Abstract
BACKGROUND AND PURPOSE: Currently, the effect of the volume of cement used during sacroplasty on the restoration of pelvic strength and stiffness is unknown. The purpose of this study was to measure that effect in a sacral insufficiency fracture model.
MATERIALS AND METHODS: Twenty-five osteoporotic cadaveric pelves were potted, and sacral fractures were produced. Specimens were divided into 4 groups: group 0 + 0 (control), no sacroplasty; group 3 + 0, sacroplasty (posterior approach), 3 mL of a bone cement injected bilaterally into the fracture site at S1; group 3 + 3, sacroplasty (posterior approach), 3 mL of the same cement injected bilaterally into the fracture site at S1 and S2; and group 6 + 3, sacroplasty (posterior approach), 6 mL of the same cement injected bilaterally at S1 and 3 mL injected bilaterally at S2. Cement position and extravasation were documented with CT. Specimens were tested to failure to assess the strength and stiffness after sacroplasty.
RESULTS: There were no significant differences in strength or stiffness restoration between control and treatment groups.
CONCLUSIONS: Sacroplasty does not restore the strength or stiffness of the sacrum in a cadaveric model regardless of the volume or location of cement.
Sacroplasty is being used to stabilize sacral insufficiency fractures.1-3 No randomized controlled trials have compared the outcomes after sacroplasty with those after nonoperative treatment. The mechanism by which sacroplasty may give pain relief is unknown. One hypothesis is that the injected cement reduces movement at the fracture site and, thus, pain, by augmenting the strength and stiffness of the sacrum3-5 and that increasing the amount of cement will increase fracture stability.
Finite-element analysis suggests that sacroplasty reduces motion at the fracture site.4 A cadaveric model of sacral insufficiency fractures has been developed to facilitate biomechanical testing of the effects of sacroplasty.6 The optimal amount of cement injected during sacroplasty is unknown, but it is thought to be a balance between achieving mechanical stability and avoiding cement extravasation. The purpose of our study was to measure the effect of cement volume on the restoration of strength and stiffness to the pelvis via sacroplasty.
Materials and Methods
Twenty-five cadaveric pelves with attached lumbar spines were obtained from the Maryland State Anatomy Board. Each specimen was confirmed to be osteoporotic by dual-energy x-ray absorptiometry (DEXA) (Lunar DPX-NT; Hologic, Bedford, Mass) of L1-L4. Osteoporosis was defined as a total t-score of −2.5 or less, in accordance with the World Health Organization.7 Specimens were examined with CT (Aquilion CT 16; Toshiba, New York, NY) at 1-mm intervals to rule out pre-existing fracture, instrumentation, and pathologic lesions.
The specimens were kept frozen at −20°C in double bags until the time of use. Each specimen was defrosted at room temperature for 12 hours before preparation for testing. The spine of each specimen was transected through the L1-L2 disk, leaving L2-L5 attached to the pelvis. The specimens were denuded of soft tissue, except for the ligaments and disks of the L2-L5 vertebrae and the sacrospinous and sacrotuberous ligaments of the pelvis. The pelvis was oriented in the standing position by aligning the anterior iliac crest with the symphysis in the vertical plane. A plumb line was used to check vertical alignment. The lumbar vertebrae L2-L4 were potted in a 4-inch-diameter polyvinyl chloride pipe with a polymethylmethacrylate (PMMA) cement (Fastray; Bosworth, Skokie, Ill). The specimen then was mounted on a servohydraulic testing machine (8500; MTS, Eden Prairie, Minn). Impressions of the ischial tuberosities were made by using the PMMA in aluminum trays to distribute the reaction force through the tuberosities.
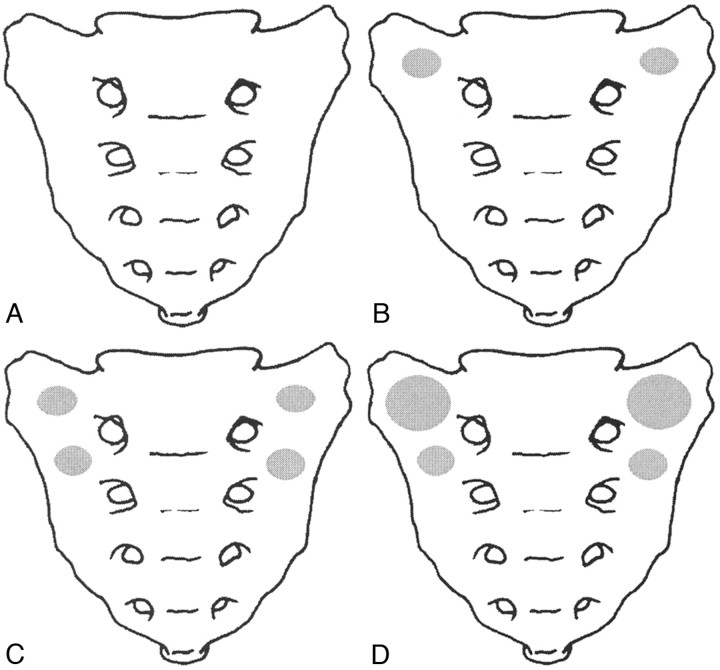
Simulated sacral insufficiency fractures were then produced as described by Waites et al.6 Specimens were loaded axially through the spine at 1 mm/s, until a fracture was observed in the sacrum or a sudden decrease in reaction load was noted from the load-versus-deformation trace. The test was stopped immediately on recognition of a fracture, and the fracture load was recorded. A digital video recording (ZR800; Canon USA, Jamesbury, NJ) of the anterior aspect of each specimen was made during loading to aid in identifying the onset and location of the fracture. An axial load was applied to each specimen during CT scanning to confirm that a fracture had been produced.6 The fracture position, fracture pattern, existence of air within the bone, and location of cortical disruption were recorded. After verification of the presence of fractures via CT scans, the specimens were randomly assigned to 4 groups (Fig 1): group 0 + 0 (n = 6, control), no cement, no sacroplasty; group 3 + 0 (n = 6), sacroplasty via a posterior approach, 3 mL of a PMMA bone cement (SpinePlex; Stryker, Kalamazoo, Mich) injected bilaterally into the fracture site at S1; group 3 + 3 (n = 7), sacroplasty via a posterior approach, 3 mL of the same bone cement injected bilaterally into the fracture site at S1 and S2; and group 6 + 3 (n = 6), sacroplasty via a posterior approach, 6 mL of the same bone cement injected bilaterally into the fracture site at S1 and 3 mL injected bilaterally at S2.
Fig 1.
Schematic of the 4 sacroplasty groups. A, Group 0 + 0 (control), no sacroplasty, no cement. B, Group 3 + 0 (sacroplasty), 3 mL of bone cement injected bilaterally at S1. C, Group 3 + 3 (sacroplasty), 3 mL of bone cement injected bilaterally at S1 and S2. D, Group 6 + 3 (sacroplasty), 6 mL of bone cement injected bilaterally at S1 and 3 mL injected bilaterally at S2.
All sacroplasty procedures were performed in an identical manner. The specimen was placed prone, and the fluoroscopy beam was oriented parallel with the sacroiliac joint of the side to be treated. An 11-gauge bone-biopsy needle with a trocar was introduced into the fracture site in the ala of S1 or S2. The trocar was inserted through the posterior cortex of the sacrum at the level appropriate for the injection. Needle position was confirmed with inlet- and outlet-view fluoroscopy. A 3.5-mm screw was placed into the S1 neural foramina during needle positioning to assist in visualizing the foramina. The cement was mixed according to the manufacturer's instructions and injected into each side under fluoroscopic monitoring. Cement was injected slowly in an attempt to minimize extravasation. Once the cement set, the procedure was repeated on the contralateral side. Bilateral injections were conducted even if the specimen exhibited only a unilateral fracture. In this manner, the sacroplasty procedure was standardized to reduce experimental variables. Each specimen was again scanned via CT to document cement placement and any extravasation.
All specimens were then tested to failure on the MTS machine with the same loading paradigm as outlined previously and again scanned with CT to establish the mode of failure. CT images were reviewed by 2 orthopedic surgeons (A.M.R., S.C.M.), and the fracture position and pattern were recorded.
The effect of treatment on failure load and stiffness was checked with an analysis of variance with repeated measures. Restoration strength and stiffness were defined as the ratio of treated value divided by the initial value. We checked intergroup differences for significance (P < .05) by using a Tukey test and for a correlation between the t-score and failure load by using linear regression.
Results
The initial crush resulted in 13 bilateral and 12 unilateral vertical sacral fractures, all in Denis Zone 1.8 Three specimens also had horizontal fracture lines, 2 at S1 and 1 at S2. There were no significant differences among groups in terms of age, sex distribution, bone mineral attenuation, t-score, or fracture pattern (Table 1).
Table 1:
Specimen demographics
| Group | Age at Time of Death (yr) | Female/Male Ratio | Bone Mineral Attenuation (g/cm2) | t-Score | Fracture Pattern (unilateral/bilateral) |
|---|---|---|---|---|---|
| 0 + 0 (n = 6) | 84.2 | 5:1 | 0.65 | −3.61 | 3:3 |
| 3 + 0 (n = 6) | 82.4 | 4:2 | 0.62 | −3.62 | 2:4 |
| 3 + 3 (n = 7) | 78.14 | 5:2 | 0.64 | −3.57 | 3:4 |
| 6 + 3 (n = 6) | 84.33 | 4:1 | 0.54 | −4.36 | 4:2 |
Failure load and stiffness values for treated specimens were significantly lower than those in the intact state (Table 2). There was no correlation between bone mineral attenuation and failure load (R2 = 0.07). The strength restoration ratios and stiffness restoration ratios were not significantly different among treatment groups (Table 2).
Table 2:
Mean strength and gross stiffness values
| Group | Initial Failure Load (N)* | Treated Failure Load (N)* | Strength Restoration (%) | Initial Stiffness (N/mm)† | Treated Stiffness (N/mm)† | Stiffness Restoration (%) |
|---|---|---|---|---|---|---|
| 0 + 0 (n = 6) | 3349 | 1970‡ | 62 | 396 | 202‡ | 56 |
| 3 + 0 (n = 6) | 3044 | 1737 | 60 | 404 | 181 | 49 |
| 3 + 3 (n = 7) | 2488 | 1751 | 75 | 314 | 191 | 69 |
| 6 + 3 (n = 6) | 2692 | 1668 | 62 | 374 | 179 | 51 |
Standard error of the mean (SEM) = 234 N.
SEM = 39 N/mm.
Specimens in the control group, though not treated, underwent the same reloading protocols as those in the treated groups.
Among the 25 specimens, there were 26 injections of 3 mL of PMMA into the S1 ala, 12 injections of 6 mL into the S1 ala, and 26 injections of 3 mL into the S2 ala. Cement extravasation was seen in 15 of the twenty-six 3-mL S1 injections, 8 of the twelve 6-mL S1 injections, and 12 of the twenty-six 3-mL S2 injections. The location of the extravasations varied, and leakage from many of the injection sites occurred in multiple directions.
The failure mechanism of the sacroplasty was examined. All failures occurred at the bone-cement interface, and no failure was through the cement.
Discussion
We did not find a dose-response association between injected cement volume and restoration of strength and stiffness. The 3 treatment groups did not differ significantly from the control group. We had originally hypothesized that sacroplasty would result in stabilization (ie, restoration of strength and stiffness) so as to prevent painful micromotion, but we were unable to show that sacroplasty altered the biomechanics of the fractured sacrum. Recently, finite-element modeling has suggested that sacroplasty results in a reduction in fracture-site micromotion but does not affect overall stiffness.4 In our current study, we measured overall stiffness and could not discern any localized stiffening. Future studies are planned to obtain kinematic data on the anterior aspect of the pelvis in the area of the sacral ala to measure more precisely fracture-site motion.
Whatever stabilization may occur through sacroplasty, it seems not to be strongly related to the amount of cement injected. This result suggests that one might achieve appropriate stabilization with modest amounts (≤3 mL) of cement and thereby reduce the risk of extravasation. Pommersheim et al3 noted that sacral insufficiency fractures with gaping fracture lines may represent a contraindication to sacroplasty because of the high risk of extrusion into the soft tissues and surrounding structures. In our current study, the injection of 6 and 3 mL of cement led to an amount of extravasation that would not be acceptable clinically. Because our pelves were denuded of soft tissue, extravasation may have been more common than would be expected with the soft tissues intact.
Case series in the literature report good symptomatic relief after sacroplasty.1-3 The results of our current study, however, raise questions regarding the proposed mechanism by which sacroplasty reduces pain (eg, by reducing micromotion, increasing stiffness, and reducing strain). Sacroplasty may reduce pain by another mechanism, or it may be that our method of testing was not sensitive enough to differentiate between the control and sacroplasty groups. We used a simple load-to-failure test mimicking a fall onto the buttocks. Lumbar spine loads during upright standing are approximately 800 N9 but are reportedly <2.5 times body weight during walking.10 The postsacroplasty failure loads measured in the current study were approximately 2.5 times body weight and are on the order of what might be experienced during activities of daily living. We did measure the stiffness of the constructs to assess how sacroplasty affects the behavior of the sacrum at a submaximal load in the physiologic range, and again, we found no difference. This reduction of micromotion has been cited as a mechanism of action in finite-element analysis of sacroplasty.4,5 To assess whether this mechanism is the case in our cadaveric model, one needs to measure strain across the fracture site while the construct is being cyclically stressed at physiologic loads equal to those experienced during rehabilitation.
Conclusions
In conclusion, neither the amount nor the location of the cement seems to affect the restoration of strength to the sacrum in a model sacral insufficiency fracture. Future studies are needed to test whether sacroplasty may reduce micromotion at the fracture site, leading to pain relief.
Footnotes
S.C.M. was supported by the Dennis A. Jahnigen Career Development Award from the American Geriatrics Society and Atlantic Philanthropies.
References
- 1.Frey ME, DePalma MJ, Cifu DX, et al. Efficacy and safety of percutaneous sacroplasty for painful osteoporotic sacral insufficiency fractures: a prospective, multicenter trial. Spine 2007;32:1635–40 [DOI] [PubMed] [Google Scholar]
- 2.Garant M. Sacroplasty: a new treatment for sacral insufficiency fracture. J Vasc Interv Radiol 2002;13:1265–67 [DOI] [PubMed] [Google Scholar]
- 3.Pommersheim W, Huang-Hellinger F, Baker M, et al. Sacroplasty: a treatment for sacral insufficiency fractures. AJNR Am J Neuroradiol 2003;24:1003–07 [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson DE, Cotton JR. Mechanical analysis of percutaneous sacroplasty using CT image based finite element models. Med Eng Phys 2007;29:316–25. Epub 2006 May 24 [DOI] [PubMed] [Google Scholar]
- 5.Whitlow CT, Yazdani SK, Reedy ML, et al. Investigating sacroplasty: technical considerations and finite element analysis of polymethylmethacrylate infusion into cadaveric sacrum. AJNR Am J Neuroradiol 2007;28:1036–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waites MD, Mears SC, Mathis JM, et al. The strength of the osteoporotic sacrum. Spine 2007;32:E652–55 [DOI] [PubMed] [Google Scholar]
- 7.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO Study Group. World Health Organ Tech Rep Ser 1994;843:1–129 [PubMed] [Google Scholar]
- 8.Denis F, Davis S, Comfort T. Sacral fractures: an important problem—retrospective analysis of 236 cases. Clin Orthop Relat Res 1988;227:67–81 [PubMed] [Google Scholar]
- 9.Sato K, Kikuchi S, Yonezawa T. In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine 1999;24:2468–74 [DOI] [PubMed] [Google Scholar]
- 10.Cappozzo A. Compressive loads in the lumbar vertebral column during normal level walking. J Orthop Res 1984;1:292–301 [DOI] [PubMed] [Google Scholar]