Abstract
SUMMARY: Lupus panniculitis (LP) or lupus erythematosus profundus (LEP) is a lupus-associated dermatologic condition predominantly affecting young to middle-aged women in which the deep dermis and subcutaneous fat are mainly involved. The lesions are usually located on the forehead, cheeks, proximal aspect of the limbs, and buttocks, though cases have occasionally been reported with orbital, breast, and salivary gland involvement. Descriptions of imaging findings of LP are very scarce in the literature. We describe the CT scan imaging features of the case of a patient with head and neck LP.
Lupus panniculitis (LP) is a very rare condition the imaging features of which are extremely scarce in the literature. We describe the CT scan imaging features of this rare disease entity in a patient.
Case Report
A 28-year-old African American woman developed tender indurated lesions on the left arm and in the right face, extending from the parotid area around the angle of the jaw toward the chin. Two years previously, biopsy results of an indurated area on her face revealed no evidence of a neoplasm. She had been previously diagnosed with systemic lupus erythematosus (SLE) when she was found to have positive antinuclear antibody results in the context of a pregnancy ending in a stillbirth.
Biopsy results of the lesion on her arm revealed panniculitis. It was recommended that she initiate treatment with steroids in combination with mycophenolate for lupus panniculitis. After 2 months, she experienced swelling of the bilateral neck. On physical examination, the lesion in the area of the right parotid was unchanged. There were new subcutaneous nodules in the neck bilaterally and in the submental area. The skin overlying these nodules was fixed to them. There was no palpable adenopathy.
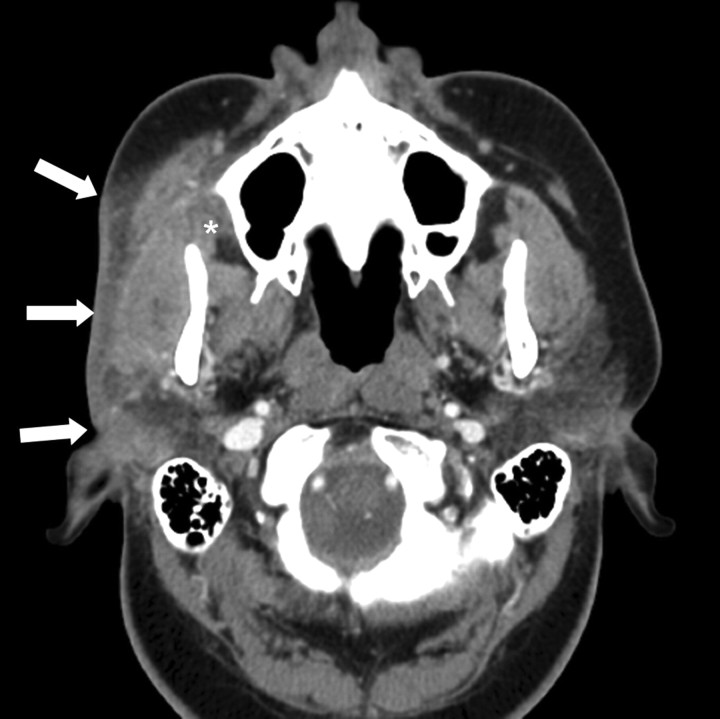
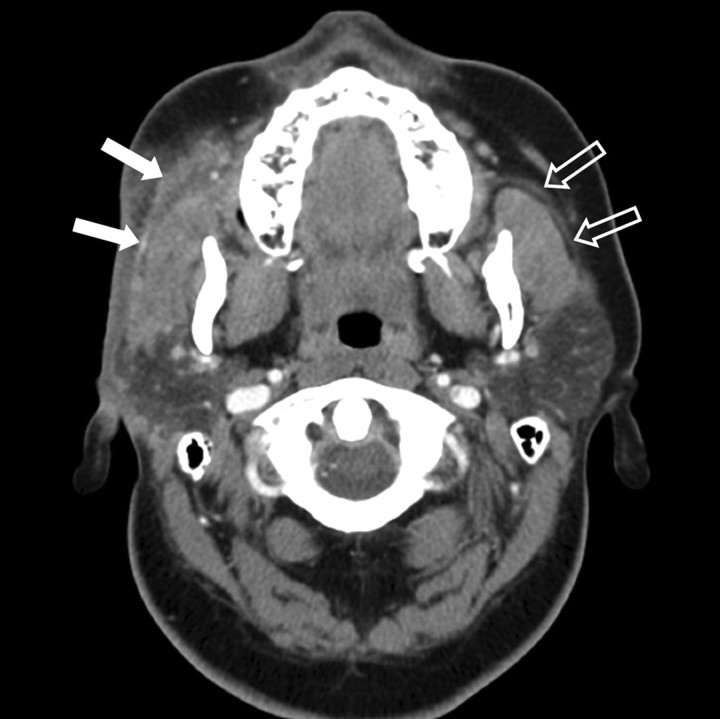
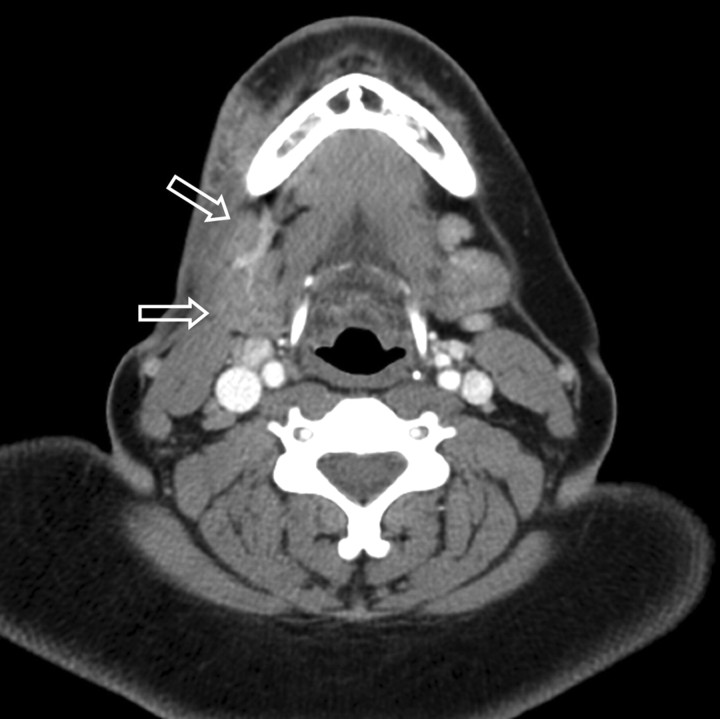
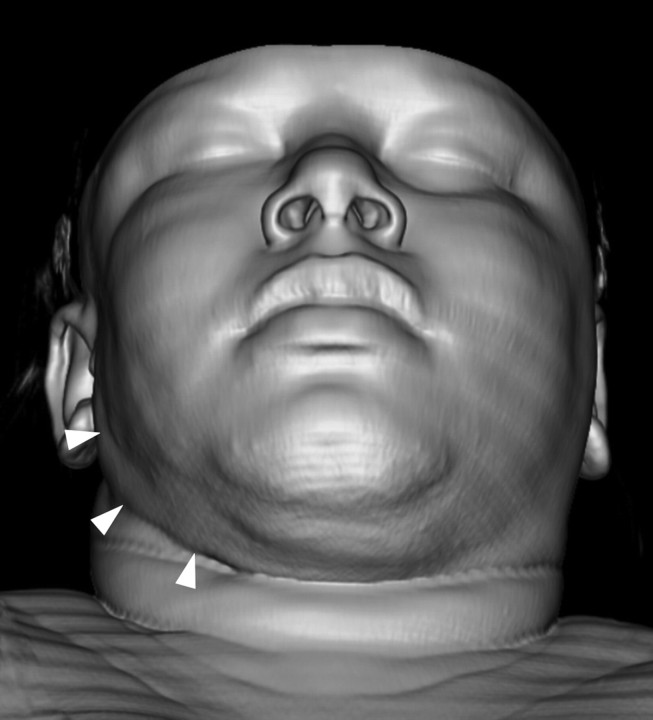
A contrast-enhanced CT scan of the neck demonstrated diffuse, superficial soft tissue infiltration of the right masticator space fat, extending into the fat overlying the anterior and lateral margins of the parotid gland. The lesion was both superficial and deep to the platysma muscle. There was extension into the posterior buccal space fat (Fig 1). The soft tissue infiltration lay superficial to both the masseter and temporalis muscles. The fat plane between the muscles was also ill defined. The right parotid duct was within the lesion (Fig 2). The process extended inferiorly into the right submandibular space, infiltrating the fat plane surrounding the right submandibular gland (Fig 3). The lesion produced an indrawing, cicatrizing effect rather than an outward bulge, which was illustrated on 3D surface-rendered images of the face (Fig 4). There was no adjacent osseous abnormality.
Fig 1.
Axial contrast-enhanced CT scan shows diffuse, ill-defined inflammatory infiltration (white arrows) of the subcutaneous fat superficial to the masseter muscle and parotid gland. This extends into the pterygomaxillary fissure (asterisk), the parotid gland, and the masseter muscle. The process extends both superficial and deep to the platysma muscle and into the posterior buccal space fat.
Fig 2.
Axial contrast-enhanced CT scan shows ill-defined fat plane between the muscles. The proximal right parotid duct (white arrows) is embedded within the lesion. Note normal left parotid duct (outlined arrows). Note flattened right facial contour, reduced bulk of right-sided subcutaneous fat compared with the left.
Fig 3.
Axial contrast-enhanced CT scan at an inferior level shows extension of the infiltrative lesion into the right submandibular space obscuring the fat surrounding the right submandibular gland. Note that the lesion produces an indrawing cicatrizing effect rather than an outward bulge.
Fig 4.
Surface-shaded 3D reconstruction of the facial soft tissues clearly demonstrates the indrawing cicatrizing effect from LP on the right side (arrowheads).
There were mildly enlarged right intraparotid and level II lymph nodes and scattered bilateral shotty cervical lymph nodes, more prominent on the right side. The left parotid and submandibular salivary glands appeared normal. Extranodal lymphoid tissue in the Waldeyer ring and thyroid gland also appeared normal.
Discussion
LP is a rare skin condition, the clinical findings of which were originally described by Kaposi1 in 1883. The terms lupus erythematosus profundus (LEP) and lupus panniculitis were introduced by Irgang2 in 1940. The disorder represents a variant of lupus erythematosus (LE), which predominantly affects the deep dermis and subcutaneous fat in young to middle-aged women. It may occur independently or in association with discoid lupus erythematosus (DLE) or SLE.3 The clinical manifestations include nodules and hardened subcutaneous plaques, often adhering to the overlying skin, that heal after a long period with atrophy and residual scarring. Persistent lesions do not usually become ulcerated, but painful ulcerations may occur. The overlying skin may be intact or show DLE lesions. These nodules and plaques are usually located on the forehead, cheeks, proximal aspect of the limbs, and buttocks, though cases have occasionally been reported with orbital,4 breast, and salivary gland5 involvement where diagnosis can be problematic, particularly when the disease is not associated with manifestations of SLE or DLE.
Descriptions of the histopathologic findings in LEP have varied. Histologic examination revealed lobular lymphocytic panniculitis with hyaline necrosis of the fat and presence of lymphoid follicles, occasionally with germinal centers.6 Older lesions may contain calcifications. More than half of the cases demonstrate changes characteristic of LE in the dermis and epidermis, with epidermal atrophy, vacuolar degeneration of the basement membrane, and perivascular and periadnexal lymphocytic infiltrates. These findings are more frequent when the lesions are associated with discoid lupus.5 Ackermann et al7 divided the histopathologic features of LEP in 3 stages (early, mature, and late). According to these and other authors,6 the presence of lymphocytic nuclear dust within a patchy lymphoplasmacytic infiltrate in the lobules of the subcutaneous fat is a clue for the histopathologic diagnosis of LEP. Mucin deposition in the dermis, a finding frequently associated with skin lesions of LE, also represents a possible diagnostic clue.
The clinical and histopathologic differential diagnosis of LEP in the florid phase includes many conditions such as erythema nodosum, erythema induratum of Bazin, pancreatic panniculitis, cold panniculitis, poststeroid panniculitis, and morphea profunda in the late stages.7,8 The most difficult differential diagnosis is subcutaneous panniculitis-like T-cell lymphoma (SPTCL), a peculiar cytotoxic a/b CD8þ T-cell lymphoma with exclusive involvement of subcutaneous tissues. From a clinical standpoint, LEP and SPTCL are indistinguishable. The most useful histopathologic criteria to distinguish LEP from SPTCL are the presence of involvement of the epidermis, lymphoid follicles with reactive germinal centers, mixed-cell infiltrate with prominent plasma cells, clusters of B lymphocytes, and polyclonal T-cell receptor gamma gene rearrangement.6
Descriptions of imaging findings of LP are very scarce in the literature. CT scans of LEP showing periorbital edema9 and increased attenuation surrounding the submandibular glands suggesting inflammation10 have been reported, but there is no detailed imaging assessment of a LEP case in the literature, to the best of our knowledge. We analyzed the CT scan of our biopsy-proved LP case with the aid of 3D reconstructions of facial soft tissues. The diffuse soft tissue infiltration of the subcutaneous fat produced an ill-defined appearance of fat planes between the adjacent muscles, salivary glands, and the surrounding the proximal parotid duct. There were few adjacent shotty lymph nodes, and there was no bony involvement. It is notable that the lesion produced a cicatrizing effect rather than an outward bulge, illustrated by the 3D surface rendering of the facial soft tissues. This was an interesting and potentially diagnostically useful finding because CT scans of facial cellulitis or tumor typically demonstrate outward bulging of the skin and soft tissues accompanying the underlying soft tissue infiltrative changes. This cicatrizing pattern might thereby be useful to exclude SPTCL.
We report the case of this patient, as the imaging literature of head and neck panniculitis is very sparse. LEP should be considered in the differential diagnosis of infiltrative soft tissue lesions in patients with a history of lupus, especially when no definite clinical features of cellulitis and no abscesses were detected on CT scan.
References
- 1.Kaposi M. Pathologie und Therapie der Hautkrankheiten. 2nd ed. Vienna: Urban & Schwarzenberg;1883. :642
- 2.Irgang S. Lupus erythematosus profundus: report of an example with clinical resemblance to Darier-Roussy sarcoid. Arch Dermatol Syph 1940;42:97–108 [Google Scholar]
- 3.Tuffanelli DL. Lupus erythematosus panniculitis (profundus): clinical and immunologic studies. Arch Dermatol 1971;103:231–42 [PubMed] [Google Scholar]
- 4.Jordan DR, McDonald H, Olberg B, et al. Orbital panniculitis as the initial manifestation of systemic lupus erythematosus. Ophtalmic Plast Reconstr Surg 1993;9:71–75 [DOI] [PubMed] [Google Scholar]
- 5.Pérez-Pastor G, Valcuende F, Tomás G, et al. Lupus erythematosus panniculitis presenting as palpebral edema and parotiditis. Actas Dermosifiliogr 2007;98:549–52 [PubMed] [Google Scholar]
- 6.Massone C, Kodama K, Salmhofer W, et al. Lupus erythematosus panniculitis (lupus profundus): clinical, histopathological, and molecular analysis of nine cases. J Cutan Pathol 2005;32:396–404 [DOI] [PubMed] [Google Scholar]
- 7.Ackermann AB, Chongchinant N, Sanchez J, et al., eds. Histologic Diagnosis of Inflammatory Skin Disease, 2nd ed. Baltimore: Williams and Wilkins;1997. :529
- 8.Stork J, Vosmik F. Lupus erythematosus panniculitis with morphea-like lesions. Clin Exp Dermatol 1994;19:79. [DOI] [PubMed] [Google Scholar]
- 9.Braun RP, French LE, Massouyé I, et al. Periorbital oedema and erythema as a manifestation of discoid lupus erythematosus. Dermatology 2002;205:194–97 [DOI] [PubMed] [Google Scholar]
- 10.Ogura N, Fujisaku A, Jodo S, et al. Lupus erythematosus profundus around the salivary glands: a case resembling submandibular salivary gland disease. Lupus 1997;6:477–79 [DOI] [PubMed] [Google Scholar]