Abstract
BACKGROUND AND PURPOSE: The major goal of acute ischemic stroke treatment is fast and sufficient recanalization. Percutaneous transluminal balloon angioplasty (PTA) and/or placement of a stent might achieve both by compressing the thrombus at the occlusion site. This study assesses the feasibility, recanalization rate, and complications of the 2 techniques in an animal model.
MATERIALS AND METHODS: Thirty cranial vessels of 7 swine were occluded by injection of radiopaque thrombi. Fifteen vessel occlusions were treated by PTA alone and 15, by placement of a stent and postdilation. Recanalization was documented immediately after treatment and after 1, 2, and 3 hours. Thromboembolic events and dissections were documented.
RESULTS: PTA was significantly faster to perform (mean, 16.6 minutes versus 33.0 minutes for stent placement; P < .001), but the mean recanalization rate after 1 hour was significantly better after stent placement compared with PTA alone (67.5% versus 14.6%, P < .001). Due to the self-expanding force of the stent, vessel diameter further increased with time, whereas the recanalization result after PTA was prone to reocclusion. Besides thromboembolic events related to the passing maneuvers at the occlusion site, no thrombus fragmentation and embolization occurred during balloon inflation or stent deployment. Flow to side branches could also be restored at the occlusion site because it was possible to direct thrombus compression.
CONCLUSIONS: Stent placement and postdilation proved to be much more efficient in terms of acute and short-term vessel recanalization compared with PTA alone.
The aim of stroke treatment is the fast and effective recanalization of occluded intracranial arteries. The systemic or local application of thrombolytic drugs has been successful, but the procedure is time-consuming and increases the risk of intracranial hemorrhage.1–4 The recently introduced embolectomy technique seems to reduce the time for recanalization. However, the mechanical approach and the repeated retrieval attempts necessary to remove the thrombus increase the stress to the vessel wall. Therefore, studies on mechanical embolectomy have shown a new spectrum of complications during interventional stroke treatment, such as subarachnoid hemorrhage and vessel rupture.5,6
A few clinical studies have evaluated the feasibility of percutaneous transluminal balloon angioplasty (PTA) alone or in combination with thrombolysis.7–10 Recent advances in technology have also allowed placement of stents in intracranial arteries. Stent placement for acute stroke treatment has been reported only by 1 group so far.11,12 Both approaches (ie, PTA and stent placement) promise a fast recanalization without repetitive passing and retrieval attempts and might omit the application of thrombolytic drugs. Instead of mechanical retrieval, both achieve recanalization by compressing the thrombus to the vessel wall. The few data on the recanalization rate of these approaches are promising. However, the fate of the thrombotic material after these interventions7–12 has not been assessed until now.
We performed the present study to obtain more knowledge about the usability of PTA and/or stent placement for acute stroke treatment. The efficiency of PTA alone or stent placement with subsequent PTA (postdilation) for acute vessel recanalization was compared in an animal model. The immediate results and the reocclusion rate with time were assessed. The use of a radiopaque thrombus illustrated the thrombus and demonstrated the effect on perforating vessels due to thrombus compression.13
Materials and Methods
Animal Model and Animal Care
The experiments were performed in accordance with the international guideline for animal testing and were approved by the responsible local authorities.
Seven swine, ranging in weight from 44 to 48 kg, were used in this study. Sedation was induced by 0.05-mg/kg atropine and 15-mg/kg midazolam, and endotracheal intubation was performed. General anesthesia was maintained by a 2% isoflurane inhalant. Vital parameters were continuously recorded and confined to narrow margins: arterial blood pressure was kept between 80 and 120 mm Hg; mean arterial blood pressure, at 90–95 mm Hg; heart rate, between 60 and 95 beats per minute; expired carbon dioxide level, at 30–35 mm Hg; and body temperature, above 34°C.13 After preparation of the groin of the anesthetized swine, the external iliac artery and vein were exposed and a central venous catheter and an 8F catheter sheath (Arrow; Arrow International, Reading, Pa) was inserted. At the end of the surgical procedure, 500-mg acetylsalicylic acid was given intravenously and the animal was transferred to the angiography suite. After the experiments, the animals were euthanized with an intravenous injection of 20-mmol potassium chloride.
Experimental Material
Experiments were performed in the neuroangiography lab equipped with a biplane high-resolution angiography system (CAS 500; Toshiba, Tokyo, Japan; 1024 × 1024 pixel matrix). This system allowed direct measurement of vessel diameters on the biplane images. Data were collected digitally and stored on a CD.
Angiographic material and contrast media were the same as those used in humans. PTA was performed with an over-the-wire balloon catheter developed for intracranial intervention in humans (Gateway PTA balloon catheter; Boston Scientific/Target Therapeutics, Fremont, Calif). The balloons were firmly mounted and inflated via a second lumen. Balloons were undersized (ie, measuring 80% of the diameter of the initial vessel) to avoid vessel rupture during PTA. Balloons ranged from 9 to 20 mm in length and from 1.5 to 2.5 mm in diameter.
Stent placement was performed by using a self-expanding intracranial stent (Wingspan; Boston Scientific, Natick, Mass). The stent was designed for the treatment of intracranial stenosis, and the open-cell design of the mesh should allow perfusion of smaller branches at the level of the stented vessel segment. The stents were mounted on a 3.5F stent-delivery catheter that was also an over-the-wire system. Stent size was equal to or greater than the initial vessel diameter.
Thrombus Generation
Thrombi were created by mixing 10-mL autologous blood of the animal (whole-blood thrombus) with 1-g barium sulfate and 0.25-mL bovine thrombin solution (Dade; Dade Behring, Newark, Del). After incubation at room temperature for 1 hour, thrombi were cut to pieces of 10 mm in length, which were injected into the target artery. This setting produces a red or “whole blood” thrombus with a rather soft consistency compared with clot used by other study groups.14 For further details, see previous studies.13,15 Due to the radiopacity gained by the added barium sulfate, thrombus movement and behavior could be observed during angiography and fluoroscopy. This enabled the documentation of thrombus movement during the procedure and thromboembolic events, even of small fragments.
Experimental Design
For vessel occlusion, the lingual artery (LA), ascending pharyngeal artery, and maxillary artery with luminal diameters of 2–3 mm were selectively embolized. These vessels matched in diameter and, to some extent, the course of frequently occluded vessels in humans (ie, the middle cerebral and basilar arteries).13,16 Furthermore, the distance between the arterial access in the groin and the cranial arteries is similar to that in humans. In swine, the rete mirabile impedes catheter access to the cerebral arteries. Moreover, the latter have a diameter of ≤1 mm, making them inappropriate for mechanical revascularization therapy.17
Four or 5 vessels were occluded consecutively in each swine, with a total of 30 occlusions in 7 animals. Complete vessel occlusion was documented by angiography immediately after thrombus application. The thrombus was allowed 5 minutes for embedding under blood-flow conditions. Recanalization was attempted in 15 vessels by PTA alone and in 15 vessels by stent placement and subsequent PTA.
For mechanical recanalization, the occluding thrombus was first passed with a 2.5F microcatheter (Renegade; Boston Scientific) equipped with a microguidewire (SilverSpeed 14; ev3, Irvine, Calif). After navigating the microcatheter to the distal vessel segment, we introduced an exchange wire (Transend 300 Floppy, Boston Scientific). The microcatheter was exchanged with either the balloon-catheter or the stent-delivery catheter. In case of stent deployment, postdilation with the balloon-catheter was performed. The recanalization result was documented by follow-up angiography immediately after intervention and after 60 minutes.
To document early reocclusion, we performed follow-up angiography after 2 hours in 12 of the 15 occlusions and after 3 hours in 5 of the 15 occlusions for each group. Follow-up of all occlusions at 2 and 3 hours was not possible due to an unacceptably prolonged examination interval for each animal (eg, 3-hour follow-up of a fifth occlusion in a testing animal).
Each procedure was performed by an experienced endovascular neuroradiologist (G.S., J.G., and C.B.). All 3 operators performed the same number of interventions (ie, PTA and stent placement with postdilation).
Angiographic Evaluation
Each vessel diameter was measured before embolization and during the follow-up angiography. The recanalization rate was given as a percentage of the initial diameter. Furthermore, to achieve data comparable with other studies, we quantified recanalization according to the Thrombolysis in Myocardial Infarction (TIMI) trial criteria18: TIMI 0 = vessel remains occluded, TIMI 1 = minimal recanalization, TIMI 2 = partial recanalization, and TIMI 3 = complete recanalization.
For each of the 2 revascularization techniques (PTA alone and stent placement with postdilation), we evaluated the following criteria:
) Primary end point: recanalization rate of the cranial vessels 1 hour after the intervention (P1).
- ) Secondary end points:
- Recanalization rate at 2 (P2) and 3 hours (P3) after the intervention.
- Application time: interval between the start of the mechanical procedure and the time that PTA or stent placement was performed.
- Evaluation of the thrombus-device interaction, direction of thrombus compression, and possible side branch occlusions.
- Assessment of complications (dislocation of thrombotic material in a distal direction, embolization of thrombus in previously unaffected vessels, and vessel dissection or perforation).
Statistical Evaluation
The Student t test was used for between-group analysis of vessel diameter and application time of the devices. Variance analysis of repeated measures and the Student t test were performed to compare the recanalization success immediately after application and at 1, 2, and 3 hours after intervention. The Mann-Whitney U test was used to compare TIMI grades achieved by both procedures. P values < .05 were considered significant.
Results
Thirty cranial vessels were occluded. Mean vessel diameter before occlusion was 2.14 ± 0.3 mm in the PTA group and did not differ significantly from the mean vessel diameter in the stent placement group (2.28 ± 0.4 mm; P = .92; Table 1).
Table 1:
Results of PTA and stenting with postdilation for vessel recanalization
| PTA | Stent | Level of Significance | |
|---|---|---|---|
| Total No. of attempts | 15 | 15 | NA |
| Mean vessel diameter (mm) | 2.14 (±0.3) | 2.28 (±0.4) | NS |
| Mean time (min) per attempt | 16.6 (±5.0) | 33.0 (±15.1) | P < .001* |
| Total incidence of embolic events | 3/16 (20%) | 2/16 (13%) | NS |
| Mean recanalization rate (%) | |||
| Immediately after PTA/stenting | 20.5% | 61.8% | P < 0.001† |
| P1 | 14.6% | 67.5% | P < 0.001† |
| P2 | 12.5% | 65.3% | P < 0.001† |
| P3 | 6.0% | 71.6% | P < 0.001† |
Note:—NA indicates not applicable; NS, not significant; PTA, percutaneous transluminal angioplasty; P1, 1 hour postintervention.
Student t test.
Variance analysis of repeated-measures.
Recanalization after PTA
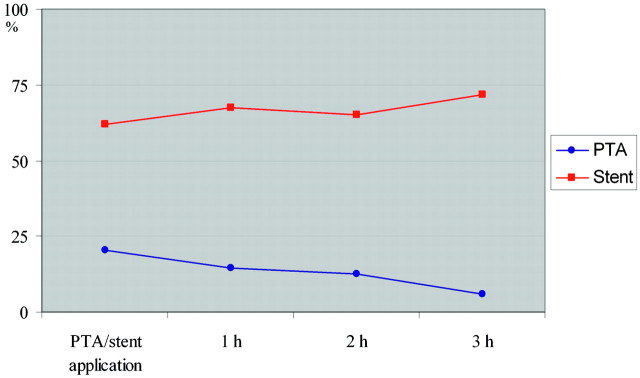
PTA was feasible in all occlusions. The mean recanalization rate immediately after PTA was 20.5% of the initial vessel diameter (range, 0%–56%) decreasing to 14.6% after 1 hour (P1), 12.5% after 2 hours (P2, 12 vessels; range, 0%–40%), and 6.0% after 3 hours (P3, 5 vessels; range, 0%–20%; Fig 1).
Fig 1.
Graph shows the mean recanalization rate after PTA and stent placement during the study interval.
None of the occlusions achieved a recanalization of ≥75%, and only 3 occlusions reached a recanalization of >50% immediately after PTA. Of these 3 occlusions, 2 remained at that level at the primary end point (P1). Six occlusions showed no recanalization immediately after PTA or after 1 hour. Of the 9 vessels with initial partial recanalization, only 2 vessels demonstrated an increased diameter at follow-up, whereas 7 vessels decreased in diameter. Complete reocclusion was observed in 4 vessels and occurred during the first hour in 1 and between P1 and P2 in 3 vessels.
Recanalization after Stent Placement and Postdilation
Stent deployment was feasible in 14 occlusions. In 1 case, the stent could not be passed across the occlusion site. The mean recanalization rate immediately after stent placement and postdilation was 61.8% of the initial vessel diameter (range, 0%–100%), increasing to 67.5% (range, 0%–100%) at P1. Follow-up revealed a mean vessel diameter of 65.3% at P2 (12 vessels; range, 0%–100%) and of 71.6% at P3 (5 vessels; range, 60%–91%; Fig 1).
Eight occlusions (53%) achieved a recanalization rate of ≥75% immediately after stent placement and postdilation. At the primary end point of the study, 13 vessels (87%) were still patent, all showing a recanalization rate of ≥50%. One vessel revealed a reocclusion within the first hour. The mean recanalization rate decreased slightly between P1 and P2 by 2.2% and increased within the next hour (to the 3-hour follow-up) by 6.3%.
Comparison of PTA Alone with Stent Placement and Postdilation
Mean application time was significantly shorter in the PTA group (16.6 ± 5 minutes) compared with the stent placement group (33 ± 15.1 minutes, P < .001). The mean recanalization rate after stent placement was significantly higher compared with PTA immediately after the procedure and during the time course of the study (P < .001). TIMI grades at P1, P2, and P3 differed significantly between the PTA and stent placement group (Table 2). Moreover, the mean recanalization rate after stent placement slightly increased with time, whereas it decreased after PTA (Fig 1). These results are summarized in Table 1.
Table 2:
Recanalization results at different time points after PTA and stenting with postdilation according to the TIMI classification
| PTA |
Stent |
P Value* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TIMI 0 | TIMI 1 | TIMI 2 | TIMI 3 | TIMI 0 | TIMI 1 | TIMI 2 | TIMI 3 | ||
| 0 hours† (n = 30) | 6 | 1 | 8 | 0 | 2 | 2 | 2 | 9 | .006 |
| P1* (n = 30) | 7 | 4 | 4 | 0 | 2 | 0 | 6 | 7 | .001 |
| P2* (n = 24) | 6 | 2 | 4 | 0 | 1 | 0 | 6 | 5 | .002 |
| P3* (n = 10) | 3 | 2 | 0 | 0 | 0 | 0 | 2 | 3 | .007 |
Note:—TIMI indicates Thrombolysis in Myocardial Infarction trial criteria.
Mann-Whitney U test.
Immediately.
Thrombus-Device Interaction and Complications of PTA
In all 15 occlusions, the balloon could be navigated across the occlusion site. In 3 occlusions (20%), minor fragments of the thrombus were dislocated distally into the parent artery during the passing maneuvers. Remarkably, passing of the microguidewire and of the balloon catheter always took place between the vessel wall and the thrombus. A penetration of the thrombus never occurred. Consequently, the thrombus was compressed and displaced to the opposite vessel wall during balloon inflation. Thrombus was also pressed into side branches at the occlusion site. After balloon deflation, the thrombus partially restored its shape, but occlusion of the side branches persisted (Fig 2). Reperfusion of side branches was observed when the side branch was located ipsilateral to the balloon (Fig 3). After balloon deflation and during the retrieval of the balloon-catheter and the microguidewire, no thrombus fragmentation or thromboembolic events occurred. There was no vessel dissection after balloon inflation. However, vasospasm at the site of the PTA was frequently seen but resolved without further treatment within minutes. PTA could be performed in all occlusions, and no device failure (eg, rupture) occurred.
Fig 2.
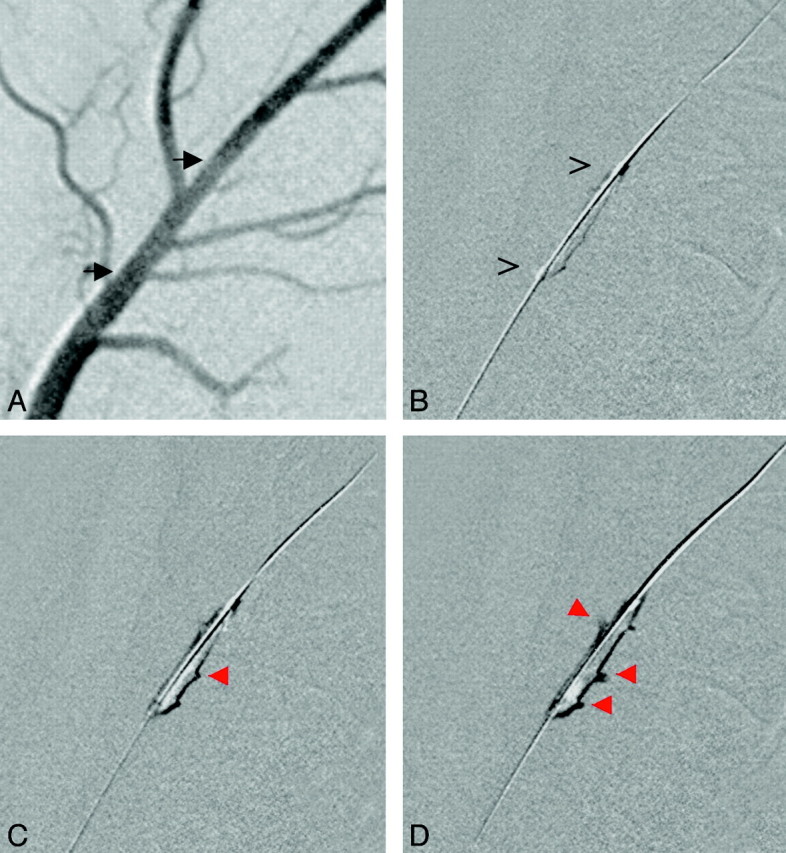
PTA performed for an occlusion of the LA. A, Angiogram before thrombus injection depicts side branches. Black arrows delineate the prospective position of the occluding thrombus. B, The microguidewire and PTA balloon are placed at the thrombus (open arrows). C and D, During balloon inflation (C), the thrombus is compressed and penetrates into side branches (red arrows) of the LA located contralateral to the balloon (D).
Fig 3.
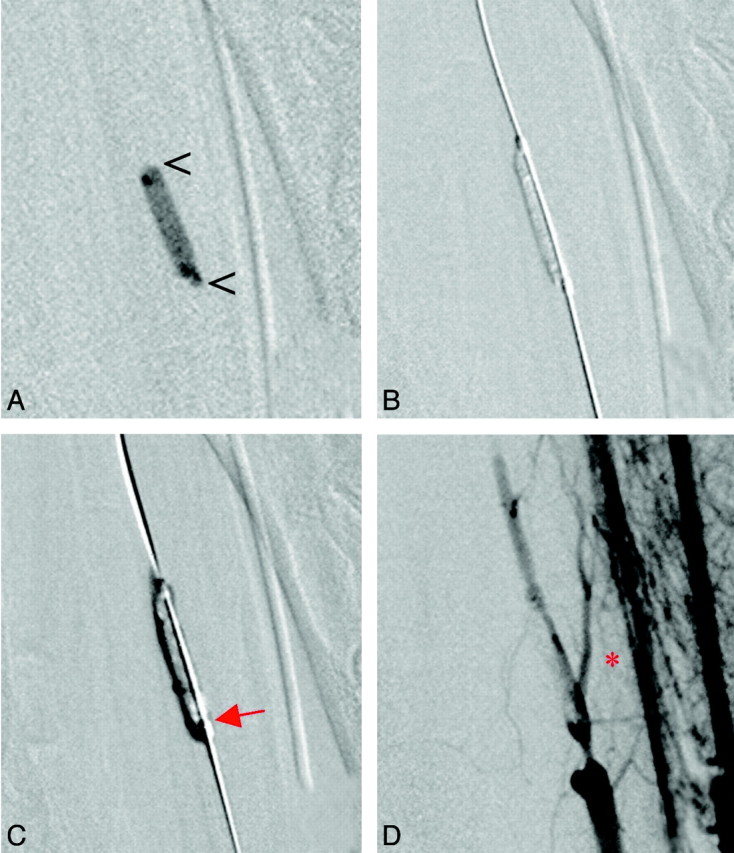
A, The radiopaque thrombus (open arrowheads) is visualized in a branch of the maxillary artery. B, The microguidewire and balloon catheter have passed between the thrombus and the vessel wall. After balloon inflation, the thrombus is pressed to the contralateral side of the vessel. C, Due to insufficient length of the balloon (red arrow), a second PTA at the proximal end of the occlusion is performed. D, Follow-up angiogram reveals flow in the parent artery and reperfusion of a side branch ipsilateral to balloon placement (red asterisk).
Thrombus-Device Interaction and Complications of Stent Placement
Concordant with the observations in the PTA group, the microguidewire and stent catheter always passed between the vessel wall and thrombus. In 2 cases, the microguidewire or microcatheter sheared off a small fragment of the thrombus and displaced it distally into the parent artery. In 1 case, the microguidewire had been placed successfully across the occlusion site but the stent catheter failed to pass the thrombus due to high friction and vessel tortuosity. The vessel diameter was 2.0 mm and, therefore, at the lower margin of the selected vessels. In the remaining 14 cases, the stent catheter was navigated across the occlusion site despite the tortuosity of the vessels (Fig 4).
Fig 4.
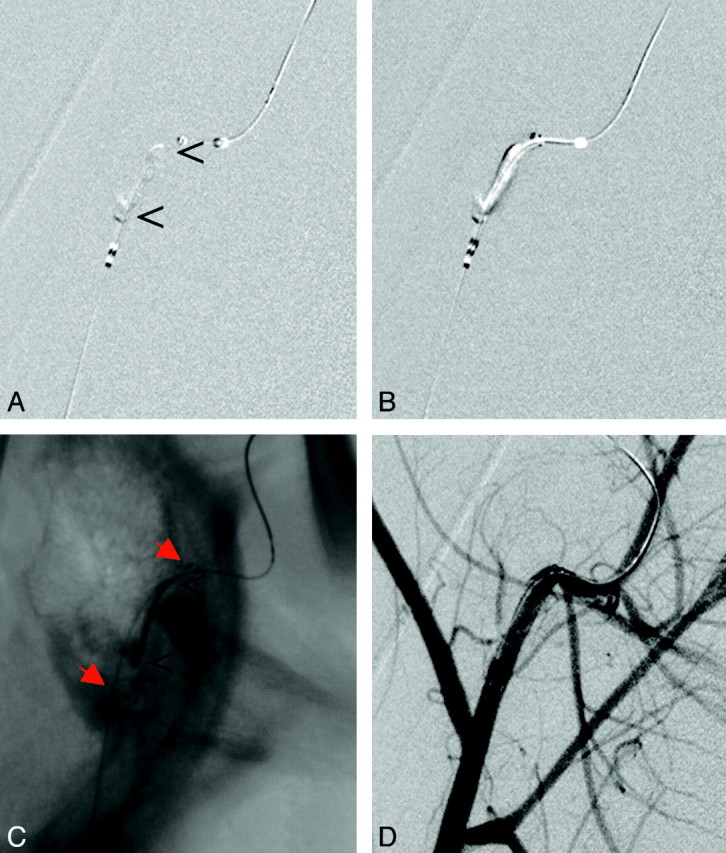
A, Occlusion of the maxillary artery in proximity to a sharp bend of the vessel. B and C, The stent (red arrows) is placed across the thrombus (B) and deployed (C). Again, the thrombus (open arrowheads) is compressed to the side contralateral to the initial passing procedure and is stabilized there (C). D, Follow-up angiogram reveals nearly complete recanalization.
During deployment, the self-expandable stent revealed a tendency to move distally. No thromboembolic events occurred during stent placement or postdilation. The retrieval of the deployment system and microguidewire across the stented segment was uncomplicated and did not displace the stent or parts of the thrombus. Side branches of the parent artery were occluded by thrombus if the initial passing of the microguidewire was performed on the contralateral side between the vessel wall and thrombus. In case of ipsilateral passage, the side branches were preserved and reperfusion was achieved (Fig 5).
Fig 5.
Stent placement performed for an occlusion of the LA. A, Assessment of vessel status before intervention (black arrowheads mark the prospective position of the thrombus). B and C, The microguidewire and stent catheter have passed the occlusion site (open arrowheads) on the left side (caudally, red arrow) on this lateral projection. D and E, The stent is deployed (open arrowheads), and the follow-up angiogram reveals reperfusion of an ipsilateral side branch (asterisk, E). Another side branch proximal to the occlusion is perfused through the mesh of the stent (curved red arrow, E).
Comparable with the PTA group, temporary vasospasm was frequently found at the proximal and distal end of the postdilation site. No dissection or vessel rupture occurred during stent deployment or after inflation of the balloon.
Discussion
Early revascularization predicts good outcome after acute ischemic stroke.19,20 Both therapies for acute ischemic stroke that are approved by the United States Food and Drug Administration (ie, intravenous thrombolysis and mechanical revascularization with the Merci Retriever System) have several drawbacks: Thrombolysis is a long procedure with a moderate recanalization rate and increases the risk of intracranial hemorrhage.1,21,22 More important, the narrow time window of only 3 hours for intravenous thrombolysis after stroke onset excludes most patients with stroke.23 The time window is extended to 8 hours for mechanical revascularization with the Merci Retriever System.5,6 However, even mechanical thrombectomy failed to remove the thrombus in several cases, and the repetitive passing and retrieval attempts increase the risk of severe complications.
Because reocclusion occurs after thrombolysis or occlusion persists in a larger subset of patients with stroke, some authors have advocated adjuvant PTA for rescue revascularization.7,8 Lum et al10 found that PTA as a complementary procedure offered additional recanalization in resistant clots. On the other hand, some authors found PTA to be ineffective for recanalization and proposed stent placement for acute stroke treatment.11 Both approaches have a common alternative concept: fast revascularization by compressing the thrombus to the vessel wall. However, until now, no study evaluated the fate of the thrombotic material during and after PTA or the placement of a stent.
This study assessed both techniques, PTA and stent placement, as feasible and relatively safe. Apart from transitory vasospasm, no severe complications such as dissection or vessel rupture were observed. No thrombus fragmentation was encountered during stent expansion or balloon inflation, indicating the stability of the thrombotic material and sufficient coverage of the thrombus by the stent struts. Although passing of the occlusion site with a microguidewire, microcatheter, and a device catheter is necessary with both procedures, distal dislocation of thrombotic material occurred as the only thromboembolic event. However, distal displacement of small thrombotic fragments during passing maneuvers seems to be acceptable and might be treated by additional application of local thrombolysis when encountered in human stroke. In only 1 case, the stent catheter could not be passed beyond the thrombus due to the high friction between the delivery system and the vessel wall and the thrombus. In all other cases, passage of the occluding thrombus was possible, and the recanalization procedure could be performed. The high technical success rate conforms to that reported for stent deliveries with and without PTA performed for acute ischemic stroke (100%)12 and for atherosclerotic intracranial stenosis (98%).24 We noticed a tendency for distal migration of the stent during deployment. This tendency could be controlled by performing the procedure under fluoroscopy and had no impact on recanalization result. Moreover, the length of the stents was always sufficient to cover the length of the occluding thrombus.
Remarkably, PTA achieved only a minor degree of revascularization compared with stent placement (mean recanalization rate at the primary end point, 14.6% vs. 67.5%). Complementary to the results of the PTA group, mean recanalization after stent placement increased with time by 9.8%. One explanation might be the further expansion due to the radial force of the stent. Moreover, once the thrombus was covered by the stent and compressed to the vessel wall, no further dislocation of thrombotic material was found. The self-expanding force of the stent stabilized the recanalization result by preventing re-expansion of the thrombus. However, the high incidence of reocclusion after PTA found in this study confirms the findings of other authors who recommended stabilization of the recanalization result by stent placement.11 Overall, these results favor stent placement for acute ischemic stroke treatment.
On the other hand, Nakano et al9 reported that PTA was successful for acute ischemic stroke treatment. In this study, additional thrombolytic agents were applied, and PTA was performed for local thrombotic occlusions of the underlying stenosis. However, when treating thrombus rather than atherosclerotic stenosis, reocclusion due to thrombus aggregation and thrombus expansion might occur.7 This could have occurred in our model using embolic occlusions. Additionally, no thrombolytic drugs were administered in our study. Hence, lower recanalization rates after PTA were seen in our study compared with those in the clinical literature. It remains unknown whether the achieved reperfusion after PTA and the consecutively increased accessible surface of the thrombus would facilitate chemical thrombolysis to a larger extent.
In-stent thrombus aggregation is a major concern; therefore, application of aspirin is mandatory. This was performed in our study and is routinely used after stent placement in humans, mostly in combination with clopidogrel.11,24 Because aspirin was also given in animals treated by PTA, other factors have to be responsible for the poor revascularization result after PTA. Without additional use of thrombolytics, it seems of the utmost importance to re-establish sufficient flow at the site of PTA or stent placement immediately after the mechanical procedure. Due to sufficient immediate recanalization, flow was sufficient. Sufficient flow prevents thrombus aggregation. In our study, the mean recanalization rate immediately after PTA was 20.5%, which is supposed to be insufficient. On the other hand, stent placement achieved a mean recanalization rate of 61.8% immediately after the intervention, allowing sufficient antegrade flow to occur. Hence, the reocclusion rate due to thrombus formation after stent placement was low.
Another issue in the treatment of occluded brain vessels is the fate of important side branches (ie, the lenticulostriate and pontine perforating arteries). Whereas lenticulostriate infarction occurs early in the course of middle cerebral artery mainstem occlusion, pontine perforating arteries might still be perfused in basilar artery occlusion. Our study revealed that side branches can be salvaged when PTA or stent placement is performed. By choosing a position of the microguidewire ipsilateral to branching vessels, one can direct the compression of the thrombus (to the contralateral wall). The adequate position of the microguidewire has to be controlled on both planes using angiography before stent deployment or balloon inflation. The risk of permanent perforator occlusion in humans can be reduced by this means. Additionally, this technique appears to be appropriate to preserve major vessel branches.
From the procedural point of view, recanalization by stent placement can be achieved relatively fast compared with other mechanical thrombectomy techniques because repetitive attempts are not performed. However, the application time of stent deployment and postdilation was significantly longer compared with PTA alone (33 minutes versus 16.6 minutes) but still shorter compared with the mean time to recanalization of mechanical thrombectomy devices in a previous in vivo study (42.1 minutes).15 Moreover, stent placement was followed by PTA in the present study, increasing procedure time. In the clinical setting, stent placement alone might be sufficient to achieve recanalization, obviating PTA and, therefore, reducing the time to recanalization.
The significantly better result after stent placement compared with PTA alone might be influenced by the study design, allowing oversizing of the stent, whereas the PTA balloon had to be underzised compared with the vessel diameter. Nevertheless, the striking superiority of stent placement compared with PTA alone was also shown in a large study on recanalization of the coronary arteries.25 Despite the difference that coronary artery occlusions are usually caused by local thrombosis of atherosclerotic vessels and cerebral vessel occlusions are most often caused by emboli, the concept of fast recanalization with immediate stabilization of the result by placement of a stent appears very convincing.
Limitations
Compared with the young vessels in the animal model, arteries of patients with stroke will show more atherosclerosis, stenosis, and elongation. Therefore, the technical feasibility of the 2 presented techniques might be overestimated, and complications like dissection or vessel perforation might easily be underestimated.
The study was designed to evaluate the mechanical recanalization effect of both procedures, but it neglected possible differences in the coagulation status and spontaneous fibrinolysis between the 2 species (ie, swine and humans). Furthermore, the barium sulfate in the thrombotic material, crucial for the visualization of thrombus-device interaction, might influence the spontaneous thrombolysis of the clot.
To summarize, PTA and stent placement are feasible treatment options for acute vessel occlusions and exhibit a low complication and device-failure rate. In the animal model, the recanalization rate after PTA alone is insufficient and decreases with time. Treatment with self-expanding stents achieves sufficient reperfusion and stabilizes the revascularization result. Important side branches at the occlusion site can be preserved by the ipsilateral placement of the microguidewire and stent, resulting in a compression and dislocation of the thrombus to the contralateral vessel wall.
References
- 1.Tissue plasminogen activator for acute ischemic stroke: the National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995;333:1581–87 [DOI] [PubMed] [Google Scholar]
- 2.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: The PROACT II study—a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 1999;282:2003–11 [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, Schroth G, Nedeltchev K, et al. Intra-arterial thrombolysis in 100 patients with acute stroke due to middle cerebral artery occlusion. Stroke 2002;33:1828–33 [DOI] [PubMed] [Google Scholar]
- 4.Brekenfeld C, Remonda L, Nedeltchev K, et al. Symptomatic intracranial haemorrhage after intra-arterial thrombolysis in acute ischaemic stroke: assessment of 294 patients treated with urokinase. J Neurol Neurosurg Psychiatry 2007;78:280–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–38 [DOI] [PubMed] [Google Scholar]
- 6.Smith WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke: results of the multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, part I. AJNR Am J Neuroradiol 2006;27:1177–82 [PMC free article] [PubMed] [Google Scholar]
- 7.Ueda T, Hatakeyama T, Kohno K, et al. Endovascular treatment for acute thrombotic occlusion of the middle cerebral artery: local intra-arterial thrombolysis combined with percutaneous transluminal angioplasty. Neuroradiology 1997;39:99–104 [DOI] [PubMed] [Google Scholar]
- 8.Ringer AJ, Qureshi AI, Fessler RD, et al. Angioplasty of intracranial occlusion resistant to thrombolysis in acute ischemic stroke. Neurosurgery 2001;48:1282–88 [DOI] [PubMed] [Google Scholar]
- 9.Nakano S, Iseda T, Yoneyama T, et al. Direct percutaneous transluminal angioplasty for acute middle cerebral artery trunk occlusion: an alternative option to intra-arterial thrombolysis. Stroke 2002;33:2872–76 [DOI] [PubMed] [Google Scholar]
- 10.Lum C, Stys PK, Hogan MJ, et al. Acute anterior circulation stroke: recanalization using clot angioplasty. Can J Neurol Sci 2006;33:217–22 [DOI] [PubMed] [Google Scholar]
- 11.Levy EI, Ecker RD, Horowitz MB, et al. Stent-assisted intracranial recanalization for acute stroke: early results. Neurosurgery 2006;58:458–63 [DOI] [PubMed] [Google Scholar]
- 12.Levy EI, Mehta R, Gupta R, et al. Self-expanding stents for recanalization of acute cerebrovascular occlusions. AJNR Am J Neuroradiol 2007;28:816–22 [PMC free article] [PubMed] [Google Scholar]
- 13.Gralla J, Schroth G, Remonda L, et al. A dedicated animal model for mechanical thrombectomy in acute stroke. AJNR Am J Neuroradiol 2006;27:1357–61 [PMC free article] [PubMed] [Google Scholar]
- 14.Levy EI, Sauvageau E, Hanel RA, et al. Self-expanding versus balloon-mounted stents for vessel recanalization following embolic occlusion in the canine model: technical feasibility study. AJNR Am J Neuroradiol 2006;27:2069–72 [PMC free article] [PubMed] [Google Scholar]
- 15.Gralla J, Schroth G, Remonda L, et al. Mechanical thrombectomy for acute ischemic stroke: thrombus-device interaction, efficiency, and complications in vivo. Stroke 2006;37:3019–24 [DOI] [PubMed] [Google Scholar]
- 16.Marder VJ, Chute DJ, Starkman S, et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke 2006;37:2086–93 [DOI] [PubMed] [Google Scholar]
- 17.Reinert M, Brekenfeld C, Taussky P, et al. Cerebral revascularization model in a swine. Acta Neurochir Suppl 2005;94:153–57 [DOI] [PubMed] [Google Scholar]
- 18.TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial: phase I findings. N Engl J Med 1985;312:932–36 [DOI] [PubMed] [Google Scholar]
- 19.Ringelstein EB, Biniek R, Weiller C, et al. Type and extent of hemispheric brain infarctions and clinical outcome in early and delayed middle cerebral artery recanalization. Neurology 1992;42:289–98 [DOI] [PubMed] [Google Scholar]
- 20.Alexandrov AV, Demchuk AM, Felberg RA, et al. High rate of complete recanalization and dramatic clinical recovery during tPA infusion when continuously monitored with 2-MHz transcranial Doppler monitoring. Stroke 2000;31:610–14 [DOI] [PubMed] [Google Scholar]
- 21.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II): Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–51 [DOI] [PubMed] [Google Scholar]
- 22.Neumann-Haefelin T, du Mesnil de Rochemont R, Fiebach JB, et al. Effect of incomplete (spontaneous and postthrombolytic) recanalization after middle cerebral artery occlusion: a magnetic resonance imaging study. Stroke 2004;35:109–14, Epub 2003 Dec 11 [DOI] [PubMed] [Google Scholar]
- 23.Barber PA, Zhang J, Demchuk AM, et al. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology 2001;56:1015–20 [DOI] [PubMed] [Google Scholar]
- 24.Bose A, Hartmann M, Henkes H, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke 2007;38:1531–37. Epub 2007 Mar 29 [DOI] [PubMed] [Google Scholar]
- 25.Grines CL, Cox DA, Stone GW, et al. Coronary angioplasty with or without stent implantation for acute myocardial infarction: Stent Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med 1999;341:1949–56 [DOI] [PubMed] [Google Scholar]