralization in the anterior segment and right lateralization in the posterior segment; the lateralization was stronger for the percentage of activated voxels according to the 2 thresholds than for the BOLD percent change. At the permissive threshold, a significant activation cluster was found in the left parahippocampal gyrus for 94% of subjects and in the right parahippocampal gyrus for all subjects.
For the face-encoding task, analysis of the BOLD percent change revealed a larger response in the right hemisphere (P = .0001); the effect did not reach statistical significance for the percentage of activated voxels (P = .08 for the permissive and P = .05 for the more restrictive threshold). According to all measures, there was no main effect of Axis and no interaction. The LI calculated according to all 3 measures indicated a clearly right-lateralized activation pattern. According to the BOLD percentage change, lateralization was stronger for the anterior segment, but according to the percentage of activated voxels at the 2 thresholds, the lateralization was stronger for the posterior segment. At the permissive threshold, a significant activation cluster was found in the left parahippocampal gyrus for 69% of subjects and in the right parahippocampal gyrus for 81% of subjects.
Discussion
The hippocampus and parahippocampal gyrus are crucial for the acquisition of new memories and, according to recent evidence, also subserve perceptual functions such as spatial and object processing.19,20 fMRI may enable assessment of the functional status of the MTL in patients with TLE and support the planning of temporal lobectomy. Although several fMRI studies have investigated memory processing in healthy subjects, there is considerable need for simple paradigms eliciting robust activation of the hippocampus and related structures for use in clinical settings.
In this study, rather than aiming to isolate activations specific to memory encoding, we intended to obtain robust memory–related engagement of the hippocampus and parahippocampal gyrus. We studied a group of healthy subjects with 3 paradigms consisting of word, object, and face encoding; the high-recognition accuracy confirmed that the tasks were easy to perform and therefore were applicable to the study of patients with TLE, who are characterized by heterogeneous memory performance. Both second-level group analysis and an ROI-based analysis were performed; with both analyses, activations were left-lateralized with words, bilateral with objects, and right-lateralized with faces. Significant activations of the hippocampus and parahippocampal gyrus were found for most subjects. In particular, for words, the left hippocampus was activated in 94% of subjects; for faces, the right hippocampus was activated in 94% of subjects; and for objects, the left and right hippocampi were, again, activated in 94% of subjects. It is reassuring that the hippocampal activations were observed both in the second-level group analysis and at the level of individual subjects, as confirmed by the ROI-based counts of activated voxels.
Both analyses showed that the head of the hippocampus was always activated, on the left side with words and objects, and on the right side with faces (Figs 2 and 3). The body and tail were engaged by objects, to a lesser extent by faces, and only marginally by words. These results are in line with the hypothesis that the anterior portion of the hippocampus has a crucial role in memory encoding.10,16,23–26 Moreover, our results are concordant with the view that the hippocampus is involved not only in forming associations between multiple items but also in the encoding of single items.2,16
As demonstrated by the ROI-based analysis, parahippocampal activations were observed for all 3 tasks at the level of individual subjects (Fig 3), but, as shown by the second-level analysis (Fig 2), at the group level, parahippocampal activations reached significance for words and objects only. Overall, activations were essentially bilateral and involved both the anterior and posterior regions. In particular, for word encoding, activations were not lateralized according to the BOLD percent change, in contrast with hippocampal activations; the percentage of activated voxels, however, did show left lateralization for the anterior part. The interpretation of this difference in the lateralization of hippocampal and parahippocampal activations remains unclear, a possibility being that the right parahippocampal gyrus is engaged in a novelty response related to the changing visual characteristics of words presented with unpredictable timing.18 For object encoding, both analyses indicated left lateralization for the anterior segment and right lateralization for the posterior segment, in accordance with the lateralization of hippocampal activations; the determinant of this difference in lateralization remains unclear. For face encoding, the ROI-based analysis revealed a right-lateralized activation that was not evident in the second-level group analysis. Taken together, these results are concordant with the hypothesis that the parahippocampal gyrus also has a central role in word and object encoding.10,17,36–38
Discrepancies between the 2 types of analyses may be due to the fact that the traditional second-level analysis gives an overview of the activations on the basis of their voxel-level significance at the group level. By contrast, with the ROI-based analysis the count of activated voxels is based on the voxel-level significance of activations at the subject level; it enables the measurement of lateralization and provides quantitative indexes.
In the ROI-based analysis, the percentage of activated voxels and the BOLD percent change provide complementary information: although, at a given threshold, the former is representative of the spatial extent of activation, the latter is more directly representative of the strength of hemodynamic engagement. In regions where activations tend to be widespread but are too feeble to reach the significance threshold in a voxel, the BOLD percent change may be more sensitive. Our results, in fact, showed that in the hippocampus, where activations are more difficult to observe, the effects of Axis and Hemisphere were stronger for the BOLD percent change than for the percentage of activated voxels. By contrast, for the parahippocampal gyrus, the percentage of activated voxels revealed more effects than the BOLD percent change; it is important to note that the effects did not depend on the selected threshold.
Only a limited number of paradigms have been developed to study memory in clinical settings, and most event-related fMRI studies of memory have been conducted directly contrasting remembered vs forgotten items, with the purpose of visualizing only the activations specific to the memory processes.5,10 Here, however, we had a different aim: to evaluate paradigms activating the hippocampus and parahippocampal gyrus to assess their functional reactivity in patients with MTL pathology. For the intended preoperative planning purpose, demonstrating the presence of activation in MTL regions that appear pathologic on structural imaging suggests their sparing to the neurosurgeon, irrespective of whether these activations are specifically encoding related or they are part of a more extended network subserving the perception and comprehension of a given stimulus.19,20 Therefore, we maximized sensitivity to activation at the price of limited functional specificity and used encoding tasks, which are generally agreed to elicit stronger activation compared with retrieval tasks.30,39 Because the approach used in our study does not need an approximately equal number of remembered and forgotten items, these paradigms may be relatively robust with respect to the variable level of memory performance observed in patients.
Our study has some limitations that need to be taken into account. First, it was performed on a 1.5T scanner; however, although the incremental gain from higher-field strength would probably have improved assessment of the lateralization of the activations, our study is relevant because clinical fMRI is still performed at 1.5T in many centers. The results obtained in our study should be confirmed at higher-field strength. Second, an event-related design was used, resulting in lower statistical power and therefore longer task duration with respect to an equivalent blocked design; however, it has been shown that the localization of memory-related activations is more reliable with event-related designs, and the tasks, despite being 10 minutes long, are relatively undemanding because of slow pacing (1 stimulus every 11 s).32 Third, ROIs were drawn only once, and interrater reliability was therefore not calculated; however, provision of detailed anatomic criteria limited the subjectivity in tracing, and the ROIs were independently checked by 2 authors who always confirmed that they adhered to the anatomic specifications. Fourth, the recognition performance was lower for faces than for words and objects, and more generally, we did not perform a direct comparison with other paradigms used in previous studies. As a consequence, we are unable to conclude that the proposed paradigms are superior to the existing ones; however, the performance on the word and object tasks appeared very high (98%), and the performance on the face task, though lower, was still higher (80%) than reported in other similar studies.9,10,16 Fifth, a standard echo-planar sequence was used, though susceptibility artifacts, manifesting as areas of signal intensity dropout, can be significant in the temporal lobe because of proximity to the skull base. We reduced field inhomogeneities with second-order shimming and minimized their effect by placing the frequency-encoding direction along the craniocaudal direction, in which the field gradient is generally largest, and the phase-encoding direction along the mediolateral direction, in which the field gradient is generally smallest.40 Adequate visualization of the hippocampus and parahippocampal gyrus was always confirmed by visual inspection. Finally, although we present data obtained using 3 different measures (BOLD percent change and percentage of activated voxels at 2 thresholds), our results were not conclusive regarding which provides the most representative quantification of the true lateralization of function. Although there was generally good agreement between the 3 measures in terms of the side of greater activation, results are discordant for the parahippocampal gyrus with the word-encoding task. To our knowledge, there is only 1 previous study comparing BOLD percent change and percentage of activated voxels that reported partial concordance between the measures, essentially in line with our findings.24
More work is needed to confirm to what extent the lateralization of fMRI activations elicited by these paradigms can be used to predict postsurgical outcome. Validation work on patient populations, with use of the Wada test and postsurgical outcome data as reference standards, is necessary before these paradigms can be considered for clinical use.
Conclusions
Our event-related fMRI study shows that simple encoding tasks for words, objects, and faces elicit robust hippocampal and parahippocampal activations in healthy subjects. As expected, left lateralization was observed for words, right lateralization was observed for faces, and activations were bilateral for objects. In particular, by dividing the hippocampus into anterior and posterior portions, we showed that during memory encoding the head was more strongly engaged than the body and tail, irrespective of the type of material. Data for the LI, calculated with the BOLD percent change and the percentage of activated voxels at 2 thresholds, are given, thus providing 2 measures of activation: the intensity and the spatial extent of activation. The combination of these 3 undemanding memory tasks deserves consideration as a tool to assess the functional status of the medial temporal lobe in clinical settings.
Acknowledgments
We would like to thank all volunteers for participating in our study and Dr. Carlo Marras for helpful discussions. We are grateful to 2 anonymous reviewers for the insightful advice they provided on an earlier version of this manuscript.
Footnotes
Indicates article with supplemental on-line tables
References
- 1.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 1957;20:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosc 2008;9:182–94 [DOI] [PubMed] [Google Scholar]
- 3.Frisk V, Milner B. The role of the left hippocampal region in the acquisition and retention of story content. Neuropsychologia 1990;28:349–59 [DOI] [PubMed] [Google Scholar]
- 4.Smith ML, Milner B. The role of the right hippocampus in the recall of spatial location. Neuropsychologia 1981;19:781–93 [DOI] [PubMed] [Google Scholar]
- 5.Richardson MP, Strange BA, Duncan JS, et al. Preserved verbal memory function in left medial temporal pathology involves reorganisation of function to right medial temporal lobe. Neuroimage 2003;20:112–19 [DOI] [PubMed] [Google Scholar]
- 6.Baxendale S, Thompson PJ, Duncan JS. Improvements in memory function following anterior temporal lobe resection for epilepsy. Neurology 2008;71:1319–25 [DOI] [PubMed] [Google Scholar]
- 7.Giovagnoli AR, Avanzini G. Learning and memory impairment in patients with temporal lobe epilepsy: relation to the presence, type and location of brain lesion. Epilepsia 1999;40:904–11 [DOI] [PubMed] [Google Scholar]
- 8.Kelley WM, Miezin FM, McDermott KB, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron 1998;20:927–36 [DOI] [PubMed] [Google Scholar]
- 9.Golby AJ, Poldrack RA, Spencer D, et al. Material-specific lateralization in the medial temporal lobe and prefrontal region during memory encoding. Brain 2001;124:1841–54 [DOI] [PubMed] [Google Scholar]
- 10.Powell HW, Koepp MJ, Symms MR, et al. Material-specific lateralization of memory encoding in the medial temporal lobe: blocked versus event-related design. Neuroimage 2005;27:231–39 [DOI] [PubMed] [Google Scholar]
- 11.Detre JA, Maccotta L, King D, et al. Functional MRI lateralization of memory in temporal lobe epilepsy. Neurology 1998;50:926–32 [DOI] [PubMed] [Google Scholar]
- 12.Szaflarski JP, Holland SK, Schmithorst, et al. High-resolution functional MRI at 3T in healthy and epilepsy subjects: hippocampal activation with picture encoding task. Epilepsy Behav 2004;5:244–52 [DOI] [PubMed] [Google Scholar]
- 13.Weber B, Kugler F, Elger CE. Comparison of implicit memory encoding paradigms for the activation of mediotemporal structures. Epilepsy Behav 2007;10:442–48 [DOI] [PubMed] [Google Scholar]
- 14.Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. J Neurosci 2006;26:9162–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Achim AM, Bertrand MC, Montoya A, et al. Medial temporal lobe activations during associative memory encoding for arbitrary and semantically related object pairs. Brain Research 2007;1161:46–55 [DOI] [PubMed] [Google Scholar]
- 16.Chua EF, Schacter DL, Rand-Giovannetti E, et al. Evidence for a specific role of the anterior hippocampal region in successful associative encoding. Hippocampus 2007;17:1071–80 [DOI] [PubMed] [Google Scholar]
- 17.Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol 2002;88:982–90 [DOI] [PubMed] [Google Scholar]
- 18.Strange BA, Otten LJ, Josephs O, et al. Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. J Neurosci 2002;22:523–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee ACH, Bussey TJ, Murray EA, et al. Perceptual deficits in amnesia: challenging the medial temporal lobe ‘mnemonic’ view. Neuropsychologia 2005;43:1–11 [DOI] [PubMed] [Google Scholar]
- 20.Lee ACH, Scahill VL, Graham KS. Activating the medial temporal lobe during oddity judgment for faces and scenes. Cer Cortex 2008;18:683–96 [DOI] [PubMed] [Google Scholar]
- 21.Small SA, Nava AS, Perera GM, et al. Circuit mechanisms underlying memory encoding and retrieval in the long axis of the hippocampal formation. Nat Neurosci 2001;4:442–49 [DOI] [PubMed] [Google Scholar]
- 22.Greicius MD, Krasnow B, Boyett-Anderson JM, et al. Regional analysis of hippocampal activation during encoding and retrieval: An fMRI study. Hippocampus 2003;13:164–74 [DOI] [PubMed] [Google Scholar]
- 23.Kohler S, Crane J, Milner B. Differential contributions of the parahippocampal place area and the anterior hippocampus to human memory for scenes. Hippocampus 2002;12:718–23 [DOI] [PubMed] [Google Scholar]
- 24.Parsons MW, Haut MW, Lemieux SK, et al. Anterior medial temporal lobe activation during encoding of words: fMRI methods to optimize sensitivity. Brain Cogn 2006;60:253–61 [DOI] [PubMed] [Google Scholar]
- 25.Richardson MP, Strange BA, Duncan JS, et al. Memory fMRI in left hippocampal sclerosis: optimizing the approach to predicting postsurgical memory. Neurology 2006;66:699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kircher T, Weis, Leube, et al. Anterior hippocampus orchestrates successful encoding and retrieval of non-relational memory: An event-related fMRI study Eur Arch Psychiatry Clin Neurosci 2008;258:363–72 [DOI] [PubMed] [Google Scholar]
- 27.Kjelstrup KB, Solstad T, Brun VH, et al. Finite scale of spatial representation in the hippocampus. Science 2008;321:140–44 [DOI] [PubMed] [Google Scholar]
- 28.Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus 1998;8:608–19 [DOI] [PubMed] [Google Scholar]
- 29.Witter MP, Wouterlood FG, Naber PA, et al. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci 2000;911:1–24 [DOI] [PubMed] [Google Scholar]
- 30.Binder JR, Bellgowan PSF, Hammeke TA, et al. A comparison of two fMRI protocols for eliciting hippocampal activation. Epilepsia 2005;46:1061–70 [DOI] [PubMed] [Google Scholar]
- 31.Vincent JL, Snyder AZ, Fox MD, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol 2006;96:3517–31 [DOI] [PubMed] [Google Scholar]
- 32.Powell HW, Koepp MJ, Richardson M, et al. The application of functional MRI of memory in temporal lobe epilepsy: A clinical review. Epilepsia 2004;45:855–63 [DOI] [PubMed] [Google Scholar]
- 33.Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol 1980;6:174–215 [DOI] [PubMed] [Google Scholar]
- 34.Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. Oxford, UK: Academic Press;2008
- 35.Asano S, Mihara B, Kirino T, et al. Anatomical constraints on visualization of the human hippocampus using echo-planar imaging. Neuroradiology 2004;46:535–40 [DOI] [PubMed] [Google Scholar]
- 36.Brewer JB, Zhao Z, Desmons JE, et al. Making memories: brain activity that predicts how well visual experience will be remembered. Science 1998;281:1185–87 [DOI] [PubMed] [Google Scholar]
- 37.Brewer JB & Moghekar A. Imaging the medial temporal lobe: exploring new dimensions. Trends Cogn Sci 2002;6:217–23 [DOI] [PubMed] [Google Scholar]
- 38.Wagner AD, Schacter DL, Rotte M, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science 1998;281:1188–91 [DOI] [PubMed] [Google Scholar]
- 39.Gabrieli JDE, Brewer JB, Desmond JE, et al. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science 1997;276:264–66 [DOI] [PubMed] [Google Scholar]
- 40.Chen NK, Dickey CC, Yoo SS, et al. Selection of voxel size and slice orientation for fMRI in the presence of susceptibility field gradients: application to imaging of the amygdala Neuroimage 2003;19:817–25 [DOI] [PubMed] [Google Scholar]