Abstract
BACKGROUND AND PURPOSE: The detection of time-related maturational changes of the olfactory bulb (OB) on MR imaging may help early identification of patients with abnormal OB development and anatomic-based odor-cueing anomalies.
MATERIALS AND METHODS: Two separate reviewers retrospectively analyzed coronal T2-weighted spin-echo MR images of the frontobasal region in 121 patients. There were 22 patients who underwent MR imaging examinations several times, accounting for a total of 156 studies. Age range was 1 day to 19.6 years. OBs were bilaterally identified in all cases and categorized according to their shape and signal intensity.
RESULTS: Three different anatomic patterns were identified. In pattern 1 (median age, 15 days; age range, 1–168 days), the OBs were round to oval with a continuous external T2-hypointense rim and a prominent T2-hyperintense central area. In pattern 2 (median age, 287 days; age range, 4 days–22 months), the OBs were U shaped, with thinning and concave deformation of the superior layer. A hyperintense central area on T2-weighted images was still visible. In pattern 3 (median age, 5.2 years; age range, 107 days–19.6 years), the OBs were small, round, or J shaped with a more prominent lateral part. No difference in signal intensity between the central area and the peripheral layer was identified anymore.
CONCLUSIONS: The OBs show time-related maturational changes on MR imaging. There is a progressive reorganization of the peripheral neuronal layers and signal intensity changes of the central area, which are completed at the end of the second year, paralleling cerebral maturational changes.
Normal anatomy of the olfactory pathways and many congenital or acquired anomalies have been repetitively described.1–6 Despite considerable advance in knowledge regarding the complex neuronal network associated with olfactory stimulation, information describing the normal maturation process in the neonatal period and childhood is scarce. There is experimental evidence that anomalies of the face are linked to abnormal development of the olfactory placode and bulbs.7 The importance of early detection of congenital anomalies of the development of the olfactory bulbs (OBs) is not only to identify patients with odor-coding deficiencies, but also to trigger thorough examination of the frontobasal area and midline structures, which are embryologically related, and to look for associated cerebral malformations. Because it is recognized that OBs and nerves are not real cranial nerves but extensions of the telencephalic vesicles, we postulated that their maturation should parallel cerebral maturation and be detected by MR imaging. Therefore, the purpose of our study was to identify and characterize age-dependent maturational changes in the OBs and tracts.
Materials and Methods
Patients
Two reviewers retrospectively analyzed all routine brain MR examinations done at our institution between January 2004 and January 2007. The local ethics committee approved the study. Examinations were excluded in cases of preterm neonates, incomplete visualization of the OBs, and evidence of brain malformation or generalized brain pathologic conditions.
A total of 121 children were considered eligible for the study, of which 22 children had 2 or more consecutive studies. There were a total of 156 cranial MR imaging examinations, of which 57 were multiple follow-ups. There were 66 boys (55%) and 53 girls (44%). Age range was 1 day to 19.6 years (median, 373 days). Written informed consent before examination was obtained for all patients.
Image Data Analysis
Cranial MR imaging examinations were performed on a clinical 1.5T MR imaging system (Picker 1.5T; Picker International, Cleveland, Ohio) and included coronal T2-weighted fast spin-echo (FSE) sequence, which is part of our routine examination protocol. Scan parameters were TR, 8490 ms; TE, 119 ms; matrix, 256 × 384; FOV, 160 mm; section thickness, 3 mm; NEX, 2; and number of sections, 31. The OBs were identified as prominent structures located above the cribriform plate with a posterior thinning into the olfactory tracts, which could be followed in all cases up to their frontobasal and, sometimes, to their limbic projectional areas. Visual inspection on coronal T2-weighted FSE images were consensually analyzed by 2 observers (F.F. and J.F.S.), and the OBs were discriminated according to their shape and signal intensity pattern.
Median age and demographic distribution graphs were performed with JMP software (JMP IN, version 5.1.2; SAS, Cary, NC).
Results
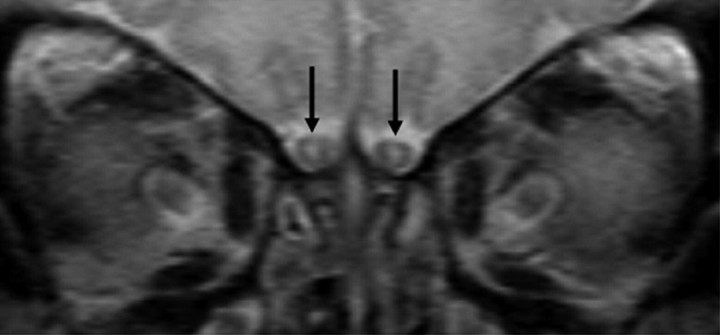
Immediately after birth, at a median age of 15 days (age range, 1–168 days), the OBs had a round to oval shape in 69 studies. At that stage, a continuous external T2-hypointense rim that demonstrated the same signal intensity as that of the cortical layer of cerebral hemispheres, and a central T2-hyperintensity correlating with the signal intensity of the cerebral white matter were seen in all cases (Fig 1).
Fig 1.
Pattern 1 of OBs (black arrows) shows a continuous external neuronal layer and a central area of T2-hyperintense unmyelinated white matter.
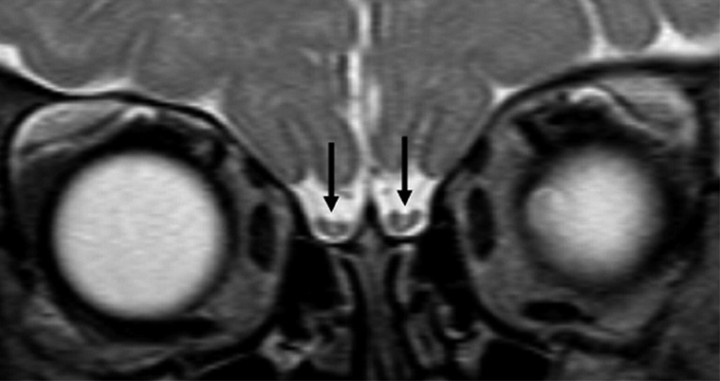
At a median age of 287 days (age range, 4 days–22 months) the OBs demonstrated thinning and slight concave deformation of their superior aspect, leading to a U-shaped appearance in 48 studies. The periphery still showed identical signal intensity to the cerebral cortex, and the central area also showed similar signal intensity compared with cerebral white matter (Fig 2).
Fig 2.
Pattern 2 of OBs (black arrows) shows a thinning of the superior aspect and a still T2-hyperintense, not yet fully myelinated, central white matter area.
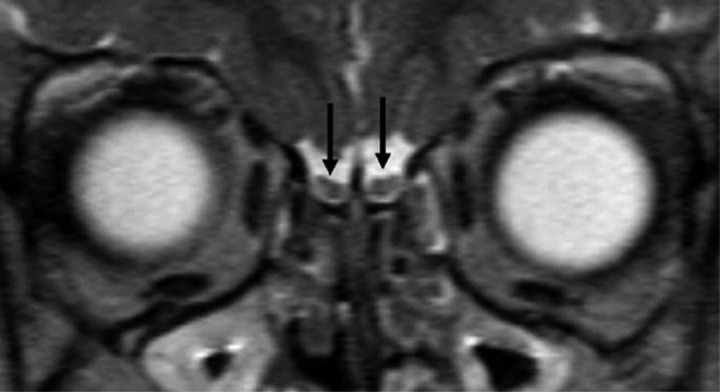
At a median age of 5.2 years (age range, 107 days–19.6 years), the OBs demonstrated an adult-type form that was smaller than those of the previous stages, with a predominantly round or slightly J-shaped aspect in 39 studies. In many cases, the lateral part of the bulbs was more prominent than the medial part. The central part was no longer distinct from the periphery and, as in the previous stages, showed a signal intensity that was identical to the myelinated cerebral white matter (Fig 3).
Fig 3.
Pattern 3 of OBs (black arrows) shows a slightly J-shaped form with a more prominent lateral neuronal layer and a now fully myelinated central white matter area.
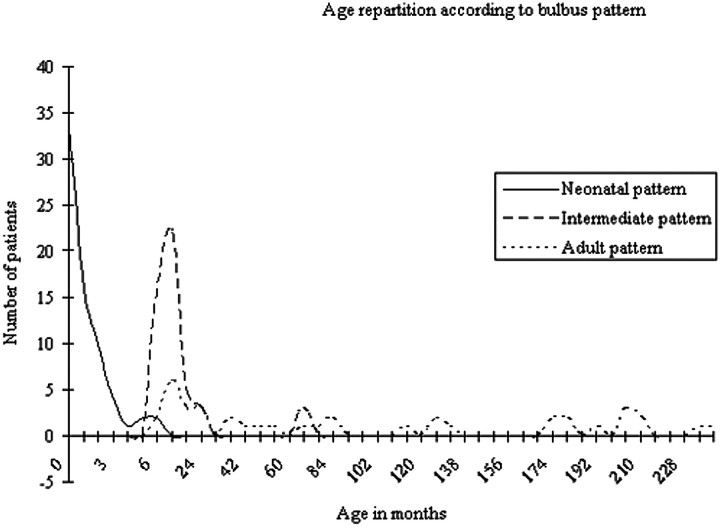
No correlation with the sex of the children was found. Age distribution according to the form and signal intensity pattern of the OB can be seen in Fig 4.
Fig 4.
Demographics and age repartition according to the MR imaging pattern of OBs.
Discussion
MR imaging has been successfully used to analyze the normal adult anatomy of the OBs1–4 and to depict abnormal olfactory pathways.5–7 Embryonic and fetal maturational changes have been studied in a fixed specimen.8 To date, only 1 study has reported on the appearance of fetal OBs in vivo.9 Our study focused on postnatal development, and our results demonstrate that OBs are well-organized structures that undergo a predefined and predictable maturational process, with rapidly developing olfactory activity ensuing.
Olfactory activity has been demonstrated to begin in utero, with an active intake of chemosensory information as part of the normal fetal experience that shapes the development of subsequent chemosensory sensitivities and preferences. The immature fetal brain is able to register amniotic odor or cues.10 Neonates actively encode odor cues associated with their mothers’ breast and discriminate the odor of their mother's skin or milk from those of other mothers. This ability allows them to modify their feeding behavior according to the milk flavor.11–13 In general, the neonate brain is extremely receptive to the odor environment. During the first years of life, the role of olfaction is probably very important to mother-child bonding.14
In opposition to other cranial nerves, with the exception of the optic nerve, the OB is a true neocerebral extension. The primordial of the olfactory apparatus appears in late somite embryos as 2 ectodermal thickenings, the olfactory or nasal placodes situated above the stomatodeum and below and lateral to the forebrain. By the proliferation of the surrounding mesoderm, these placodes become depressed to form the olfactory pits that represent the future site of the olfactory nerve. Further growth of the mesoderm causes the epithelium of each nasal placode to lie in the medial and lateral walls of the upper fifth of the corresponding nasal cavity. Conversely, the neuroblasts in the olfactory epithelium itself differentiate into nerve cells and give origin to olfactory nerve fibers that grow toward the apical region of the corresponding cerebral hemisphere. In their course, the fibers pierce the roof of the cartilaginous nasal capsule, which has developed around the primitive nasal cavity. They penetrate into the apical region of the hemisphere, which projects ventrally toward the olfactory placode, which in turn will become the elongated OBs. Eventually, the extension of the ventricular cavity into the OBs will become obliterated.
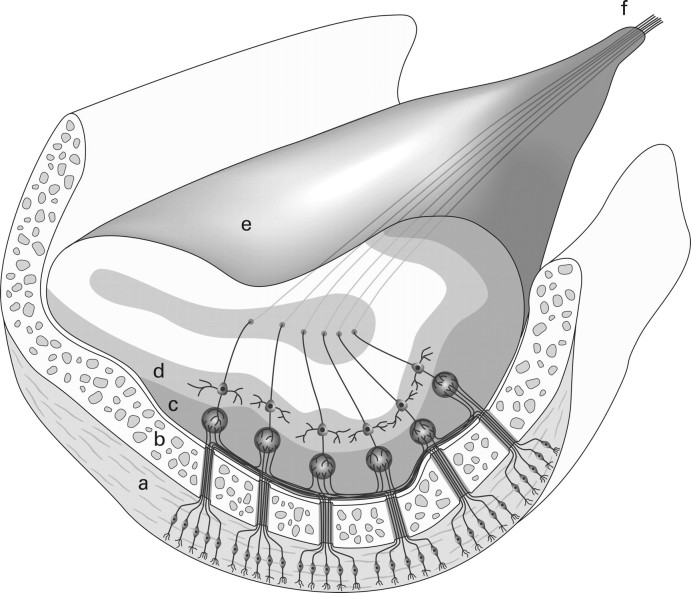
Reflecting their embryologic origin, the external layer of the OBs, as suggested by our study, consists of a rim of neurons originally derived from the cortex of the telencephalic vesicle. Cytoarchitectural studies have shown that in rodents, peripheral neurons are, to some extent, derived from migratory neurons originating from the prestriatal subventricular zone but can also originate from progenitor cells organized around the core of the OBs.15,16 A similar migratory pathway has only been identified once in humans,17 but this finding is controversial.18 These peripheral cells will eventually serve as a relay to the olfactory neurons from the nasal cavity. Therefore, considering that primary olfactory neurons project from beneath and from the side into the OB, the first maturational change we could recognize was a thinning of the upper aspect of the bulb itself, which might reflect reorganization of those neuronal layers that do not receive olfactory inputs. At the periphery of the OB, the external olfactory nerve layer and the glomerular cells layer located immediately beneath show a prominent lateral and inferior protrusion, whereas the superior aspect is extremely thin to nonexistent (Fig 5). The asymmetric laminar neuronal distribution has been demonstrated in rodents and is seen more pronounced in adult humans.19 This is in contrast to embryonic and fetal human specimens, in which an even, circular distribution of the peripheral layer of the immature OB is seen.8 In the later stages of postnatal development, we observed that the lateral part of the OBs (which becomes somewhat J shaped) will become more prominent than its medial counterpart. This can be attributed to the fact that the lateral stria as a posterior extension of the OB is the predominant anatomic bundle that projects to the primary olfactory centers of the limbic system.20
Fig 5.
Schematic drawing with a coronal cut through the OB, demonstrating asymmetric neuronal layering in an adult OB. a, Nasal olfactory epithelium. b, Cribriform plate. c, Glomerular layer. d, Mitral cell layer. e, Olfactory bulb. f, Olfactory tract.
Considering the central area of the OBs, it not only consists of bundles of axons that project to the primary olfactory area but also consists of a rich extracellular matrix and multiple interneurons coming from the anterior part of the ganglionic eminence (aGE). These axons and synaptic networks undergo progressive myelination that is reflected by the fact that the signal intensity of the center of the OBs parallels the signal intensity of the cerebral white matter. On the other hand, there is reduction in an extracellular matrix and a corresponding reduction of signal intensity on T2-weighted images. Both processes lead to almost identical signal intensity between the central and peripheral layers after the normal adult-type pattern is reached. As shown in Fig 3, pattern 2 was seen only up to 22 months after birth, confirming that the adultlike pattern 3 is seen in all cases after completion of the second year of life. This parallels the visual end of myelination, as seen on T2-weighted images for the cerebral white matter. It has been postulated that this central T2-hyperintensity could represent fluid-filled remnants of the ventricular cavity extension into the OBs as in other mammals,17 which has been disputed18 and is not reflected by our results.
The interpretation of our results is subject to some limitations. First, although all 3 maturation steps of the OBs do always occur, there is a large interindividual variability in the timing of maturation that seems to be less regular or predictable than cerebral maturation. For instance, the adultlike pattern 3 could be seen as early as 3 months after birth but was the single pattern recorded in children older than 2 years. Moreover, in all children with repeated MR imaging examinations, it was possible to show intraindividual age-related changes. Serial examinations of the same children clearly depicted all 3 presented patterns as an organized temporal process (Fig 6). This finding strengthens the hypothesis of a consistent maturational process, albeit not as strictly timed as in the cerebral hemispheres.
Fig 6.
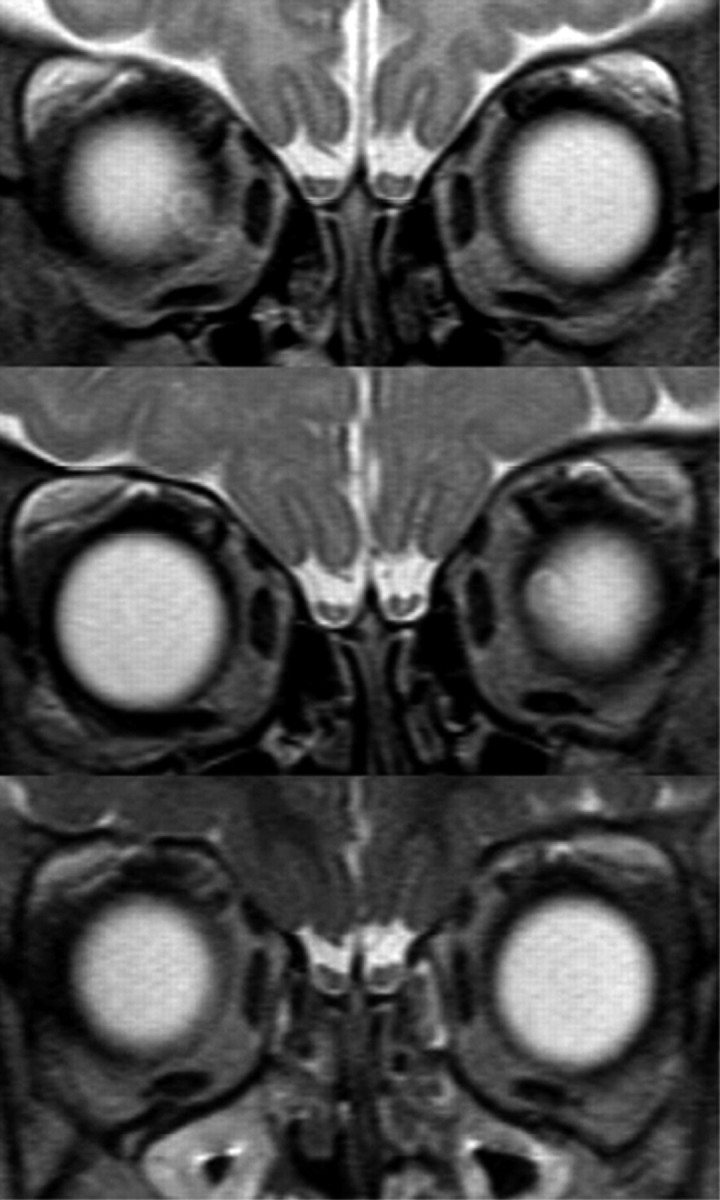
Serial MR imaging examinations (from top to bottom: ages 2 months, 8 months, and 18 months) in the same child show all 3 maturational steps.
In conclusion, we were able to demonstrate that OBs show time-related maturational changes. There was a progressive reorganization of the peripheral neuronal layers and central structures, leading to an adultlike aspect in all children no later than the end of the second year, paralleling maturational changes of cerebral white matter.
References
- 1.Suzuki M, Takashima T, Kadoya M, et al. MR imaging of olfactory bulbs and tracts. AJNR Am J Neuroradiol 1989;10:955–57 [PMC free article] [PubMed] [Google Scholar]
- 2.Yousem DM, Geckle RJ, Bilker WB, et al. Olfactory bulb and tract and temporal lobe volumes. Normative data across decades. Ann N Y Acad Sci 1998;855:546–55 [DOI] [PubMed] [Google Scholar]
- 3.Held P, Seitz J, Fründ R, et al. MRI detection of olfactory bulb and tract. J Neuroradiol 2000;27:112–18 [PubMed] [Google Scholar]
- 4.Iida Y, Naito M, Asahina N, et al. Magnetic resonance imaging of the olfactory apparatus. Arch Otolaryngol Head Neck Surg 1994;120:869–72 [DOI] [PubMed] [Google Scholar]
- 5.Castillo M, Mukherji SK. Magnetic resonance imaging of the olfactory apparatus. Top Magn Reson Imaging 1996;8:80–86 [PubMed] [Google Scholar]
- 6.Li C, Yousem DM, Doty RL, et al. Neuroimaging in patients with olfactory dysfunction. AJR Am J Roentgenol 1994;162:411–18 [DOI] [PubMed] [Google Scholar]
- 7.Cohen MM Jr. Malformations of the craniofacial region: evolutionary, embryonic, genetic, and clinical perspectives. Am J Med Genet 2002. :115:245–68 [DOI] [PubMed] [Google Scholar]
- 8.Müller F, O'Rahilly R. Olfactory structures in staged human embryos. Cells Tissues Organs 2004;178:93–116 [DOI] [PubMed] [Google Scholar]
- 9.Azoulay R, Fallet-Bianco C, Garel C, et al. MRI of the olfactory bulbs and sulci in human fetuses. Pediatr Radiol 2006;36:97–107 [DOI] [PubMed] [Google Scholar]
- 10.Delaunay-El Allam M, Marlier L, et al. Learning at the breast: preference formation for an artificial scent and its attraction against the odor of maternal milk. Infant Behav Dev 2006;29:308–21 [DOI] [PubMed] [Google Scholar]
- 11.Schaal B, Marlier L, Soussignan R. Olfactory function in the human fetus: evidence from selective neonatal responsiveness to the odor of amniotic fluid. Behav Neurosci 1998;112:1438–49 [DOI] [PubMed] [Google Scholar]
- 12.Cernoch JM, Porter RH. Recognition of maternal axillary odors by infants. Child Dev 1985;56:1593–98 [PubMed] [Google Scholar]
- 13.Varendi H, Porter RH, Winberg J. Does the newborn baby find the nipple by smell? Lancet 1994;344:989–90 [DOI] [PubMed] [Google Scholar]
- 14.Hall B. Changing composition of human milk and early development of an appetite control. Lancet 1975;1:779–81 [DOI] [PubMed] [Google Scholar]
- 15.Shapiro EM, Gonzalez-Perez O, Manuel García-Verdugo J, et al. Magnetic resonance imaging of the migration of neuronal precursors generated in the adult rodent brain. Neuroimage 2006;32:1150–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, Martin LJ. Olfactory bulb core is a rich source of neural progenitor and stem cells in adult rodent and human. J Comp Neurol 2003;459:368–91 [DOI] [PubMed] [Google Scholar]
- 17.Curtis MA, Kam M, Nannmark U, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 2007;315:1243–49 [DOI] [PubMed] [Google Scholar]
- 18.Sanai N, Berger MS, Garcia-Verdugo JM, et al. Comment on “Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension”. Science 2007;318:393 ; author reply 393 [DOI] [PubMed] [Google Scholar]
- 19.Maresh A, Gil DR, Whitman MC, et al. Principles of glomerular organization in the human olfactory bulb–implications for odor processing. PLoS ONE. 2008;3:e2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shipley MT, Reyes P. Anatomy of the human olfactory bulb and central olfactory pathways. In: Laing DG, Doty RL, Breipohl W (eds). The Human Sense of Smell. Berlin: Springer-Verlag;1991. :29–60