Abstract
BACKGROUND AND PURPOSE: Multidetector CT angiography (MDCTA) is emerging as the favored initial diagnostic examination in the evaluation of patients presenting with spontaneous intraparenchymal hemorrhage (IPH). This study aims to evaluate the diagnostic accuracy and yield of MDCTA for the detection of vascular etiologies in adult patients presenting to the emergency department with IPH.
MATERIALS AND METHODS: We conducted a retrospective study of 623 consecutive adult patients presenting to the emergency department with IPH, who were evaluated with MDCTA during a 9-year period. CT angiograms were reviewed by 2 neuroradiologists to determine the IPH site and the presence of a vascular etiology. Patients with associated subarachnoid hemorrhage in the basal cisterns were excluded from the study. Medical records were reviewed for risk factors and correlation with final diagnosis. The diagnostic accuracy of MDCTA compared with conventional angiography, intraoperative evaluation, and pathologic findings was determined, when available. Multiple-variable logistic regression analysis was performed to determine clinical and radiologic factors that predict a higher yield of MDCTA.
RESULTS: MDCTA demonstrated a vascular etiology in 91 patients (14.6%), with a sensitivity of 96%, specificity of 99%, and diagnostic accuracy of 98%. We found independent, statistically significant higher yields of MDCTA in patients with the following characteristics: 1) age younger than 46 years (47%); 2) lobar (20%) or infratentorial (16%) IPH, especially lobar IPH with associated intraventricular hemorrhage (25%); 3) female sex (18%); or 4) neither known hypertension nor impaired coagulation at presentation (33%).
CONCLUSIONS: MDCTA is an accurate diagnostic examination in the evaluation of adult patients presenting with spontaneous IPH and should be performed in all patients with the aforementioned clinical and radiologic characteristics.
Spontaneous, nontraumatic intraparenchymal cerebral hemorrhage (IPH) accounts for 10%–15% of cases of acute stroke.1 Although most spontaneous IPHs are caused by hypertension, amyloid angiopathy, or impaired coagulation, many are also caused by vascular lesions such as arteriovenous malformations (AVMs), ruptured intracranial aneurysms, and dural venous sinus thromboses.1–3 Accurate identification of a vascular lesion as the IPH cause is important because these have a high rate of rehemorrhage, with further increased morbidity and mortality, and are potentially treatable.1,2,4–6 Prior studies have identified clinical and noncontrast CT (NCCT) features that increase the likelihood of identifying a vascular etiology with conventional angiography: age younger than 40–50 years,7–10 absence of pre-existing hypertension,7,11,12 presence of associated subarachnoid (SAH) or intraventricular hemorrhage (IVH),8,10,12–14 and temporal or frontal lobe location.8,13 However, Halpin et al15 found vascular abnormalities with conventional angiography in 24% of patients deemed unlikely to have positive findings on angiography based on NCCT features alone, 13% of hypertensive patients, 31% of patients with basal ganglia hemorrhage, and 18% of patients with posterior fossa hemorrhage and, thus, recommended performing conventional catheter angiography in all patients presenting with nontraumatic intraparenchymal hemorrhage. Similarly, Matsumoto et al16 found coexisting unruptured intracranial aneurysms with conventional angiography in 21% of women and 9% of men presenting with hypertensive intracerebral hemorrhage and, thus, recommended performing angiographic investigations in all of these patients, particularly women.
As a result of its increasing availability, rapidity of acquisition, lower cost, favorable risk profile compared with conventional angiography, and diagnostic accuracy for the detection of vascular lesions,17–21 multidetector CT angiography (MDCTA) is emerging as the favored initial diagnostic examination in the evaluation of patients presenting with spontaneous IPH in the emergency department. This study aims to evaluate the diagnostic accuracy and yield of MDCTA for the detection of vascular etiologies in adult patients presenting to the emergency department with spontaneous IPH.
Materials and Methods
Patient Selection
Our study was approved by the institutional review board of the hospital and conducted with Health Insurance Portability and Accountability Act compliance. We conducted a retrospective study of all consecutive patients who presented to our emergency department from January 1, 2000, until November 1, 2008, with the following inclusion criteria: 1) ≥18 years of age, 2) evidence of spontaneous IPH on an NCCT examination of the head, and 3) evaluation with a CT angiogram (CTA) of the intracranial circulation within 24 hours of presentation. Patient exclusion criteria were the presence of the following: 1) associated SAH in the basal cisterns, 2) loss of gray-white matter differentiation in a vascular territory suggesting a pre-established acute ischemic stroke, 3) a known intracranial vascular abnormality or mass lesion, or 4) known probable cerebral amyloid angiopathy according to the Boston criteria.22
Image Acquisition
NCCT and MDCTA acquisitions were performed according to standard protocols on 16- or 64-section helical CT scanners (LightSpeed; GE Healthcare, Waukesha, Wis). NCCT was performed in a head holder by using an axial technique with 120–140 kilovolt (peak), 170 mA, 2-second scanning time, and 5-mm section-thickness reconstruction. MDCTA was subsequently performed by scanning from the base of the C1 vertebral body to the vertex using the following parameters: pitch, 0.5; collimation, 1.25 mm; maximal mA, 350; kilovolt (peak), 120; FOV, 22 cm; and 65 to 85 mL of nonionic contrast material administered by power injector at 4–5 mL per second into an antecubital vein with either a fixed 25-second delay between the onset of contrast injection and the start of scanning (the delay was increased to 40 seconds in patients with atrial fibrillation), or SmartPrep software, an automatic contrast bolus triggering technique (GE Healthcare). The resulting 1.25-mm-thick axial source images were digitally archived. Standard maximum intensity projection (MIP) images of the major intracranial vessels were created by the 3D laboratory.
Image Analysis
The NCCTs were reviewed by 2 neuroradiologists blinded to the clinical data and final diagnosis to determine, by consensus reading, the following: 1) the IPH location (categorized as lobar, deep gray matter, or infratentorial), 2) the presence of associated IVH or SAH, and 3) the probability of an underlying vascular etiology for the IPH (categorized as low, high, or indeterminate). At least 2 weeks after review of the NCCT, the CTA source and MIP images were reviewed by the same 2 neuroradiologists blinded to the NCCT categorization, clinical data, and final diagnosis, to determine, by consensus reading, the presence of an underlying vascular etiology for the IPH.
A “high-probability” NCCT was defined as an examination in which either enlarged vessels and/or a filling defect with associated calcifications along the margins of the IPH or hyperattenuation within a major dural venous sinus and/or a cortical vein potentially draining the region of the IPH was identified. A “low-probability” NCCT was defined as an examination in which none of the findings of a high-probability NCCT were present and the IPH was located within the deep gray matter or pons. An “indeterminate” NCCT was defined as an examination in which the findings of neither a high- nor low-probability NCCT were identified.
A “positive” CTA was defined as a study in which an underlying vascular etiology for the IPH was identified. For the diagnosis of dural venous sinus and/or cortical vein thrombosis, nonopacification of the affected venous structure on the first-pass CTA was confirmed by either persistent nonopacification on delayed images (if obtained) and/or corresponding hyperattenuation in the NCCT.
Medical Record Review
Medical records were reviewed for patient age, sex, presence of known hypertension, impaired coagulation, and history of recent cocaine use. Given that prior studies have found significant differences in the yield of conventional angiography according to patient age,7–10 we divided our patient population into 3 groups according to age: group 1, patients 18–45 years of age; group 2, patients 46–70 years of age; and group 3, patients 71 years of age and older.
Patients were classified as hypertensive if they had a history of hypertension on medical records or were taking antihypertensive medications at presentation. Patients were classified as having impaired coagulation if, at presentation, they were receiving daily antiplatelet therapy with aspirin (at least 81 mg) and/or clopidogrel,23 had a platelet count of <50,000 cells per cubic millimeter of blood, were on anticoagulation with warfarin and had an international normalized ratio >3.0,24 or were on anticoagulation with heparin and had an activated partial thromboplastin time of >80 seconds.
Correlation was made with reports of subsequent conventional angiography and MR imaging examinations, as well as surgical and pathologic findings, to establish a final diagnosis. The reports of subsequent conventional angiography examinations and intraoperative and pathologic findings were defined as gold standards for the purpose of evaluation of the diagnostic accuracy of MDCTA. In cases in which no confirmatory examination was performed, if a vascular etiology was identified on the CTA, it was considered the final diagnosis—however, these examinations were not included in the evaluation of diagnostic accuracy.
Statistical Analysis
Statistical analysis was performed utilizing the SAS 9.1 software package (SAS Institute, Cary, NC). After performing univariate statistical analysis using the Pearson χ2 test, we constructed a multiple variable logistic regression model to determine the correlation of patient age group, sex, known hypertension, impaired coagulation, IPH location, and presence of associated IVH, with the identification of a vascular etiology on MDCTA for all patients in the population, the 3 IPH locations separately, and the 3 different age groups separately. A P value ≤.05 was considered statistically significant. We calculated standard parameters of diagnostic accuracy for MDCTA compared with defined gold standards along with 95% confidence intervals.
Results
From January 1, 2000 until November 1, 2008, a total of 775 adult patients presented to our emergency department with IPH on an NCCT examination and were evaluated with MDCTA of the intracranial circulation within 24 hours of presentation. One hundred fifty-two patients were excluded from the study (19.6%): 91 due to the presence of associated SAH within the basal cisterns, 74 of whom had an underlying vascular etiology (81%); 36 due to the presence of a known vascular or mass lesion; 14 due to loss of gray-white matter differentiation in a vascular territory suggesting a pre-established acute ischemic stroke; and 11 due to known amyloid angiopathy. Hence, a total of 623 patients met the inclusion criteria of our study, with a mean age of 65 years (range, 18–94 years), 335 of whom were men (53.8%) and 288 women (46.2%).
NCCT Examination Categorization and Comparison with Final Diagnosis
Of the 623 NCCTs, 183 were categorized as low-probability for the presence of an underlying vascular etiology (29.4%, 172 due to deep gray matter and 11 due to pontine IPH location) and 19 were categorized as high probability for the presence of an underlying vascular etiology (3%, 8 due to enlarged vessels and/or filling defects with associated calcification along the margins of the IPH and 11 due to hyperattenuation within a dural venous sinus and/or cortical vein overlying the IPH). The remaining 421 NCCTs were categorized as indeterminate for the presence of an underlying vascular etiology (67.6%). Of the 183 low-probability NCCT examinations, 179 were true-negative and 4 were false-negative (1 pontine, 1 thalamic and 2 basal ganglia AVMs). Of the 19 high-probability NCCT examinations, 16 were true-positive and 3 were false-positive. Hence, for the prediction of a vascular etiology for the IPH, high- and low-probability NCCT examinations demonstrated a positive predictive value of 84.2% and a negative predictive value of 97.8%, respectively. However, 72 of the 421 indeterminate NCCTs had an underlying vascular etiology for the IPH (17.1%).
CTA Examination Categorization and Diagnostic Accuracy Compared with Gold Standards
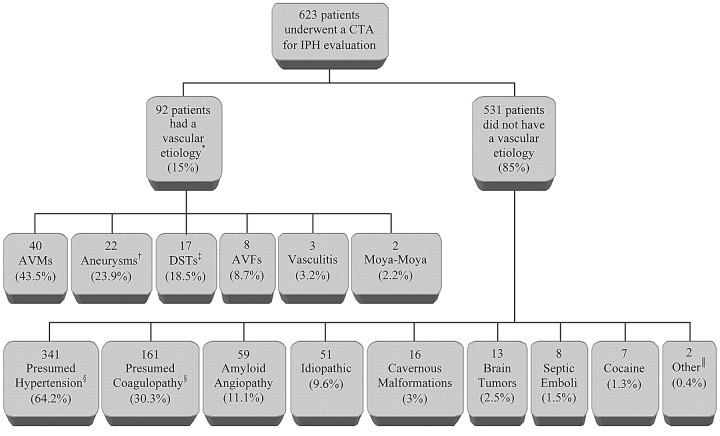
A total of 91 CTAs were categorized as positive for the presence of an underlying vascular etiology for the IPH (14.6%), with the following diagnoses: 40 AVMs (43.9%, Figs 1 and 2), 18 saccular aneurysms with intraparenchymal rupture (19.8%; mean maximum aneurysm sac size, 9.1 mm; range, 5–14 mm, Fig 3), 17 dural venous sinus and/or cortical vein thromboses (18.7%, Fig 4), 8 arteriovenous fistulas (AVFs, 8.8%), 3 pseudoaneurysms (3.3%), 3 cases of vasculitis (3.3%, Fig 5), and 2 cases of Moyamoya disease (2.2%, Fig 6).
Fig 1.
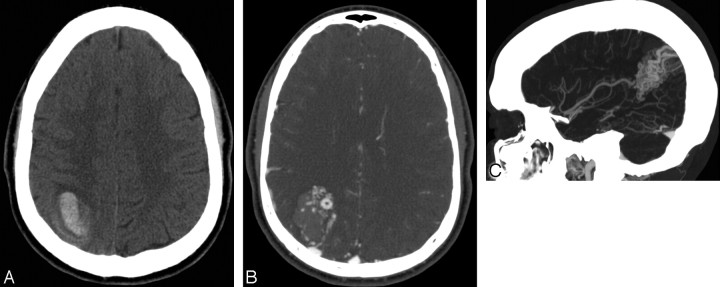
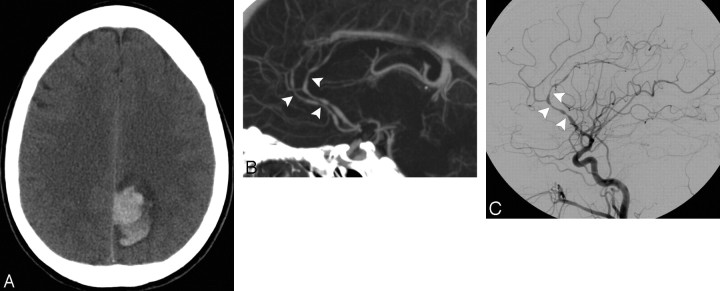
A 20-year-old normotensive man with intact coagulation who presented with severe headache after weight lifting. A, Indeterminate NCCT demonstrates an acute right parietal IPH with surrounding edema. There is no evidence of IVH or SAH (not shown). B, CTA source image demonstrates a tangle of abnormal vessels in the anteromedial aspect of the IPH with an associated enlarged cortical vein draining to the superior sagittal sinus, consistent with an AVM. C, CTA MIP image in the sagittal plane demonstrates a tangle of abnormal vessels in the right parietal lobe with an enlarged feeding artery arising from the right middle cerebral artery, consistent with an AVM. This lesion was confirmed with conventional angiography.
Fig 2.
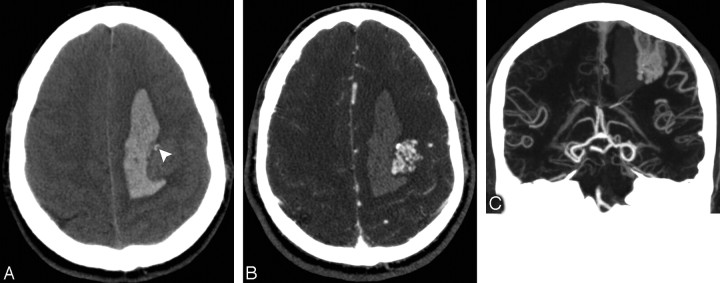
A 37-year-old normotensive man with intact coagulation who presented with acute onset of right hemiparesis. A, High-probability NCCT demonstrates an acute left frontal IPH with a peripheral filling defect and associated calcifications (arrowhead). There is no evidence of IVH or SAH (not shown). B, CTA source image demonstrates a tangle of abnormal vessels in the lateral aspect of the IPH, consistent with an AVM. C, CTA MIP image in the coronal plane demonstrates a tangle of abnormal vessels in the left frontal lobe with enlarged feeding arteries arising from the left middle cerebral artery and superficial venous drainage to the superior sagittal sinus, consistent with an AVM. This lesion was confirmed with conventional angiography.
Fig 3.
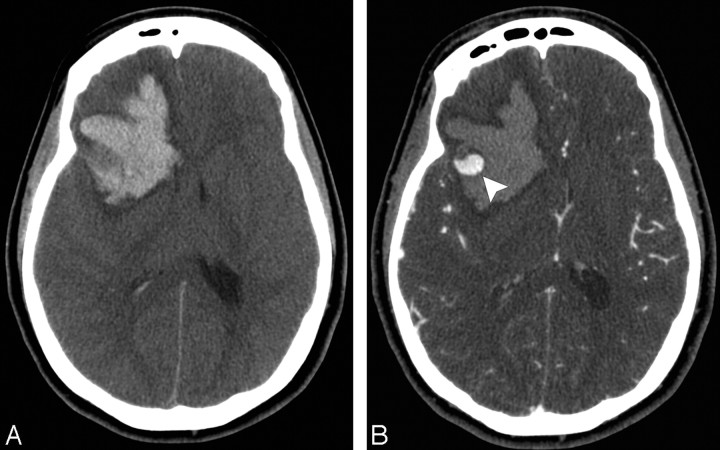
A 22-year-old normotensive woman with intact coagulation who presented with altered mental status. A, Indeterminate NCCT demonstrates an acute right fronto-temporal IPH with associated IVH and surrounding edema. There is no evidence of SAH (not shown). B, CTA source image demonstrates an arterially-enhancing structure in the lateral aspect of the IPH measuring 13 mm (arrowhead), consistent with a right middle cerebral artery aneurysm. This lesion was confirmed at surgery.
Fig 4.
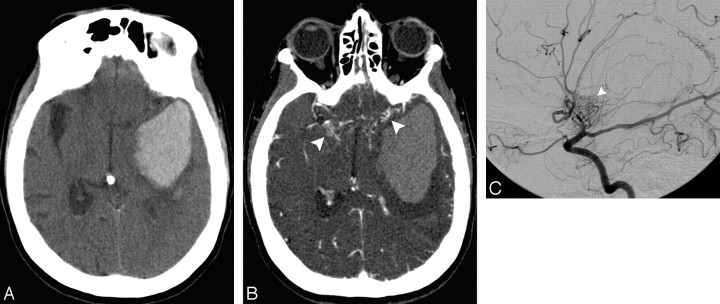
A 48-year-old normotensive woman with intact coagulation and a history of oral contraceptive use who presented with worsening headaches followed by a generalized tonic-clonic seizure. A and B, High probability NCCT demonstrates an acute left temporal IPH with associated hyperattenuation in the left transverse sinus evidenced in the coronal reformation (arrowhead). There is no associated IVH or SAH. There is also hyperattenuation within the left vein of Labbe (not shown). C, MIP image after calvarial segmentation of a CT venogram performed immediately after the CTA demonstrates nonopacification of the left transverse and sigmoid sinuses (arrowheads), with adequate opacification of the contralateral venous sinuses, consistent with dural venous sinus thrombosis.
Fig 5.
A 25-year-old normotensive woman with intact coagulation who presented with severe headache after recent cocaine use. A, Indeterminate NCCT demonstrates an acute left parietal IPH with surrounding edema. There is no evidence of IVH or SAH (not shown). B, CTA MIP image in the sagittal plane demonstrates multiple areas of segmental narrowing in the anterior cerebral and pericallosal arteries bilaterally (arrowheads), consistent with vasculitis. C, Arterial phase of a catheter angiogram in the lateral projection following contrast injection of the left common carotid artery demonstrates multiple areas of segmental narrowing in the left anterior cerebral and pericallosal arteries (arrowheads), consistent with vasculitis.
Fig 6.
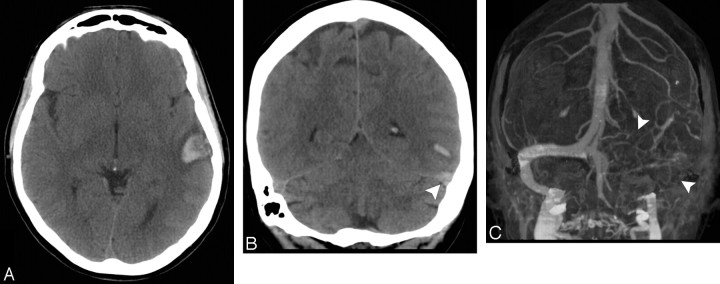
A 53-year-old normotensive woman with intact coagulation who presented with severe headache, right-sided weakness, and worsening aphasia. A, Indeterminate NCCT demonstrates an acute left temporal IPH with associated IVH and surrounding edema. There is no evidence of SAH (not shown). B, CTA source image demonstrates nonopacification of the distal supraclinoid segments of the internal carotid arteries and M1 segments of the middle cerebral arteries bilaterally, with numerous associated lenticulostriate collateral vessels (arrowheads), consistent with Moyamoya disease. C, Arterial phase of a catheter angiogram in the lateral projection following contrast injection of the left common carotid artery demonstrates complete occlusion of the distal supraclinoid segment of the left internal carotid artery and M1 segment of the left middle cerebral artery, with a “puff-of-smoke” appearance produced by an extensive network of lenticulostriate collateral vessels (arrowhead), consistent with Moyamoya disease.
Following MDCTA, a catheter angiogram was performed in 108 patients (17.3%), surgical evacuation of the hematoma with direct inspection of the cavity and pathologic evaluation of the surgical specimen was performed in 132 patients (21.2%), and autopsies were performed in 24 patients (3.9%). Fifty-four patients had >1 gold-standard examination performed; in 1 patient, these examinations were found to be in disagreement and the surgical examination was used as the gold standard. Hence, there were a total of 210 patients in whom the CTA was compared with at least 1 gold standard (33.7%). The results of diagnostic accuracy for MDCTA compared with these gold standards are summarized in Table 1. For the detection of an underlying vascular etiology for the IPH, MDCTA demonstrated a sensitivity of 96.1%, a specificity of 98.5%, and an overall diagnostic accuracy of 97.6%. The 3 false-negative CTAs were the following: 1 AVM with a subcentimeter nidus, 1 subcentimeter AVF, and a 2-mm distal middle cerebral artery pseudoaneurysm. All patients with false-negative CTAs were younger than 46 years of age, normotensive, and had normal coagulation. One of the false-positive CTAs was interpreted as demonstrating an AVM that was not demonstrated at conventional angiography and was found to be a cavernous malformation with an associated developmental venous anomaly on a subsequent MR imaging examination. The second false-positive CTA was interpreted as demonstrating an AVF overlying the IPH; however, no arteriovenous shunting was demonstrated at conventional angiography, and the enlarged cortical vein was considered to be a normal variant.
Table 1:
Diagnostic accuracy of multidetector CT angiography in IPH
| Findings | Confirmed Vascular Lesion* | Confirmed No Vascular Lesion | Total |
|---|---|---|---|
| Positive CTA | 73 | 2 | 75 |
| Negative CTA | 3 | 132 | 135 |
| Total | 76 | 134 | 210 |
| Sensitivity | 96.1% (88.1%–99.0%)† | ||
| Specificity | 98.5% (94.2%–99.7%)† | ||
| Accuracy | 97.6% | ||
| PPV | 97.3% (89.8%–99.5%)† | ||
| NPV | 97.8% (93.2%–99.4%)† |
Note:—PPV indicates positive predictive value; NPV, negative predictive value; IPH, intraparenchymal hemorrhage; CTA, CT angiography.
Fifty-one vascular lesions were confirmed by conventional angiography, 54 during direct surgical evaluation of the hematoma cavity and pathologic evaluation of the surgical specimen and 4 at postmortem examination (33 vascular lesions were confirmed by >1 type of examination but counted only once for the purpose of this analysis).
95% confidence interval.
Clinical and Radiologic Predictors of a Positive CTA Examination
The results of univariate and multiple variable logistic regression analysis for the diagnostic yield of MDCTA in the entire patient population, patients with lobar IPH, and the 3 different patient age groups are summarized in Tables 2–4, respectively. For infratentorial and deep gray matter IPH, no independent, statistically-significant differences among patients with different characteristics were found. Patients with IPH and 1) less than 46 years of age (47%), 2) lobar (20%) or infratentorial (16%) IPH location, especially lobar IPH with associated IVH (25%), 3) female sex (18%), or 4) neither hypertension nor impaired coagulation at presentation (33%), had independent, statistically significant higher incidences of vascular etiologies on MDCTA compared to patients without these characteristics (Table 5). Among cases of lobar IPH, those limited to either the parietal (30%) or temporal (29%) lobes had higher incidences of vascular etiologies than IPHs affecting other lobes or >1 lobe. Importantly, patients younger than 46 years of age with infratentorial (67%) or lobar (58%) IPH, with neither hypertension nor impaired coagulation (65%), or female sex (63%) had particularly high incidences of vascular etiologies on MDCTA.
Table 2:
Diagnostic yield of multidetector CT angiography in IPH
| No. Patients (%) | Positive CTAs* (%) | P Value† | |
|---|---|---|---|
| All patients | 623 (100) | 91 (14.6) | n/a |
| Sex | .04‡ | ||
| Male | 335 (53.8) | 40 (11.9) | |
| Female | 288 (46.2) | 51 (17.7) | |
| Age group§ | <.01‡ | ||
| 1, 18–45 years | 87 (14.0) | 41 (47.1) | |
| 2, 46–70 years | 263 (42.2) | 39 (14.8) | |
| 3, 71–94 years | 273 (43.8) | 11 (4.0) | |
| Hemorrhage site | <.01‡ | ||
| Lobar | 382 (61.3) | 76 (19.9) | |
| Deep gray matter | 173 (27.8) | 4 (2.3) | |
| Infratentorial | 68 (10.9) | 11 (16.2) | |
| Known HTN | <.01 | ||
| Yes | 368 (59.1) | 21 (5.7) | |
| No | 255 (40.9) | 70 (27.5) | |
| Impaired coagulation | <.01 | ||
| Yes | 204 (32.7) | 8 (3.9) | |
| No | 419 (67.3) | 83 (19.8) | |
| Neither HTN nor impaired coagulation | <.01‡ | ||
| Yes | 207 (33.2) | 69 (33.3) | |
| No‖ | 416 (66.8) | 22 (5.3) | |
| IVH | .68‡ | ||
| Yes | 282 (45.3) | 43 (15.3) | |
| No | 341 (54.7) | 48 (14.1) |
Note:—HTN indicates hypertension; n/a, not applicable; IVH, intraventricular hemorrhage.
Includes 2 false-positive CTAs.
From univariate analysis with the Pearson χ2 test.
Independent predictor in multiple variable logistic regression analysis.
Group 1: 21 AVMs, 8 aneurysms, 7 dural sinus thromboses, 3 AVFs, 1 case of vasculitis, and 1 case of Moyamoya disease; group 2: 15 AVMs, 9 dural sinus thromboses, 8 aneurysms, 4 AVFs, 2 cases of vasculitis, and 1 case of Moyamoya disease; group 3: 5 aneurysms, 4 AVMs, 1 AVF, and 1 dural sinus thrombosis.
Includes patients with either HTN and/or impaired coagulation.
Table 4:
Diagnostic yield of multidetector CT angiography in different age groups
| Group 1 (18–45 years) |
Group 2 (46–70 years) |
Group 3 (71–94 years) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % Positive CTAs* | P Value† | No. | % Positive CTAs* | P Value† | No. | % Positive CTAs | P Value† | |
| All patients in group | 87 | 47.1 | n/a | 263 | 14.8 | n/a | 273 | 4 | n/a |
| Sex | <.01 | .24 | .06 | ||||||
| Male | 49 | 34.7 | 164 | 12.8 | 122 | 1.6 | |||
| Female | 38 | 63.2 | 99 | 18.2 | 151 | 6 | |||
| Hemorrhage site | <.01 | <.01‡ | .24 | ||||||
| Lobar | 60 | 58.3 | 155 | 21.3 | 167 | 4.8 | |||
| Deep gray matter | 21 | 9.5 | 75 | 1.3 | 77 | 1.3 | |||
| Infratentorial | 6 | 66.7 | 33 | 15.2 | 29 | 6.9 | |||
| Known HTN | <.01 | <.01 | 0.09 | ||||||
| Yes | 23 | 4.3 | 158 | 9.5 | 187 | 2.7 | |||
| No | 64 | 62.5 | 105 | 22.9 | 86 | 7 | |||
| IC | .13 | .04 | .03 | ||||||
| Yes | 6 | 16.7 | 77 | 7.8 | 121 | 0.8 | |||
| No | 81 | 49.4 | 186 | 17.7 | 152 | 6.6 | |||
| Neither HTN nor IC | <.01 | <.01 | <.01 | ||||||
| Yes | 60 | 65 | 90 | 26.7 | 57 | 10.5 | |||
| No§ | 27 | 7.4 | 173 | 8.7 | 216 | 2.3 | |||
| IVH | .26 | .21‡ | .22 | ||||||
| Yes | 49 | 42.9 | 111 | 18 | 133 | 2.3 | |||
| No | 38 | 52.6 | 152 | 12.5 | 140 | 5.7 | |||
Note:—No. indicates the number of patients; IC, impaired coagulation.
Includes 1 false-positive CTA.
From univariate analysis with the Pearson χ2 test.
Independent predictor in multiple variable logistic regression analysis.
Includes patients with either HTN and/or impaired coagulation.
Table 5:
Summary of independent clinical and radiologic predictors of a higher yield of multidetector CT angiography in IPH
| % Positive CTAs* | |
|---|---|
| Age <46 years | 47 |
| Neither known HTN nor IC | 33 |
| Female sex | 18 |
| Lobar/Infratentorial IPH | 20/16 |
| Lobar IPH with IVH | 25 |
Includes 2 false-positive CTAs.
Summary of Final IPH Etiologies in the Patient Population
In our population of 623 patients presenting to the emergency department, there were a total of 92 patients with a vascular etiology for the IPH (14.8%; mean age, 48 years; range, 18–83 years), 89 of whom were identified on MDCTA (96.7%). Hence, there were 531 patients without a vascular etiology for the IPH (85.2%; mean age, 68 years; range, 18–94 years), 300 of whom underwent subsequent MR imaging (56.5%). Two hundred twenty-three of these MR examinations were negative (74.3%). The remaining MR examinations demonstrated evidence of probable amyloid angiopathy according to the Boston criteria22 in 47 patients (all with lobar hemorrhage), hemorrhagic cavernous malformations in 12 patients, brain tumors in 11 patients (6 primary and 5 metastatic), septic emboli secondary to bacterial endocarditis in 6 patients, and posterior reversible encephalopathy syndrome in 1 patient. In addition, pathologic examinations demonstrated evidence of amyloid angiopathy in 12 patients, hemorrhagic cavernous malformations in 4 patients, septic emboli in 2 patients, metastases in 2 patients, and a small distal embolic hemorrhagic infarction in 1 patient. A history of recent cocaine use was elicited in 12 patients, 4 of whom had positive CTAs and 1 who had a hemorrhagic cavernous malformation (Fig 7).
Fig 7.
Summary of final IPH etiologies in our emergency department patient population. *Eighty-nine of 92 cases were detected on MDCTA (96.7%). †Includes 18 saccular aneurysms as well as 2 iatrogenic and 2 mycotic pseudoaneurysms with intraparenchymal rupture. ‡Includes 15 dural venous sinus and 2 isolated cortical vein thromboses. §127 patients had both hypertension and impaired coagulation (23.9%). Forty-two patients were discharged with a new diagnosis of hypertension. ‖Includes 1 IPH due to a distal embolic hemorrhagic infarction and 1 IPH due to posterior reversible encephalopathy syndrome. DST indicates dural sinus thrombosis.
Overall, evidence of amyloid angiopathy was demonstrated in 59 of the 221 patients with lobar IPH and no vascular etiology who underwent either subsequent MR imaging and/or pathologic examination (26.7%), with a mean age of 76 years (range, 62–94 years). In addition, the mean age of patients with hemorrhagic cavernous malformations was 44 years (range, 18–77 years) and of those with brain tumors was 59 years (range, 34–76 years).
Discussion
In our patient population, a vascular etiology for the IPH was identified in 14.8% of patients, which is lower than reported in previous conventional angiography studies, which ranged from 27.5 to 52.5%.7,8,10,11,14,15 This is likely due to the older mean age in our patient population (65 years), compared with 42–52 years in prior studies,7,8,10,11,14,15 and the higher prevalence of hypertension and impaired coagulation in our patient population (59% and 33%, respectively), compared with 13%–60% for hypertension, and 7%–14% for impaired coagulation in prior studies.7–15 These differences in patient demographics likely reflect the lower threshold for performing a CTA compared with a conventional angiogram due to the lower risk of MDCTA in older patients and the emerging role of MDCTA as an important screening examination in the evaluation of patients presenting to the emergency department with IPH.
Systematic evaluation of the NCCT is helpful in the identification of a small proportion of high (3%) and low (29%) probability examinations with high positive (84%) and negative (98%) predictive values for the presence of an underlying vascular etiology for the IPH, respectively. However, in most patients (68%), the NCCT does not demonstrate specific characteristics and is indeterminate for the presence of an underlying vascular etiology for the IPH. Indeed, in our study population, if a CTA had only been performed in the 3% of patients with a high-probability NCCT, 76 of the 92 patients with a vascular etiology for the IPH would not have been diagnosed (83%). These results reflect an overall low sensitivity of the NCCT for the prediction of an underlying vascular abnormality for the IPH. Our results are similar to those reported by Halpin et al,15 who found an angiographic yield of 84% in patients with a high-probability NCCT examination but also found that 24% of patients with a low-probability NCCT had an underlying vascular etiology. Hence, NCCT findings alone should not be used to reliably select patients for MDCTA evaluation.
Compared with defined gold standards, MDCTA demonstrated high sensitivity (96%), specificity (99%), and overall diagnostic accuracy (98%) for the detection of vascular etiologies in patients with IPH. However, the sensitivity of MDCTA was lower for small AVMs/AVFs and distal aneurysms. Our results of diagnostic accuracy for MDCTA in the setting of IPH are similar to those reported in prior studies that have assessed the accuracy of this technique for detecting aneurysms and venous sinus thrombosis.19–21 However, our calculated parameters of diagnostic accuracy for MDCTA only measured the presence or absence of a vascular etiology for the IPH; other important features relating to AVMs/AVFs, such as the number and origin of arterial feeders, presence of intranidal and/or feeding artery aneurysms, and the presence of draining vein stenoses and deep versus superficial venous drainage, were not measured. In addition, although 34% of patients in our study population underwent a gold standard examination after MDCTA, patients with a positive CTA were more likely to undergo these examinations (82%) than patients with a negative CTA (25%), which is reflective of the clinical practice of our institution. However, of the 397 patients with a negative CTA who did not undergo a gold standard examination, 270 had either known hypertension and/or impaired coagulation on admission (68%), 57 had MR imaging examinations that demonstrated an etiology for the IPH (14.4%), and 5 had a history of recent cocaine use (1.3%). Thus, only 65 patients in this group had neither clinical nor MR imaging findings to explain the IPH (16.3%).
We identified patients with clinical and radiologic characteristics that predict a higher yield of MDCTA for the presence of an underlying vascular etiology for the IPH (Table 5). Our results are similar to those of most prior conventional angiography studies, in which yields of 50%–89% in patients younger than 40–50 years,7–9,10,15 significant differences in the yield of conventional angiography for different IPH locations,7–15 increased prevalence of aneurysms in women,16 and lower angiographic yields in patients with hypertension and/or impaired coagulation are reported.7,8,11,14,15 Given the relatively lower sensitivity of MDCTA for the detection of small vascular lesions, patients with these clinical and radiologic characteristics who have a negative initial CTA should undergo conventional angiography to exclude a small vascular lesion as the IPH etiology.
Evidence of amyloid angiopathy was demonstrated in 27% of patients with lobar IPH and no vascular etiology who underwent either subsequent MR imaging and/or pathologic examination, with a mean age of 76 years. These results are similar to those reported in prior studies, in which cerebral amyloid angiopathy has been associated with 20%–75% of lobar IPHs in older patients.22,25–27 In addition, hemorrhagic cavernous malformations and brain tumors were found, on average, in patients 21 and 6 years younger than the average patient in our study, respectively—though these were distributed over a broad age range. Hence, if the initial CTA examination is negative, a contrast-enhanced MR imaging examination with a gradient-echo susceptibility sequence should be performed to evaluate for amyloid angiopathy and brain tumors in older patients, and for cavernous malformations and brain tumors in younger patients.
The limitations of our study are its retrospective nature, the inclusion of only patients who presented with IPH and were evaluated with MDCTA (patients who, on clinical grounds, may have been deemed less likely to harbor an underlying vascular etiology for the IPH may not have undergone MDCTA), and the presence of a selection bias in the performance of gold standard examinations following MDCTA (these were performed with higher frequency in patients with positive CTAs compared to patients with negative CTAs). Thus, the overall diagnostic yield and sensitivity of MDCTA in adult patients with spontaneous IPH may be lower than the results reported in this study.
Conclusions
MDCTA is an accurate diagnostic technique for the evaluation of adult patients presenting to the emergency department with spontaneous IPH, demonstrating a vascular etiology in 14.6% of patients. MDCTA has high diagnostic yields in patients with the following: 1) a high probability NCCT (84%); 2) age younger than 46 years (47%), particularly young women (63%); 3) lobar (20%) or infratentorial (16%) IPH location, especially in lobar IPH involving either the parietal (30%) or temporal (29%) lobes and lobar IPH with associated IVH (25%); 4) female sex (18%); or 5) neither known hypertension nor impaired coagulation at presentation (33%). Given these findings, we recommend that MDCTA be performed in all patients within these subgroups. For cases of deep gray matter IPH and patients with other clinical characteristics, the decision to perform MDCTA should be based on the clinical assessment.
Table 3:
Diagnostic yield of multidetector CT angiography in lobar IPH
| No. Patients (%) | Positive CTAs* (%) | P value† | |
|---|---|---|---|
| All patients | 382 (100) | 76 (19.9) | n/a |
| Sex | 0.24‡ | ||
| Male | 189 (49.5) | 33 (17.5) | |
| Female | 193 (50.5) | 43 (22.3) | |
| Age group | <.01‡ | ||
| 1, 18–45 years | 60 (15.7) | 35 (58.3) | |
| 2, 46–70 years | 155 (40.6) | 33 (21.3) | |
| 3, 71–94 years | 167 (43.7) | 8 (4.8) | |
| Hemorrhage site | .01 | ||
| Frontal | 105 (27.5) | 20 (19.0) | |
| Temporal | 69 (18.1) | 20 (29.0) | |
| Parietal | 56 (14.7) | 17 (30.4) | |
| Occipital | 25 (6.5) | 2 (8.0) | |
| >1 Lobe | 127 (33.2) | 17 (13.4) | |
| Known HTN | <.01 | ||
| Yes | 199 (52.1) | 18 (9.0) | |
| No | 183 (47.9) | 58 (31.7) | |
| Impaired coagulation | <.01 | ||
| Yes | 114 (29.8) | 5 (4.4) | |
| No | 268 (70.2) | 71 (26.5) | |
| Neither HTN nor impaired coagulation | <.01 | ||
| Yes | 148 (38.7) | 58 (39.2) | |
| No§ | 234 (61.3) | 18 (7.7) | |
| IVH | .05‡ | ||
| Yes | 143 (37.4) | 36 (25.2) | |
| No | 239 (62.6) | 40 (16.7) |
Includes 2 false-positive CTAs.
From univariate analysis with the Pearson χ2 test.
Independent predictor in multiple variable logistic regression analysis.
Includes patients with either HTN and/or impaired coagulation.
Acknowledgments
We thank Elkan Halpern for his contribution in the statistical analysis and Eleni K. Balasalle for her contribution to the artwork for this manuscript.
Footnotes
Paper previously presented in part at: Annual Meeting of the American Society of Neuroradiology, June 9–14, 2007; Chicago, Ill.
Dr. Josser E. Delgado Almandoz is the 2009 ASNR Cornelius G. Dyke Memorial Award recipient.
References
- 1.Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med 2001;344:1450–60 [DOI] [PubMed] [Google Scholar]
- 2.Badjatia N, Rosand J. Intracerebral hemorrhage. Neurologist 2005;11:311–24 [DOI] [PubMed] [Google Scholar]
- 3.Singh T, Chakera T. Dural sinus thrombosis presenting as unilateral lobar haematomas with mass effect: an easily misdiagnosed cause of cerebral haemorrhage. Australas Radiol 2002;46:351–65 [DOI] [PubMed] [Google Scholar]
- 4.Jane JA, Kassell NF, Torner JC, et al. The natural history of aneurysms and arteriovenous malformations. J Neurosurg 1985;62:321–23 [DOI] [PubMed] [Google Scholar]
- 5.Arteriovenous malformations of the brain in adults: the Arteriovenous Malformation Study Group. N Engl J Med 1999;340:1812–18 [DOI] [PubMed] [Google Scholar]
- 6.Ondra SL, Troupp H, George ED, et al. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg 1990;73:387–91 [DOI] [PubMed] [Google Scholar]
- 7.Zhu XL, Chan MS, Poon WS. Spontaneous intracranial hemorrhage: which patients need diagnostic cerebral angiography? A prospective study of 206 cases and review of the literature. Stroke 1997;28:1406–09 [DOI] [PubMed] [Google Scholar]
- 8.Griffiths PD, Beveridge CJ, Gholkar A. Angiography in non-traumatic brain haematoma: an analysis of 100 cases. Acta Radiol 1997;38:797–802 [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Sandoval JL, Cantu C, Barinagarrementeria F. Intracerebral hemorrhage in young people: analysis of risk factors, location, causes and prognosis. Stroke 1999;30:537–41 [DOI] [PubMed] [Google Scholar]
- 10.Abu Bakar I, Shuaib IL, Mohd Ariff AR, et al. Diagnostic cerebral angiography in spontaneous intracranial haemorrhage: a guide for developing countries. Asian J Surg 2005;28:1–6 [DOI] [PubMed] [Google Scholar]
- 11.Toffol GJ, Biller J, Adams HP, et al. The predicted value of arteriography in nontraumatic intracerebral hemorrhage. Stroke 1986;17:881–83 [DOI] [PubMed] [Google Scholar]
- 12.Ohtani R, Kazui S, Tomimoto H, et al. Clinical and radiographic features of lobar cerebral hemorrhage: hypertensive versus non-hypertensive cases. Intern Med 2003;42:576–80 [DOI] [PubMed] [Google Scholar]
- 13.Loes DJ, Smoker WR, Biller J, et al. Nontraumatic lobar intracerebral hemorrhage: CT/angiographic correlation. AJNR Am J Neuroradiol 1987;8:1027–30 [PMC free article] [PubMed] [Google Scholar]
- 14.Laissy JP, Normand G, Monroc M, et al. Spontaneous intracerebral hematomas from vascular causes: predictive value of CT compared with angiography. Neuroradiology 1991;33:291–95 [DOI] [PubMed] [Google Scholar]
- 15.Halpin SF, Britton JA, Byrne JV, et al. Prospective evaluation of cerebral angiography and computed tomography in cerebral haematoma. J Neurol Neurosurg Psychiatry 1994;57:1180–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto K, Sakaki S, Abekura M, et al. Co-existence of unruptured cerebral aneurysms in patients with hypertensive intracerebral hemorrhage. Acta Neurochir (Wien) 2004;146:1085–89 [DOI] [PubMed] [Google Scholar]
- 17.Leffers AM, Wagner A. Neurologic complications of cerebral angiography: a retrospective study of complication rate and patient risk factors. Acta Radiol 2000;41:204–10 [DOI] [PubMed] [Google Scholar]
- 18.Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta-analysis. Stroke 1999;30:317–20 [DOI] [PubMed] [Google Scholar]
- 19.Goddard AJ, Tan G, Becker J. Computed tomography angiography for the detection and characterization of intra-cranial aneurysms: current status. Clin Radiol 2005;60:1221–36 [DOI] [PubMed] [Google Scholar]
- 20.Tomandl BF, Kostner NC, Schempershofe M, et al. CT angiography of intracranial aneurysms: a focus on postprocessing. Radiographics 2004;24:637–55 [DOI] [PubMed] [Google Scholar]
- 21.Linn L, Ertl-Wagner B, Seelos KC, et al. Diagnostic value of multidetector-row CT angiography in the evaluation of thrombosis of the cerebral venous sinuses. AJNR Am J Neuroradiol 2007;28:946–52 [PMC free article] [PubMed] [Google Scholar]
- 22.Knudsen KA, Rosand J, Karluk D, et al. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;56:537–39 [DOI] [PubMed] [Google Scholar]
- 23.Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Eng J Med 2006;354:1706–17 [DOI] [PubMed] [Google Scholar]
- 24.Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med 2004;141:745–52 [DOI] [PubMed] [Google Scholar]
- 25.Woo D, Sauerbeck LR, Kissela BM, et al. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke 2002;33:1190–96 [DOI] [PubMed] [Google Scholar]
- 26.Greenberg SM, Briggs ME, Hyman BT, et al. Apolipoprotein E4 is associated with the presence and earlier onset of hemorrhage in cerebral amyloid angiopathy. Stroke 1996;27:1333–37 [DOI] [PubMed] [Google Scholar]
- 27.Masuda J, Tanaka K, Ueda K, et al. Autopsy study of incidence and distribution of cerebral amyloid angiopathy in Hisayama, Japan. Stroke 1988;19:205–10 [DOI] [PubMed] [Google Scholar]