Abstract
BACKGROUND AND PURPOSE: Non-neoplastic, calcified, fibro-osseous lesions known as “calcifying pseudoneoplasms of the neuraxis” (CAPNON) are rare and can occur anywhere within the neuraxis. The radiologic and histopathologic characteristics of this unusual entity are not well understood. We present the largest series reviewing the MR imaging features of CAPNON.
MATERIALS AND METHODS: The MR and CT imaging features in 4 patients with a pathologic diagnosis of “calcifying pseudoneoplasms of the neuraxis” were retrospectively reviewed. A neuropathologist also analyzed the histopathologic features for typical and atypical patterns.
RESULTS: Imaging features were strikingly similar for all 4 patients. All lesions appeared T1 and T2 hypointense without vasogenic edema. All tumors had dense calcification, and 3 tumors showed minimal linear internal or rim enhancement on MR imaging.
CONCLUSIONS: CAPNON may mimic more common vascular malformations or neoplasms and are often not considered in the differential diagnosis of calcified lesions. CAPNON should be included in the differential diagnosis of a calcified mass with marked T1 and T2 hypointensity and limited to no enhancement. Careful CT and MR imaging evaluation can suggest this entity, and this preoperative recognition may help subsequent management decisions.
As their name suggests, calcifying pseudoneoplasms of the neuraxis (CAPNON) are non-neoplastic, calcified lesions occurring anywhere in the central nervous system. These lesions can be intra-axial or extra-axial and have been reported to occur in the brain and spine with similar frequency (Table 1). Since the original pathologic description by Rhodes and Davis,1 there have been approximately 32 reported cases.2-9 We found 32 additional cases reported in the literature: 11 spine CAPNON and 21 intracranial CAPNON. Of the intracranial lesions, most were extra-axial and were located at the skull base. Eight cases of intra-axial CAPNON are reported, and, as in our 3 patients, most of these patients presented with seizures (Table 1).
Table 1:
Reported cases of intracranial and spinal CAPNON*
| References | Pt No. | Age/Sex | Location | CT Performed | Presentation |
|---|---|---|---|---|---|
| Rhodes & Davis, 1978 | 1 | 27/F | R frontal | No | HA |
| 2 | 55/F | Brain, dura | No | Autopsy finding | |
| 3 | 60/M | L cerebellum | No | Autopsy finding | |
| 4 | 74/F | Brain, dura | No | Autopsy finding | |
| 5 | 46/M | Choroid | No | Autopsy finding | |
| 6 | 62/M | Pineal | No | Autopsy finding | |
| 7 | 83/M | Brain, dura | No | Autopsy finding | |
| Jun, 1984 | 8 | 55/M | Corpus callosum | Calc | HA, N/V |
| Garen, 1989 | 9 | 44/M | Dura, Meckel cave | Calc | Atypical facial pain |
| Bertoni, 1990 | 10 | 31/M | Jugular foramen | No | HA, hoarseness |
| 11 | 50/M | Foramen magnum | No | Neck pain | |
| 12 | 48/M | Skull base/cerebellum | No | R CN XI paralysis | |
| 13 | 23/M | Spine, T10 | No | Back pain | |
| 14 | 58/M | Spine, C2–3 | No | Back pain | |
| 15 | 32/M | L frontal | No | Epilepsy | |
| 16 | 45/F | Skull base | No | CN paralysis | |
| 17 | 58/M | Skull base | No | Hoarseness | |
| 18 | 12/M | Spine, C6 | No | Pain | |
| 19 | 32/M | Spine, L4–5 | No | Back pain | |
| 20 | 33/F | Spine, T9 | No | Back pain | |
| 21 | 68/F | Spine, L4–5 | No | R hip pain | |
| 22 | 20/F | Spine, C2 | No | Incidental | |
| 23 | 56/F | Spine, L4–5 | No | Back pain | |
| Smith, 1994 | 24 | 48/M | Spine, L2–3 | No | Sciatica |
| Tsugu, 1999 | 25 | 22/F | R parietal | Calc | Seizures |
| Tatke, 2001 | 26 | 6/M | L temporal | Calc | Seizures |
| Qian, 1999 | 27 | 33/F | L temporal | Calc | Developmental delay |
| 28 | 49/M | Spine, C1 & clivus | No | Weakness | |
| 29 | 59/M | Spine, C1–2 | No | Shuffling gait | |
| 30 | 47/F | Frontal lobe | Calc | Seizures |
Note:—CAPNON indicates calcifying pseudoneoplasms of the neuraxis; calc, densely calcified mass seen on CT; CN, cranial nerve; HA, headache; N/V, nausea and vomiting; L, left; R, right.
This table includes an additional 30 patients from the literature with intracranial and intraspinal CAPNON.
Previous reports are primarily in the neurosurgery and pathology literature and inconsistently describe the radiologic appearance; only 1 study presents MR imaging features in 2 patients.6 Although the nature of CAPNON is unclear, a reactive rather than a hamartomatous process has been favored.2 The outcome is often considered to be excellent, and gross total removal of the lesion seems to be curative.
Histopathologic features of CAPNON include variable amounts of fibrous stroma, spindle to epithelioid cells palisading around a chondromyxoid matrix, ossifications, foreign-body giant cells, and occasional psammoma bodies.10 It is interesting to note that the spectrum of these changes and the presence of each component in published examples show marked variations. Thus, some may resemble tumoral calcinosis, whereas others are similar to a rheumatoid nodule or osteoma.11
From a radiologic standpoint, CAPNON may resemble a number of neoplastic and non-neoplastic lesions, and correct recognition of the lesion may help avoid overtreatment or obviate additional costly procedures. The goal of this study was to provide additional observations on radiologic and pathologic features of CAPNON to help identify the characteristics that may allow preoperative recognition. Our series is the largest collection of cases with MR imaging to date.
Materials and Methods
Patients
After approval by our institutional review board, the department of pathology archives were reviewed to identify all fibro-osseous lesions that could be classified as calcifying pseudoneoplasms. Four such cases were identified at our institution during a period of 10 years. After review of pathologic features in these 4 patients, the clinical and imaging characteristics were analyzed. MR imaging scans were reviewed for all 4 patients. CT scans were available for 3 patients, with diagnostic angiography in the fourth patient.
We also reviewed the MR imaging findings, pathologic findings, and clinical presentation of 2 additional patients reported in the literature by Shrier et al.6
Imaging Protocol
Multidetector CT was performed on an 8- or 16-detector scanner (Lightspeed; GE Healthcare, Milwaukee, Wis). Noncontrast axial images were obtained from the vertex to the skull base with 2.5-mm section thickness, 120 kV, and 230 to 300 mAs.
All preoperative MR images were acquired with two1.5T MR imaging systems. Our standard preoperative STEALTH protocol (Medtronic, Minneapolis, Minn.) for a surgical navigation system includes axial 3D fast spin-echo T2-weighted and axial 3D spoiled gradient-recalled echo (SPGR) after intravenous administration of gadopentetate dimeglumine (0.1 mmol/kg). Imaging parameters for the axial 3D fast spin-echo T2-weighted imaging include TR, 3000 ms; TE, 100 ms; and NEX, 1. Imaging parameters for the 3D SPGR include TR 34 ms; TE, 3 ms; flip angle, 35°; NEX, 1, with 1-mm section thickness and matrix of 256 × 192.
Other preoperative and postoperative imaging included our standard brain tumor protocol on both 1.5T and 3T MR imaging systems. This protocol includes axial T2-weighted and axial spin-echo T1-weighted imaging performed before and after the administration of gadopentetate dimeglumine (0.1 mmol/kg). Imaging parameters for axial dual-echo T2-weighted sequence include TR, 2500 ms; TE, 30 and 80 ms; and NEX, 0.75. Imaging parameters for T1-weighted imaging were TR, 600 ms; TE, minimum; and NEX, 2. Both are obtained with 5-mm section thickness (skip, 1 mm) and 256 × 192 matrix.
Image and Pathology Review
On review of CT and MR imaging, tumor location, size, calcification, T1 and T2 signal intensity characteristics, extent of vasogenic edema, and enhancement patterns were noted. Our neuropathologist also reviewed the histopathologic characteristics after gross total resection.
Results
Imaging features were strikingly similar in our 4 cases. In all 4 cases, the MR imaging scans demonstrated markedly hypointense signal intensity on both T1- and T2-weighted images, with no vasogenic edema. Minimal linear internal enhancement or partial rim enhancement was seen in 3 of the 4 cases, and there was no enhancement in 1 case. The 3 intra-axial masses demonstrated dense calcification on CT scan, whereas dense calcification was also noted on the diagnostic angiogram in the fourth case. Otherwise, the results on diagnostic angiogram were normal. The MR imaging features in our 4 patients with CAPNON, in addition to the 2 previous cases reported in the literature, are summarized in Table 2.
Table 2:
MR imaging features of intracranial CAPNON*
| Pt No. | Age/Sex | Presentation | Location | Size (cm) | T1WI | T2WI | Enhancement |
|---|---|---|---|---|---|---|---|
| 1 | 16/M | Incidental | Temporal horn, extra-axial | 3.5 | Hypo | Hypo | Internal linear C+ |
| 2 | 35/M | Seizures | Temporal, intra-axial | 2 | Hypo | Hypo | Internal linear C+ |
| 3 | 49/F | Seizures | Hippocampus, intra-axial | 1 | Hypo | Hypo | No C+ |
| 4 | 59/M | Left arm numbness | Parietal, intra-axial | 1 | Hypo | Hypo | Rim C+ |
| Shrier et al | 32/F | Incidental | Temporal, intra-axial | 0.8 | Hypo | Hypo | Rim C+ |
| Shrier et al | 59/M | Neck pain | Foramen magnum, extra-axial | 2 | Hypo | Hypo | Heterogenous solid |
Note:—Hypo, indicates hypointense; C+, enhancement. All lesions showed dense calcification on CT; T1WI, T1-weighted imaging; T2WI, T2-weighted imaging.
This table includes our 4 patients and 2 additional patients reported in the literature.
All patients underwent complete resection (primary resection in 3 cases and secondary resection in 1 case) of the calcified mass with a histopathologic diagnosis of “fibro-osseous lesion.” The patients ranged in age from 16 to 59 years with 3 male and one female patient. Three adult patients presented with a history of long-standing seizures, and 1 lesion was found incidentally in the youngest patient, who had a head CT scan for trauma. Our fourth patient initially presented with seizures and underwent craniotomy at an outside hospital 19 years ago. He required no additional treatment until he presented again to our institution with left arm and leg numbness.
The first case, an incidentally found lesion, was a 3.2-cm extra-axial intraventricular mass (Figure 1). The remaining 3 patients with a history of seizures had intra-axial brain masses, ranging in size from 1 cm to 1.8 cm, located in the right parietal lobe near the postcentral gyrus, in the left hippocampus (Fig 2), and in the right temporal lobe, respectively. Preoperatively, the intra-axial temporal lobe lesions mimicked cavernous malformations or densely calcified neoplasms such as oligodendroglioma or ganglioglioma on the basis of their CT and MR imaging appearance. The intraventricular mass mimicked a meningioma but prospectively was thought to represent a pseudoneoplasm because of the lack of enhancement. The right parietal lesion had previously been resected 18 years previously, with a “benign” pathologic appearance and no specific diagnosis.
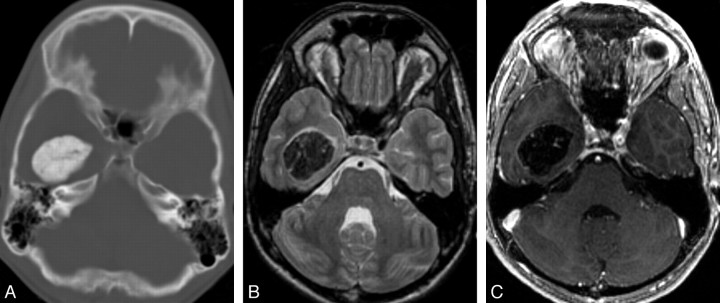
Fig 1.
Typical radiologic features of extra-axial CAPNON. A, Noncontrast CT scan shows a densely calcified mass in the right temporal horn. B, Axial T2-weighted MR image shows a uniform T2 hypointense mass centered in the right temporal horn. C, Axial T1-weighted postgadolinium sequence illustrates marked T1 hypointensity with scattered linear areas of enhancement that correspond to the strands of T2 hyperintensity. This appearance was seen in both of our larger lesions and may correspond to the vascular stromal elements seen within these lesions.
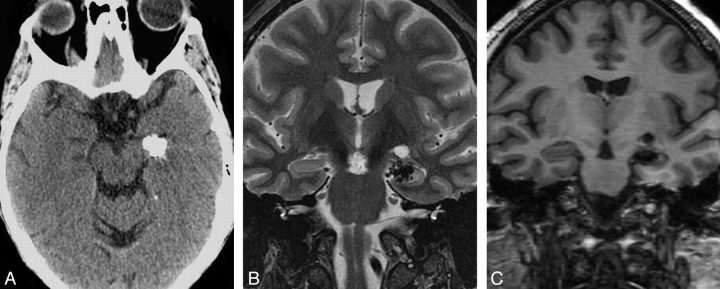
Fig 2.
Typical radiologic features of intra-axial CAPNON. A, Noncontrast CT scan shows a left hippocampal mass with attenuated calcification. B, Coronal T2-weighted sequence demonstrates T2 hypointensity with a nodular border. C, Coronal T1-weighted sequence shows the typical T1 hypointensity. Preoperatively, this lesion was thought to represent a cavernous malformation. In retrospect, the nodular contour on T2 and lack of internal T2 hyperintensity would be atypical for a cavernous malformation of this size.
Histopathologic Evaluation
All lesions demonstrated extensive calcifications and a chondromyxoid matrix with an amorphous quality; they also harbored a variable amount of spindle to epithelioid cells rimming the chondromyxoid matrix and fibrolamellar nodules (Fig 3). The spindle and epithelioid cells surrounding the matrix showed positive staining with antibodies against epithelial membrane antigen (EMA) and did not show any staining with antibodies against S-100 protein, glial fibrillary acidic protein, smooth muscle actin, or CD68. Foreign-body–type reaction with scattered giant cells was noticed in 2 of the lesions.
Fig 3.
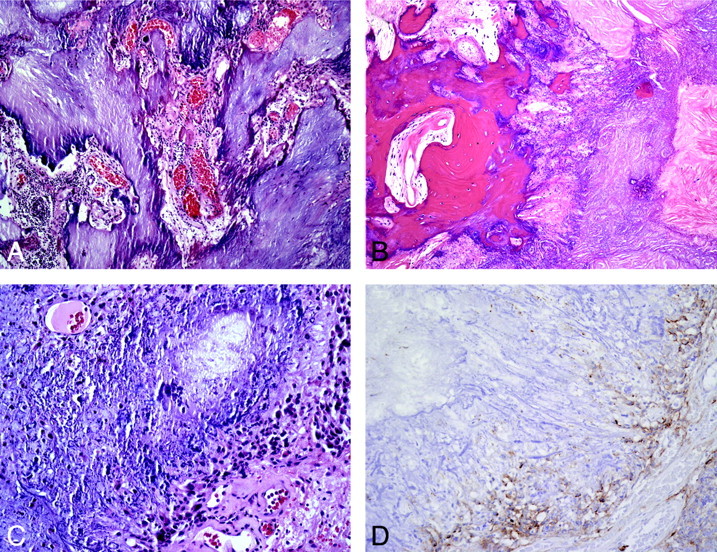
Typical histopathologic features of CAPNON. A, The typical chondromyxoid matrix of CAPNON (H&E, original magnification ×100). B, Focal osseous metaplasia is seen in all 4 cases (H&E, original magnification ×100). C, Medium-power magnification of the chondromyxoid matrix and the peripheral spindle cells (H&E, original magnification ×200). D, Immunohistochemical analysis for EMA demonstrating positive staining in the spindle cells surrounding the matrix (EMA immunohistochemistry, original magnification ×200).
In addition to the typical histopathologic features, case 1 showed extensive confluent psammomatous calcifications both within the lesion as well as the adjacent choroid plexus with osseous metaplasia. There was reactive fibroblastic proliferation and meningioangiomatosis in the surrounding tissue (Fig 4). Case 2 demonstrated architectural disarray and dysplastic changes in the adjacent neuropil with abnormal neuritic processes. There were balloonlike neurons as well as bizarre cells adjacent to the lesion, reminiscent of focal cortical dysplasia. Case 3 demonstrated a 0.5-cm calcified mass remote from the lesion in the overlying dura that was interpreted as focal osseous metaplasia. The tissue adjacent to the lesion showed scattered psammoma bodies and metaplastic bone. Case 4 demonstrated foci of hemosiderin-laden macrophages; granulation-tissue–type changes; and marked piloid gliosis with Rosenthal fibers in the surrounding tissue, which may have been related to the earlier surgical procedure. This lesion also had focal perivascular lymphocytic infiltrates and metaplastic bone formation in the adjacent neuropil.
Fig 4.
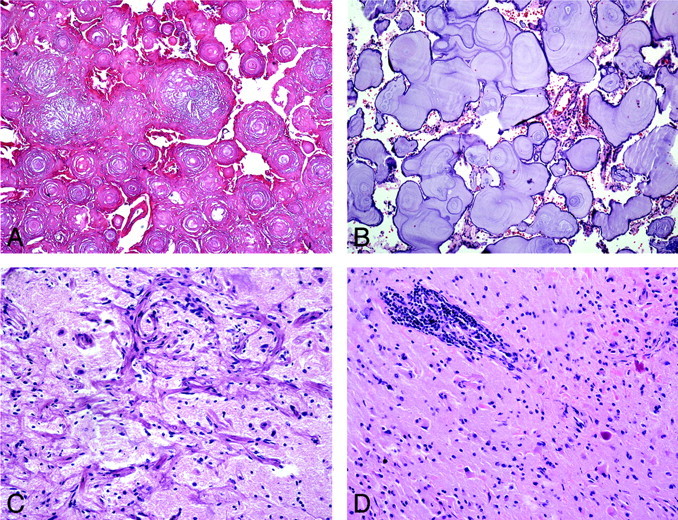
Atypical and unusual features of CAPNON. A, Areas of coalescent concentric lamellar calcifications without intervening chondromyxoid matrix or cells (H&E, original magnification ×100). B, More basophilic amorphous lamellar calcifications without intervening chondromyxoid matrix and with rare meningothelial cells (H&E, original magnification ×100). C, Adjacent cortical region showing meningioangiomatosis (H&E, original magnification ×200). D, Surrounding parenchyma with prominent perivascular lymphocytic infiltrates and Rosenthal fibers (H&E, original magnification ×200).
Discussion
CAPNON are rare lesions, with less than 30 cases reported in the literature. They do not seem to have a predilection for sex, age, or central nervous sytem location.1,2,4,5,9 The patient age ranges from 12 to 83 years.2-8,10 Most reported lesions have been extra-axial, but several intra-axial cases have also been reported.1,2 The lesions seem to be slow growing with presenting symptoms related to local compression or irritation of adjacent tissues. The underlying cause of CAPNON remains unknown, and complete surgical resection seems to be curative.2
The “classic” histopathologic features in CAPNON include a distinctive set of common elements: 1) typical chondromyxoid matrix in a nodular pattern; 2) palisading spindle to epithelioid cells; 3) variable amounts of fibrous stroma; 4) calcification, osseous metaplasia, and scattered psammoma bodies; and 5) foreign-body reaction with giant cells.10 The presence of each component is highly variable in individual lesions, and some examples may not show all of the elements above. Three of our patients had some of the above elements, and only 1 patient had all of these classic features. It has been suggested, but not proven, that CAPNON may develop as a healing response to an array of inciting factors, which can account for the variations in histopathologic features. The causal factors are not yet understood, but response to possible trauma, infection, or inflammation has been proposed. The tissue of origin most likely includes the arachnoid or fibroblasts in the choroid plexus stroma, but this has not been conclusively proven either.1
On the basis of previous reports, the radiologic differential diagnosis of these intracranial and intraspinal calcifying lesions can be broad. The primary differential consideration for the extra-axial CAPNON at the skull base is meningioma, but some authors have also included chordoma, chondrosarcoma, and vestibular schwannoma.2 However, our series suggest that this differential can be narrowed on the basis of MR imaging features. Specifically, the uniform T2 hypointensity without enhancement would be unusual for chordoma, chondrosarcoma, or vestibular schwannoma.
Important differential considerations for intra-axial calcified masses include calcifying neoplasms such as ganglioglioma and oligodendroglioma, vascular lesions such as cavernous malformation, and infections such as tuberculosis. Intraventricular masses can also raise the possibility of choroid plexus tumors, meningioma, or ependymal tumors. Our small series suggests that important MR imaging features may allow the radiologist to suggest CAPNON in the differential diagnosis. CT images of CAPNON typically show solid attenuated calcifications, and the MR imaging often shows a well-defined lesion that is uniformly hypointense on both T1- and T2-weighted images without surrounding edema. In our cases, only minimal linear internal or rim enhancement was present. The larger lesions (cases 1 and 2) best demonstrated the more serpiginous internal enhancement, which has been hypothesized to represent the vascular or stromal characteristic of these lesions (Fig 1).6 None of our cases demonstrated solid enhancement. However, an extra-axial mass presented by Shrier et al6 in the foramen magnum (Table 2) did show more solid enhancement, mimicking meningioma. None of our lesions showed significant surrounding edema. Our fourth case had surrounding T2 signal intensity, thought to represent gliosis from previous surgery, given the lack of mass effect. However, both cases presented by Shrier et al6 did have surrounding T2 prolongation thought to represent edema. Further investigation would be needed to identify the “typical” enhancement or pattern of edema in these rare lesions.
When considering the differential for calcifying intra-axial or extra-axial lesions, the uniform T1 and T2 hypointensity without solid enhancement is a key distinguishing feature. Calcified lesions with heterogeneous T2 signal intensity or T2 hyperintensity are more likely to be a calcified neoplasm and inconsistent with CAPNON. All of our cases and the previous 2 reported cases had uniform T2 hypointensity. Calcified lesions with the typical popcorn “T2 hyperintensity” and hemosiderin ring are more consistent with cavernous malformation and also distinguish themselves from CAPNON. Finally, the lack of solid enhancement helped to distinguish our intraventricular tumor from a meningioma or choroid plexus tumor.
Conclusions
In summary, CAPNON are rare, possibly reactive lesions that can occur as either extra-axial or intra-axial masses. Although the typical histopathologic features can be observed in most cases, some examples may be unusual and may be confused with calcified meningioma or tumoral calcinosis. Although the cause and pathogenesis are unclear, the histopathologic appearance is distinctive along a spectrum, and the imaging features can suggest the diagnosis. CAPNON should be considered in the imaging differential diagnosis when a heavily calcified lesion is found on CT, with hypointensity on T1-weighted and T2-weighted MR images, minimal linear rim or serpiginous internal enhancement, and limited to no edema. It is important to consider this entity to avoid aggressive surgical or diagnostic procedures.
Footnotes
Poster previously presented at: Annual Meeting of the American Society of Neuroradiology, June 2, 2008; New Orleans, La.
References
- 1.Rhodes RH, Davis RL. An unusual fibro-osseous component in intracranial lesions. Hum Pathol 1978;9:309–19 [DOI] [PubMed] [Google Scholar]
- 2.Bertoni F, Unni KK, Dahlin DC, et al. Calcifying pseudoneoplasms of the neural axis. J Neurosurg 1990;72:42–48 [DOI] [PubMed] [Google Scholar]
- 3.Chang H, Park JB, Kim KW. Intraosseous calcifying pseudotumor of the axis: a case report. Spine 2000;25:1036–39 [DOI] [PubMed] [Google Scholar]
- 4.Garen PD, Powers JM, King JS, et al. Intracranial fibro-osseous lesion. Case report. J Neurosurg 1989;70:475–77 [DOI] [PubMed] [Google Scholar]
- 5.Jun C, Burdick B. An unusual fibro-osseous lesion of the brain. Case report. J Neurosurg 1984;60:1308–11 [DOI] [PubMed] [Google Scholar]
- 6.Shrier DA, Melville D, Millet D, et al. Fibro-osseous lesions involving the brain: MRI. Neuroradiology 1999;41:18–21 [DOI] [PubMed] [Google Scholar]
- 7.Tatke M, Singh AK, Gupta V. Calcifying pseudoneoplasm of the CNS. Br J Neurosurg 2001;15:521–23 [DOI] [PubMed] [Google Scholar]
- 8.Tsugu H, Fukushima T, Takeno Y. Calcifying pseudotumor of the neural axis–case report. Neurol Med Chir (Tokyo) 1999;39:762–65 [DOI] [PubMed] [Google Scholar]
- 9.Smith DM, Berry AD, 3rd. Unusual fibro-osseous lesion of the spinal cord with positive staining for glial fibrillary acidic protein and radiological progression: a case report. Hum Pathol 1994;25:835–38 [DOI] [PubMed] [Google Scholar]
- 10.Qian J, Rubio A, Powers JM, et al. Fibro-osseous lesions of the central nervous system: report of four cases and literature review. Am J Surg Pathol 1999;23:1270–75 [DOI] [PubMed] [Google Scholar]
- 11.Burger PC, Scheithauer BW, Vogel FS. Surgical Pathology of the Nervous System and its Coverings. New York: Churchill Livingstone;2002