Abstract
BACKGROUND AND PURPOSE: Diffusion-weighted imaging (DWI) hyperintensity is hypothesized to represent irreversibly infracted tissue (ischemic core) in the setting of acute stroke. Measurement of the ischemic core has implications for both prognosis and therapy. We wished to assess the level of evidence in the literature supporting this hypothesis.
MATERIALS AND METHODS: We performed a systematic review of the literature relating to tissue outcomes of DWI hyperintense stroke lesions in humans. The methodologic rigor of studies was evaluated by using criteria set out by the Oxford Centre for Evidence-Based Medicine. Data from individual studies were also analyzed to determine the prevalence of patients demonstrating lesion progression, no change, or lesion regression compared with follow-up imaging.
RESULTS: Limited numbers of highly methodologically rigorous studies (Oxford levels 1 and 2) were available. There was great variability in observed rates of DWI lesion reversal (0%–83%), with a surprisingly high mean rate of DWI lesion reversal (24% of pooled patients). Many studies did not include sufficient data to determine the precise prevalence of DWI lesion growth or reversal.
CONCLUSIONS: The available tissue-outcome evidence supporting the hypothesis that DWI is a surrogate marker for ischemic core in humans is troublingly inconsistent and merits an overall grade D based on the criteria set out by the Oxford Centre for Evidence-Based Medicine.
Identifying threatened but still-viable brain tissue in patients with stroke remains an important goal of acute stroke imaging.1 Most stroke imaging work to date has focused on characterizing ischemic tissue as nonviable (ie, will not survive even with immediate therapy) or as viable (ie, tissue that is salvageable with appropriate therapy). Nonviable ischemic tissue is termed “core,” whereas viable ischemic tissue is termed “penumbra.” There are 2 reasons to measure the extent of core. First, the risk of cerebral hemorrhage from acute revascularization therapy appears to be related to the size of the ischemic core.2 For this reason, patients with large regions of ischemic tissue suspected of being nonviable core are usually excluded from receiving revascularization therapy. Second, most, but not all, definitions of penumbra (the tissue targeted for therapy) require subtraction of the extent of core from the total ischemic lesion extent. Thus, accurate knowledge of both the total ischemic lesion extent and the core extent is needed to measure penumbra extent accurately. If decisions about providing or withholding therapy are to be based on imaging, the imaging test must accurately discriminate core from penumbra and from nonischemic tissue.
If either in the clinical setting or during clinical research, one attempts to use core-penumbra imaging for patient selection for therapy, there are potentially serious consequences if the measurement of core extent is inaccurate. For example, if an imaging method overestimates core extent, there are potentially 2 negative effects on patient selection. First, patients who could benefit from therapy might be incorrectly excluded from receiving therapy for safety reasons if their lesion size falsely appears to exceed the accepted limit. Second, the extent of penumbra could be underestimated when the overestimated core is subtracted from total ischemic lesion size (eg, the size of the perfusion abnormality). Thus patients who could benefit might also be excluded because of lack of sufficient apparent penumbra, the target tissue. Similarly, if an imaging method underestimates core extent, patients who are unlikely to benefit from therapy, due either to large actual core or small actual penumbra, could be incorrectly selected to receive therapy with its associated risks.
Currently, many investigators consider the MR imaging diffusion-perfusion mismatch (DPM) model to be the most accurate representation of both core and penumbra.3–5 According to this model, hyperintense signal intensity on diffusion-weighted imaging (DWI) is hypothesized to represent the extent of ischemic core and the surrounding perfusion abnormality represents the penumbra. Current use of and regard for the DPM model goes beyond merely theoretic considerations, however. Many of the recent ongoing and completed major stroke therapy and imaging trials have DWI in central roles. For example, the presence of mismatch is used as an inclusion criterion in the Desmoteplase in Acute Ischemic Stroke Trial (DIAS), DIAS-2, and Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS) trials; large DWI lesions are a reason for exclusion in the ReoPro Retavase Reperfusion of Stroke Safety Study—Imaging Evaluation (ROSIE) trial; and validation of the DPM model is central to the Echo-Planar Imaging Thrombolysis Evaluation Trial (EPITHET), Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution, and MR and Recanalization of Stroke Clots Using Embolectomy (MR-RESCUE) trials.6–12 Given the wide use and acceptance of the DPM model, it seems important to assess formally the evidence on which a key component of this hypothesis is based (ie, DWI core). We studied the level of evidence supporting the hypothesis that in human patients, DWI hyperintensity identifies areas of solely irreversible infarction and thus is a surrogate for the ischemic core in acute stroke. If valid, one would expect most infarct sizes depicted on DWI to be either stable or increased on follow-up imaging. On the basis of this hypothesis and current prevailing views expressed in the literature, we expected DWI reversible lesions to represent a very small minority of observed cases.
Materials and Methods
Studies to Be Evaluated
We performed an evidence-based systematic review of the published human literature concerning tissue outcomes of stroke lesions that were initially hyperintense on DWI and made an assessment of the evidence by using the methods established by the Oxford Centre for Evidence-Based Medicine.13
The search strategy was designed to capture as many studies as possible containing information on the relationship between DWI in the acute stroke setting and final infarct volumes on follow-up imaging. To make the scope of the search as broad as possible, we reviewed studies for possible inclusion regardless of whether determining the relationship between initial DWI lesion volume and final infarct volume was a stated primary goal of the study. Our inclusion criteria were the following: 1) acute stroke presentation in adult humans, 2) DWI performed within 24 hours, 3) follow-up imaging including CT or MR imaging performed at or >24 hours from onset of symptoms, and 4) English language publications. Both prospective and retrospective studies were included. Exclusion criteria included the following: 1) case reports or review articles, 2) evaluation of primarily subcortical stroke or transient ischemic attack, and 3) evaluation of infratentorial stroke.
Search Methodology
The Ovid MEDLINE data base was searched on February 28, 2008, with the following strategy:
) “Cerebrovascular accident” OR “exp cerebral infarction” OR “brain ischemia.”
) “Diffusion magnetic resonance imaging” OR “diffusion.mp.”
) Time factors OR exp cohort studies.
) Numbers 1 AND 2 AND 3.
) Limited to humans and English language.
All studies in the data base were included in the evaluation regardless of publication date.
Study Quality and Data Analysis
Studies were evaluated for methodologic rigor by using the criteria set forth for diagnostic studies in the Oxford Centre for Evidence-Based Medicine levels of evidence.13 These criteria grade the methodologic rigor of studies from level 1 (systematic reviews, validating cohort studies) to level 5 (expert opinion). The level of evidence for each study was recorded, along with the specific details of the study that led to the assignment of that level.
In addition to the assessment of methodologic rigor, we also collected data tailored to assessment of stroke MR imaging studies, including the number of patients, time to initial MR imaging, time to follow-up imaging, collection of clinical performance data (eg, National Institutes of Health Stroke Scale [NIHSS], modified Rankin Scale [mRS]), and use of thrombolytic therapy, according to a standardized assessment form as detailed in Table 1. Where possible, the exact percentage of patients within a study demonstrating lesion growth or reversal was calculated. Approximately one third of the studies were initially assessed by both reviewers to develop the approach and to establish satisfactory concordance between reviewers. After satisfactory concordance was reached, the remaining studies (two thirds) were assessed by 1 reviewer.
Table 1:
Collected study information
|
Note:—DWI indicates diffusion-weighted imaging; NIHSS, National Institutes of Health Stroke Scale; BI, Barthel Index; mRS, modified Rankin Scale.
In studies in which there were quantitative data regarding the change in lesion volumes between initial and follow-up studies, patients were stratified into the following 3 categories: progression (patients showing ≥10% increase in lesion volumes), no change (patients showing <10% increase to <10% decrease in lesion volumes), and reversal (patients showing ≥10% decrease in lesion volumes). For studies that did not supply individual data points but described lesions qualitatively showing progression, no change, or reversal, patients were stratified according to the definitions included in the study.
Results
The initial MEDLINE search identified 389 studies. On review of the abstracts, 215 were rejected for not satisfying our outlined criteria. The remaining 174 studies, including studies in which satisfaction of inclusion or exclusion criteria was unclear on the basis of abstract review, were submitted to full text review. Of these studies, 61 met all of our criteria and were analyzed.
Studies were grouped according to the degree to which the data allowed determination of exact levels of DWI growth or reversal (Fig 1). These groups do not reflect any particular stratification of methodologic quality or outcomes but rather reflect the degree to which data were available in the individual studies that could be used to evaluate our hypothesis. Because studies were included in the analysis provided they met the inclusion/exclusion criteria and without regard to the original purpose of the study, a sizeable number of studies provided some information on growth but did not present sufficient data to determine exact rates of reversal.
Fig 1.
Results of MEDLINE search.
In 18 of the 61 studies (assigned to group A), it was possible to determine the exact prevalence of DWI growth or reversal on the basis of the data provided.14–31 In 8 of 61 studies (assigned to group B), there were limited data indicating that at least 1 patient demonstrated DWI reversal, but it was impossible to calculate the exact number of patients with lesion reversal.9,32–38 Of the 8 group B studies, 4 showed evidence of DWI reversal in a subgroup of patients; however, the number of patients in that subgroup was not explicitly stated.9,33,36,37 Three studies demonstrated evidence of some DWI reversal in figures; however, the absence of numeric data points associated with these graphs precluded precise analysis.34,35,38 One study provided a data range for change in the size of lesions that indicated at least 1 reversal; however it was not possible to determine if >1 reversal occurred.32 Finally, in 35 of 61 studies (group C), data concerning growth of some lesions was presented, but there was insufficient information provided to determine whether any instances of DWI reversal occurred.6,7,39–71 Many of these group C studies reported average lesion volumes for initial DWI and final infarct volumes for the study population as a whole but did not include individual data points or make specific comments on the presence or absence of cases of DWI reversal. Because averaging all lesion volumes together could mask instances of reversible lesions, such group C studies could not be used to measure the precise rates of DWI growth and reversal.
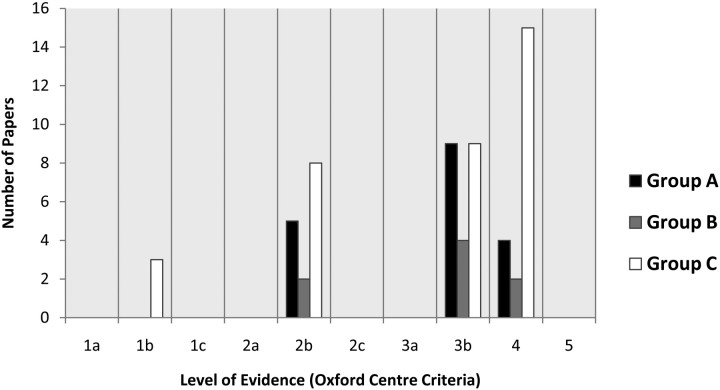
The results of the study methodology evaluation by using the Oxford Centre Criteria are presented in Fig 2. No level 1 studies were found in either group A or B. Of the 3 level 1 studies in group C, 2 were designed as therapeutic trials rather than diagnostic trials6,7 and the third study was designed to compare CT and MR imaging in the acute setting by using a quantitative scoring system and did not include quantitative lesion volumes at follow-up.64
Fig 2.
Levels of evidence based on Oxford Centre Criteria.
The methodologic details of the group A studies are presented in Table 2. Data concerning DWI lesion growth, stability, or reversal found in group A studies are presented in Table 3. The median rate of DWI reversal (>10% lesion reduction) was 22%, with a range of 0%–83%. Pooled individual patient data from the 18 studies, comprising a total of 572 patients, revealed a 24% rate of DWI reversal. Approximately half (51%) of pooled patients received either intravenous or intra-arterial thrombolytic agents (range, 0%–100%).
Table 2:
Characteristics of group A studies
| Reference | Year | Pro/Ret | Level of Evidence | No. of Patients | Time to Initial MRI | Time to Follow-Up Imaging | % of Patients Receiving Thrombolysis |
|---|---|---|---|---|---|---|---|
| Delgado-Mederos et al30 | 2007 | Ret | 4 | 113 | 6 hours | 36–48 hours | 100 |
| Rivers et al29 | 2006 | Pro | 3b | 46 | <24 hours | 1 or 3 months | 0 |
| Seitz et al28 | 2005 | Pro | 2b | 64 | <3 hours | 8 days | 45 |
| Ritzl et al27 | 2004 | Pro | 2b | 12 | <24 hours | 124 days | 58 |
| Schramm et al26 | 2004 | Pro | 3b | 22 | <6 hours | 5 days* | 59 |
| Schaefer et al25 | 2004 | Ret | 4 | 16 | <6 hours | 1–7 days | 100 |
| Fiehler et al24 | 2004 | Pro | 3b | 68 | <6 hours | 4–7 days | 40 |
| Shih et al23 | 2003 | Pro | 3b | 14 | <6 hours | 7 days | 100 |
| Schramm et al22 | 2002 | Pro | 2b | 20 | <6 hours | 5 days | 40 |
| Parsons et al21 | 2002 | Pro | 3b | 40 | <6 hours | 81 days | 48 |
| Schellinger et al20 | 2001 | Pro | 3b | 41 | <6 hours | 2–5 days | 56 |
| Kidwell et al18 | 2000 | Pro | 3b | 7 | <6 hours | 7 days | 100 |
| Parsons et al19 | 2000 | Pro | 2b | 19 | <24 hours | 30–90 days | Not specified |
| Beaulieu et al15 | 1999 | Pro | 3b | 21 | <7 hours | 30 days | 60 |
| Neumann-Haefelin et al17 | 1999 | Pro | 2b | 20 | <24 hours | 6–14 days | 20 |
| Sorensen et al16 | 1999 | Ret | 4 | 23 | <12 hours | 3–59 days | 0 |
| Barber et al31 | 1998 | Pro | 3b | 18 | <24 hours | 7–120 days | 6 |
| Tong et al14 | 1998 | Ret | 4 | 8 | <6.5 hours | 7 days | 20 |
Note:—MRI indicates MR imaging; Ret, retrospective; Pro, prospective.
Follow up imaging performed with CT.
Table 3:
Changes in lesion volumes
| Reference | Year | Patients with Lesion Progression | Patients with No Change | Patients with Lesion Reversal |
|---|---|---|---|---|
| Delgado-Mederos et al30* | 2007 | 80% | 13% | 7% |
| Rivers et al29† | 2006 | 44% | n/a | 56% |
| Seitz et al28† | 2005 | 52% | n/a | 48% |
| Ritzl et al27 | 2004 | 8% | 8% | 83% |
| Schramm et al26 | 2004 | 69% | 0% | 31% |
| Schaefer et al25 | 2004 | 88% | 6% | 6% |
| Fiehler et al24‡ | 2004 | 79% | n/a | 21% |
| Shih et al23 | 2003 | 36% | 0% | 64% |
| Schramm et al22 | 2002 | 85% | 5% | 10% |
| Parsons et al21 | 2002 | 61% | 25% | 14% |
| Schellinger et al20§ | 2001 | 100% | n/a | 0% |
| Kidwell et al18 | 2000 | 29% | 0% | 71% |
| Parsons et al19 | 2000 | 42% | 26% | 32% |
| Beaulieu et al15 | 1999 | 47% | 20% | 33% |
| Neumann-Haefelin et al17 | 1999 | 50% | 10% | 40% |
| Sorensen et al16 | 1999 | 70% | 17% | 13% |
| Barber et al31 | 1998 | 44% | 13% | 44% |
| Tong et al14‖ | 1998 | 75% | 13% | 13% |
Note:—n/a indicates not applicable.
Percentages based on author's report of stratified lesion volumes.
Percentages based on author's dichotomization of DWI volume as more or less than follow-up T2 volume.
Initial volumes determined by apparent diffusion coefficient decrease of >20%, not DWI hyperintensity.
Based on author commentary, not individual data points.
Values inferred from data presented in graphic form.
Seven of 18 studies (39%) in group A reported rates of tissue reperfusion either from assessment of vessel patency or by using perfusion imaging. Seventeen of 18 studies (94%) collected clinical performance data in the form of the NIHSS, mRS, Barthel Index (BI), or other clinical scales. Thirteen of 18 (72%) studies attempted to correlate the results of clinical performance data with lesion volumes. Only 1 study (6%) attempted to assess the degree of collateral blood flow to the affected area.
Discussion
DWI has been reported to be a highly sensitive method for imaging cerebral ischemia.72–74 Clinical observations and many published reports showing a high correlation between the extent of abnormality on DWI and final stroke volume have encouraged the view that DWI depicts regions of nonviable ischemic tissue.15,57 Contrary to this view, however, are published reports showing examples of salvageable ischemic lesions that were seen to be hyperintense on initial DWI.18,75 We undertook this study first to assess the level of available evidence in support of the currently prevailing hypothesis that DWI high-signal intensity depicts the extent of nonviable ischemic core and second to measure the reported incidence of DWI lesion reversal, which we expected to be small.
The guidelines of the Oxford Centre for Evidence-Based Medicine on assessing levels of evidence is a widely used system, which grades the methodologic rigor of studies.13,76 The inclusion criteria used in our literature search were designed to be broad enough to capture studies with data regarding DWI growth or reversal without regard to the primary purpose of the study itself. This was done to help ensure a comprehensive scope to the review. Our limitation to studies pertaining to acute presentation of supratentorial stroke in adults was designed to target the population of greatest clinical relevance. Because the accepted in vivo standard for the extent of final infarction is lesion size on follow-up T2-weighted MR or CT imaging, we included only studies with follow-up imaging.73 Because clinical diagnosis of acute stroke was an inclusion criterion and because the studies that were evaluated encompassed only patients with positive findings on DWIs, true-negative and false-negative results were not part of this evaluation. Thus, sensitivity and specificity calculations are not strictly applicable to this research.
The results of our search revealed a predominance of Oxford Centre level 3 and 4 studies, with few level 2 studies and no level 1 studies among those from which it was possible to determine precise tissue outcomes (our group A). The large number of studies in the level 3 or 4 category reflects the studies in which consecutive patients were not used, or at least not specified, and the use of retrospective studies, respectively. Assigning lower levels of evidence to some studies does not cast doubt, per se, on the validity of the results for the population studied in those particular investigations but rather raises concern for the degree to which bias might affect the generalizability of the findings. As such, conclusions drawn from level 3 and 4 studies must be validated by using methods less susceptible to bias before being accepted as universally applicable. The paucity of level 1 and 2 studies of DWI for prediction of tissue outcome highlights the need for more prospective validating studies using consecutive patients and consistent reference standards.
Despite concerns about the level of available evidence mentioned above, the existing evidence shows a substantial incidence (24% of pooled patients) of partial or complete DWI reversal. Especially notable were a few reported instances of complete reversal of individual DWI lesions. The cases of partial or complete reversal represent patients for whom the assumption would be invalid that initial diffusion hyperintensity depicts solely regions of irreversible infarction. They also represent patients who could, in theory, be inaccurately triaged for stroke therapy on the basis of prevailing assumptions. Given the wide range of reported rates of DWI reversal (0%–83%) and the high overall prevalence of partial or complete DWI reversal reported, we question use of DWI as an accurate surrogate marker for ischemic core for patients with acute stroke.
Our review of the evidence related to tissue outcomes in patients with stroke is concordant with the findings from basic and other types of human investigations of the biochemical and physiologic environments within DWI hyperintense lesions. In animal models of ischemia, it has been shown that reductions in brain apparent diffusion coefficient (ADC), the quantitative measure of diffusion abnormality, are reversible following reperfusion in a temporally dependent manner and that specific ADC thresholds do not predict tissue fate.77 Investigations of human patients have similarly demonstrated that absolute ADC thresholds do not predict response to thrombolysis or tissue fate.78 Investigation of oxygen metabolism in DWI lesions by using 15O-Positron-emission tomography by Guadagno et al79 revealed spatial variability in the cerebral metabolic rate of oxygen with individual DWI lesions and considerable variability of oxygen extraction fractions and cerebral blood flow relationships within single DWI lesions, ranging from areas with decreased flow relative to oxygen demand (“misery perfusion”) to areas with increased flow relative to demand (“absolute luxury perfusion”). These findings contribute to the idea of hyperintense DWI lesions as heterogeneous regions comprising varying biochemical and metabolic environments, which may be variably amenable to salvage rather than as homogeneous regions of ischemic core tissue.
Understanding the physical properties that result in DWI hyperintensity in stroke would be expected to contribute to our understanding of the meaning and prognostic significance of these lesions. Decreased ADC values have been observed in animals following the intracerebral administration of oubain, a selective inhibitor of Na+, K+-adenosine triphosphatase, implicating the failure of energy-dependent ion channels in the development of DWI hyperintensity.80 Many investigators have suggested that DWI hyperintensity reflects areas of trapping of intracellular fluid due to failure of these ion channels, resulting in restricted-motion water in the intracellular compartment.80–83 Others have implicated decreased movement of water molecules in the extracellular compartment.84–86 Moreover, although clearly ischemia does result in eventual bioenergetic failure, the contribution of processes other than energy failure alone to DWI hyperintensity is supported by the observation in animals that in early ischemia, the DWI lesion is initially larger than areas of adenosine triphosphate (ATP) depletion, more closely corresponding to areas of pH alteration due to anaerobic metabolism in which ATP levels remain relatively preserved.87 At present, the precise etiology of DWI lesions remains an area of investigation.
A significant limitation of many of the studies we reviewed was the inability to determine precise rates of DWI growth or reversal. Many studies reported only a single average change in lesion volume between initial and follow-up imaging for their study population as a whole. This limitation was typical of a significant portion of the studies classified by us as group C. The reporting of only average lesion sizes has the potential to hide the results from patients in the numeric minority (eg, patients with DWI reversal). Moreover, reliance on studies that report individual data points has the potential to bias the analysis in favor of studies with smaller study populations, for which this approach is more feasible. We recommend that future studies of acute stroke imaging report explicitly their rates of lesion growth and regression, even if the large size of their population precludes inclusion of individual patient data.
We found high variability in the reporting of study information specifically related to stroke, such as time to initial imaging from symptom onset, time to thrombolysis, time to follow-up imaging, rates of thrombolysis, efficacy of thrombolysis, and rates of tissue reperfusion or vessel recanalization. Correlation of these variables with rates of DWI growth and reversal is beyond the scope of the present study; this analysis is currently underway and will be reported separately. In our opinion, the most reliable data with regard to imaging of core and penumbra would be derived from studies of patients with stroke with documented arterial occlusion who received effective revascularization in a clinically relevant timeframe (eg, <6 hours from symptom onset) and in whom the precise time and degree of reperfusion are known.
In summary, review of the available literature regarding evolution of DWI lesions in humans revealed limited numbers of generalizable studies. The available data revealed high variation in the observed rates of DWI lesion reversibility, the mean of which was substantial and higher than we expected (24%). This substantial rate of DWI lesion reversal seems especially notable considering the fact that only approximately half of the pooled patients received thrombolytic therapy. Furthermore, substantial rates of DWI lesion reversal were seen in studies with low or no use of thrombolytic therapy.28,29,31 To make a final assessment, the Oxford Centre for Evidence-Based Medicine provides grades of recommendations for rating the overall level of evidence supporting a hypothesis, ranging from a grade A for a well-supported hypothesis to grade D for low levels of evidence or inconsistent results in the literature.13 These grades for final assessment are intended to summarize the evidence for a topic as a whole, and are distinct from the Oxford Centre levels of evidence discussed above, which are used to evaluate the individual papers. Overall, the level of evidence supporting the hypothesis that DWI measures solely ischemic core tissue in human stroke merits a grade D (the lowest grade of evidence) based largely on the highly inconsistent rates of DWI reversal reported in the literature. Because of the substantial incidence of partial or complete DWI reversal seen in our review, we support the modified hypothesis that in the first 24 hours after stroke onset, DWI hyperintensity in human stroke represents a combination of both reversible and irreversible ischemic tissue. Further investigations of high methodologic rigor are needed in this area to determine whether this modified hypothesis can be accepted and when and under what conditions it is appropriate to do so.
Conclusions
The available tissue-outcome evidence supporting the hypothesis that DWI hyperintensity is a surrogate marker for ischemic core is troublingly inconsistent and merits an overall grade D (the lowest grade) based on the criteria set out by the Oxford Centre for Evidence-Based Medicine. Further prospective studies specifically designed to determine whether DWI can discriminate reversible from irreversible ischemia are needed.
References
- 1.Sims J, Schwamm LH. The evolving role of acute stroke imaging in intravenous thrombolytic therapy: patient selection and outcomes assessment Neuroimaging Clin N Am 2005;15:421–40, xii [DOI] [PubMed] [Google Scholar]
- 2.Selim M, Fink JN, Kumar S, et al. Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: prognostic value of the initial apparent diffusion coefficient and diffusion-weighted lesion volume. Stroke 2002;33:2047–52 [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan A, Goyal M, Al Azri F, et al. State-of-the-art imaging of acute stroke Radiographics 2006;26 (suppl 1):S75–95 [DOI] [PubMed] [Google Scholar]
- 4.Warach S. Measurement of the ischemic penumbra with MRI: it's about time. Stroke 2003;34:2533–34 [DOI] [PubMed] [Google Scholar]
- 5.Schaefer PW, Copen WA, Lev MH, et al. Diffusion-weighted imaging in acute stroke Neuroimaging Clin N Am 2005;15:503–30, ix-x [DOI] [PubMed] [Google Scholar]
- 6.Hacke W, Albers G, Al-Rawi Y, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 2005;36:66–73 [DOI] [PubMed] [Google Scholar]
- 7.Furlan AJ, Eyding D, Albers GW, et al. Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke 2006;37:1227–31 [DOI] [PubMed] [Google Scholar]
- 8.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 2006;60:508–17 [DOI] [PubMed] [Google Scholar]
- 9.Butcher KS, Parsons M, MacGregor L, et al. Refining the perfusion-diffusion mismatch hypothesis. Stroke 2005;36:1153–59 [DOI] [PubMed] [Google Scholar]
- 10.Hacke W, Furlan AJ, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study Lancet Neurol 2009;8:141–50. Epub 2008 Dec 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MR and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE). Available at: http://clinicaltrials.gov/ct2/show/NCT00389467??term=NCT00094588&rank=1. Accessed January 1,2009
- 12.ReoPro and Retavase to Restore Brain Blood Flow After Stroke. Available at: http://www.clinicaltrials.gov/ct/gui/show/NCT00039832. Accessed January 1,2009
- 13.Oxford Centre for Evidence-based Medicine: Levels of Evidence (May 2001). Available at: http://www.cebm.net/index.aspx?o=1025. Accessed May 5,2008
- 14.Tong DC, Yenari MA, Albers GW, et al. Correlation of perfusion- and diffusion-weighted MRI with NIHSS score in acute (<6.5 hour) ischemic stroke. Neurology 1998;50:864–70 [DOI] [PubMed] [Google Scholar]
- 15.Beaulieu C, de Crespigny A, Tong DC, et al. Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: evolution of lesion volume and correlation with clinical outcome. Ann Neurol 1999;46:568–78 [DOI] [PubMed] [Google Scholar]
- 16.Sorensen AG, Copen WA, Ostergaard L, et al. Hyperacute stroke: simultaneous measurement of relative cerebral blood volume, relative cerebral blood flow, and mean tissue transit time. Radiology 1999;210:519–27 [DOI] [PubMed] [Google Scholar]
- 17.Neumann-Haefelin T, Wittsack HJ, Wenserski F, et al. Diffusion- and perfusion-weighted MRI: the DWI/PWI mismatch region in acute stroke. Stroke 1999;30:1591–97 [DOI] [PubMed] [Google Scholar]
- 18.Kidwell CS, Saver JL, Mattiello J, et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol 2000;47:462–69 [PubMed] [Google Scholar]
- 19.Parsons MW, Li T, Barber PA, et al. Combined (1)H MR spectroscopy and diffusion-weighted MRI improves the prediction of stroke outcome. Neurology 2000;55:498–505 [DOI] [PubMed] [Google Scholar]
- 20.Schellinger PD, Fiebach JB, Jansen O, et al. Stroke magnetic resonance imaging within 6 hours after onset of hyperacute cerebral ischemia. Ann Neurol 2001;49:460–69 [PubMed] [Google Scholar]
- 21.Parsons MW, Barber PA, Chalk J, et al. Diffusion- and perfusion-weighted MRI response to thrombolysis in stroke. Ann Neurol 2002;51:28–37 [DOI] [PubMed] [Google Scholar]
- 22.Schramm P, Schellinger PD, Fiebach JB, et al. Comparison of CT and CT angiography source images with diffusion-weighted imaging in patients with acute stroke within 6 hours after onset. Stroke 2002;33:2426–32 [DOI] [PubMed] [Google Scholar]
- 23.Shih LC, Saver JL, Alger JR, et al. Perfusion-weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke 2003;34:1425–30 [DOI] [PubMed] [Google Scholar]
- 24.Fiehler J, Knudsen K, Kucinski T, et al. Predictors of apparent diffusion coefficient normalization in stroke patients. Stroke 2004;35:514–19 [DOI] [PubMed] [Google Scholar]
- 25.Schaefer PW, Hassankhani A, Putman C, et al. Characterization and evolution of diffusion MR imaging abnormalities in stroke patients undergoing intra-arterial thrombolysis. AJNR Am J Neuroradiol 2004;25:951–57 [PMC free article] [PubMed] [Google Scholar]
- 26.Schramm P, Schellinger PD, Klotz E, et al. Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion-weighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours’ duration. Stroke 2004;35:1652–58 [DOI] [PubMed] [Google Scholar]
- 27.Ritzl A, Meisel S, Wittsack HJ, et al. Development of brain infarct volume as assessed by magnetic resonance imaging (MRI): follow-up of diffusion-weighted MRI lesions. J Magn Reson Imaging 2004;20:201–07 [DOI] [PubMed] [Google Scholar]
- 28.Seitz RJ, Meisel S, Weller P, et al. Initial ischemic event: perfusion-weighted MR imaging and apparent diffusion coefficient for stroke evolution. Radiology 2005;237:1020–28 [DOI] [PubMed] [Google Scholar]
- 29.Rivers CS, Wardlaw JM, Armitage PA, et al. Do acute diffusion- and perfusion-weighted MRI lesions identify final infarct volume in ischemic stroke? Stroke 2006;37:98–104. Epub 2005 Dec 1 [DOI] [PubMed] [Google Scholar]
- 30.Delgado-Mederos R, Rovira A, Alvarez-Sabin J, et al. Speed of tPA-induced clot lysis predicts DWI lesion evolution in acute stroke. Stroke 2007;38:955–60 [DOI] [PubMed] [Google Scholar]
- 31.Barber PA, Darby DG, Desmond PM, et al. Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology 1998;51:418–26 [DOI] [PubMed] [Google Scholar]
- 32.Karonen JO, Liu Y, Vanninen RL, et al. Combined perfusion- and diffusion-weighted MR imaging in acute ischemic stroke during the 1st week: a longitudinal study. Radiology 2000;217:886–94 [DOI] [PubMed] [Google Scholar]
- 33.Lansberg MG, O'Brien MW, Tong DC, et al. Evolution of cerebral infarct volume assessed by diffusion-weighted magnetic resonance imaging. Arch Neurol 2001;58:613–17 [DOI] [PubMed] [Google Scholar]
- 34.Aronen HJ, Perkio J, Karonen JO, et al. Perfusion-weighted MRI in human acute ischemic stroke: a comparison with the progression of the infarct on diffusion-weighted images Acad Radiol 2002;9 (suppl 1):S160–64 [DOI] [PubMed] [Google Scholar]
- 35.Davalos A, Blanco M, Pedraza S, et al. The clinical-DWI mismatch: a new diagnostic approach to the brain tissue at risk of infarction. Neurology 2004;62:2187–92 [DOI] [PubMed] [Google Scholar]
- 36.Derex L, Nighoghossian N, Hermier M, et al. Influence of pretreatment MRI parameters on clinical outcome, recanalization and infarct size in 49 stroke patients treated by intravenous tissue plasminogen activator. J Neurol Sci 2004;225:3–9 [DOI] [PubMed] [Google Scholar]
- 37.Fiehler J, Knudsen K, Thomalla G, et al. Vascular occlusion sites determine differences in lesion growth from early apparent diffusion coefficient lesion to final infarct. AJNR Am J Neuroradiol 2005;26:1056–61 [PMC free article] [PubMed] [Google Scholar]
- 38.Prosser J, Butcher K, Allport L, et al. Clinical-diffusion mismatch predicts the putative penumbra with high specificity. Stroke 2005;36:1700–04 [DOI] [PubMed] [Google Scholar]
- 39.Karonen JO, Vanninen RL, Liu Y, et al. Combined diffusion and perfusion MRI with correlation to single-photon emission CT in acute ischemic stroke: ischemic penumbra predicts infarct growth. Stroke 1999;30:1583–90 [DOI] [PubMed] [Google Scholar]
- 40.Wu O, Koroshetz WJ, Ostergaard L, et al. Predicting tissue outcome in acute human cerebral ischemia using combined diffusion- and perfusion-weighted MR imaging. Stroke 2001;32:933–42 [DOI] [PubMed] [Google Scholar]
- 41.Lansberg MG, Thijs VN, O'Brien MW, et al. Evolution of apparent diffusion coefficient, diffusion-weighted, and T2-weighted signal intensity of acute stroke. AJNR Am J Neuroradiol 2001;22:637–44 [PMC free article] [PubMed] [Google Scholar]
- 42.Staroselskaya IA, Chaves C, Silver B, et al. Relationship between magnetic resonance arterial patency and perfusion-diffusion mismatch in acute ischemic stroke and its potential clinical use. Arch Neurol 2001;58:1069–74 [DOI] [PubMed] [Google Scholar]
- 43.Fiebach JB, Jansen O, Schellinger PD, et al. Serial analysis of the apparent diffusion coefficient time course in human stroke. Neuroradiology 2002;44:294–98 [DOI] [PubMed] [Google Scholar]
- 44.Parsons MW, Barber PA, Desmond PM, et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol 2002;52:20–28 [DOI] [PubMed] [Google Scholar]
- 45.Rother J, Schellinger PD, Gass A, et al. Effect of intravenous thrombolysis on MRI parameters and functional outcome in acute stroke <6 hours. Stroke 2002;33:2438–45 [DOI] [PubMed] [Google Scholar]
- 46.Ehrenreich H, Hasselblatt M, Dembowski C, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med 2002;8:495–505 [PMC free article] [PubMed] [Google Scholar]
- 47.Hermier M, Nighoghossian N, Adeleine P, et al. Early magnetic resonance imaging prediction of arterial recanalization and late infarct volume in acute carotid artery stroke. J Cereb Blood Flow Metab 2003;23:240–48 [DOI] [PubMed] [Google Scholar]
- 48.Nighoghossian N, Hermier M, Adeleine P, et al. Baseline magnetic resonance imaging parameters and stroke outcome in patients treated by intravenous tissue plasminogen activator. Stroke 2003;34:458–63 [DOI] [PubMed] [Google Scholar]
- 49.Schaefer PW, Ozsunar Y, He J, et al. Assessing tissue viability with MR diffusion and perfusion imaging. AJNR Am J Neuroradiol 2003;24:436–43 [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Karonen JO, Vanninen RL, et al. Detecting the subregion proceeding to infarction in hypoperfused cerebral tissue: a study with diffusion and perfusion weighted MRI. Neuroradiology 2003;45:345–51 [DOI] [PubMed] [Google Scholar]
- 51.Kang DW, Latour LL, Chalela JA, et al. Early ischemic lesion recurrence within a week after acute ischemic stroke. Ann Neurol 2003;54:66–74 [DOI] [PubMed] [Google Scholar]
- 52.Lee YZ, Lee JM, Vo K, et al Rapid perfusion abnormality estimation in acute stroke with temporal correlation analysis. Stroke 2003;34:1686–92 [DOI] [PubMed] [Google Scholar]
- 53.Baird TA, Parsons MW, Phanh T, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke 2003;34:2208–14 [DOI] [PubMed] [Google Scholar]
- 54.Seitz RJ, Meisel S, Moll M, et al. The effect of combined thrombolysis with rtPA and tirofiban on ischemic brain lesions. Neurology 2004;62:2110–12 [DOI] [PubMed] [Google Scholar]
- 55.Nasel C, Kronsteiner N, Schindler E, et al. Standardized time to peak in ischemic and regular cerebral tissue measured with perfusion MR imaging. AJNR Am J Neuroradiol 2004;25:945–50 [PMC free article] [PubMed] [Google Scholar]
- 56.Fiehler J, Kucinski T, Knudsen K, et al. Are there time-dependent differences in diffusion and perfusion within the first 6 hours after stroke onset? Stroke 2004;35:2099–104 [DOI] [PubMed] [Google Scholar]
- 57.Na DG, Thijs VN, Albers GW, et al. Diffusion-weighted MR imaging in acute ischemia: value of apparent diffusion coefficient and signal intensity thresholds in predicting tissue at risk and final infarct size. AJNR Am J Neuroradiol 2004;25:1331–36 [PMC free article] [PubMed] [Google Scholar]
- 58.Mitsias PD, Ewing JR, Lu M, et al. Multiparametric iterative self-organizing MR imaging data analysis technique for assessment of tissue viability in acute cerebral ischemia. AJNR Am J Neuroradiol 2004;25:1499–508 [PMC free article] [PubMed] [Google Scholar]
- 59.Derex L, Hermier M, Adeleine P, et al. Clinical and imaging predictors of intracerebral haemorrhage in stroke patients treated with intravenous tissue plasminogen activator. J Neurol Neurosurg Psychiatry 2005;76:70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alsop DC, Makovetskaya E, Kumar S, et al. Markedly reduced apparent blood volume on bolus contrast magnetic resonance imaging as a predictor of hemorrhage after thrombolytic therapy for acute ischemic stroke. Stroke 2005;36:746–50 [DOI] [PubMed] [Google Scholar]
- 61.Seitz RJ, Meisel S, Moll M, et al. Partial rescue of the perfusion deficit area by thrombolysis. J Magn Reson Imaging 2005;22:199–205 [DOI] [PubMed] [Google Scholar]
- 62.Simon JE, Bristow MS, Lu H, et al. A novel method to derive separate gray and white matter cerebral blood flow measures from MR imaging of acute ischemic stroke patients. J Cereb Blood Flow Metab 2005;25:1236–43 [DOI] [PubMed] [Google Scholar]
- 63.Koga M, Reutens DC, Wright P, et al. The existence and evolution of diffusion-perfusion mismatched tissue in white and gray matter after acute stroke. Stroke 2005;36:2132–37 [DOI] [PubMed] [Google Scholar]
- 64.Barber PA, Hill MD, Eliasziw M, et al. Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry 2005;76:1528–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ay H, Koroshetz WJ, Vangel M, et al. Conversion of ischemic brain tissue into infarction increases with age. Stroke 2005;36:2632–36 [DOI] [PubMed] [Google Scholar]
- 66.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006;37:979–85 [DOI] [PubMed] [Google Scholar]
- 67.Arakawa S, Wright PM, Koga M, et al. Ischemic thresholds for gray and white matter: a diffusion and perfusion magnetic resonance study. Stroke 2006;37:1211–16 [DOI] [PubMed] [Google Scholar]
- 68.Rivers CS, Wardlaw JM, Armitage PA, et al. Persistent infarct hyperintensity on diffusion-weighted imaging late after stroke indicates heterogeneous, delayed, infarct evolution. Stroke 2006;37:1418–23 [DOI] [PubMed] [Google Scholar]
- 69.Luby M, Bykowski JL, Schellinger PD, et al. Intra- and interrater reliability of ischemic lesion volume measurements on diffusion-weighted, mean transit time and fluid-attenuated inversion recovery MRI. Stroke 2006;37:2951–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thomalla G, Sobesky J, Kohrmann M, et al. Two tales: hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia—MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 hours. Stroke 2007;38:313–18 [DOI] [PubMed] [Google Scholar]
- 71.Tei H, Uchiyama S, Usui T. Clinical-diffusion mismatch defined by NIHSS and ASPECTS in non-lacunar anterior circulation infarction. J Neurol 2007;254:340–46 [DOI] [PubMed] [Google Scholar]
- 72.Warach S, Gaa J, Siewert B, et al. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol 1995;37:231–41 [DOI] [PubMed] [Google Scholar]
- 73.Gonzalez RG, Schaefer PW, Buonanno FS, et al. Diffusion-weighted MR imaging: diagnostic accuracy in patients imaged within 6 hours of stroke symptom onset. Radiology 1999;210:155–62 [DOI] [PubMed] [Google Scholar]
- 74.Lovblad KO, Laubach HJ, Baird AE, et al. Clinical experience with diffusion-weighted MR in patients with acute stroke. AJNR Am J Neuroradiol 1998;19:1061–66 [PMC free article] [PubMed] [Google Scholar]
- 75.Grant PE, He J, Halpern EF, et al. Frequency and clinical context of decreased apparent diffusion coefficient reversal in the human brain. Radiology 2001;221:43–50 [DOI] [PubMed] [Google Scholar]
- 76.Dodd JD. Evidence-based practice in radiology: steps 3 and 4—appraise and apply diagnostic radiology literature. Radiology 2007;242:342–54 [DOI] [PubMed] [Google Scholar]
- 77.Pierpaoli C, Alger JR, Righini A, et al. High temporal resolution diffusion MRI of global cerebral ischemia and reperfusion. J Cereb Blood Flow Metab 1996;16:892–905 [DOI] [PubMed] [Google Scholar]
- 78.Loh PS, Butcher KS, Parsons MW, et al. Apparent diffusion coefficient thresholds do not predict the response to acute stroke thrombolysis. Stroke 2005;36:2626–31 [DOI] [PubMed] [Google Scholar]
- 79.Guadagno JV, Warburton EA, Jones PS, et al. How affected is oxygen metabolism in DWI lesions? A combined acute stroke PET-MR study. Neurology 2006;67:824–29 [DOI] [PubMed] [Google Scholar]
- 80.Benveniste H, Hedlund LW, Johnson GA. Mechanism of detection of acute cerebral ischemia in rats by diffusion-weighted magnetic resonance microscopy. Stroke 1992;23:746–54 [DOI] [PubMed] [Google Scholar]
- 81.Baird AE, Warach S. Magnetic resonance imaging of acute stroke. J Cereb Blood Flow Metab 1998;18:583–609 [DOI] [PubMed] [Google Scholar]
- 82.Moseley ME, Kucharczyk J, Asgari HS, et al. Anisotropy in diffusion-weighted MRI. Magn Reson Med 1991;19:321–26 [DOI] [PubMed] [Google Scholar]
- 83.Hossmann KA. Cortical steady potential, impedance and excitability changes during and after total ischemia of cat brain. Exp Neurol 1971;32:163–75 [DOI] [PubMed] [Google Scholar]
- 84.Norris DG, Niendorf T, Hoehn-Berlage M, et al. Incidence of apparent restricted diffusion in three different models of cerebral infarction. Magn Reson Imaging 1994;12:1175–82 [DOI] [PubMed] [Google Scholar]
- 85.Verheul HB, Balazs R, Berkelbach van der Sprenkel JW, et al. Comparison of diffusion-weighted MRI with changes in cell volume in a rat model of brain injury. NMR Biomed 1994;7:96–100 [DOI] [PubMed] [Google Scholar]
- 86.Qiao M, Malisza KL, Del Bigio MR, et al. Transient hypoxia-ischemia in rats: changes in diffusion-sensitive MR imaging findings, extracellular space, and Na+-K+ -adenosine triphosphatase and cytochrome oxidase activity. Radiology 2002;223:65–75 [DOI] [PubMed] [Google Scholar]
- 87.Kohno K, Hoehn-Berlage M, Mies G, et al. Relationship between diffusion-weighted MR images, cerebral blood flow, and energy state in experimental brain infarction. Magn Reson Imaging 1995;13:73–80 [DOI] [PubMed] [Google Scholar]