Abstract
BACKGROUND AND PURPOSE: The very small size of cerebral aneurysms is considered to be one of the limitations for endovascular treatment, with a high risk for intraoperative rupture. We report on treatment of very small saccular ruptured cerebral aneurysms by coil embolization. All the cases were of 2-mm aneurysms with at least 1 of the dimensions being less than 2 mm.
MATERIALS AND METHODS: We performed retrospective analysis of 7 consecutive cases of very small aneurysms treated by coil embolization in our institution between July 2006 and April 2008.
RESULTS: 3D rotational angiography (3DRA) was found to be most accurate in the detection of these aneurysms; in 2 cases, 3DRA revealed the aneurysms after results on digital subtraction angiography (DSA) were considered to be negative. Coil embolization was successfully performed in 6 cases, whereas in 1 case, spontaneous thrombosis occurred after microcatheter placement. Complete (n = 5) or near complete (n = 2) immediate occlusion was seen. A single soft coil was used in all cases with the shortest available length. Balloon assistance was used in 3 cases. Although minimal coil projection in the parent vessel was seen in 3 cases, no untoward clinical complication was seen. Follow-up DSA and MR angiography in 4 patients demonstrated persistent occlusion (n = 3) or progressive thrombosis (n = 1) of the aneurysms. All of the patients with available follow-up are independent in day-to-day activities with a modified Rankin Score (mRS) of 0 or 1.
CONCLUSIONS: Coil embolization of very small ruptured cerebral aneurysms is feasible. Careful consideration of the technical issues in treatment of these cases is essential to achieve technical success while avoiding complications.
The International Subarachnoid Aneurysm Trial documented greater efficacy of endovascular treatment of ruptured aneurysms compared with clipping, whenever both the treatments were considered feasible.1 However, there are limitations to endovascular treatment, one being a very small size (<3 mm) of the aneurysm. These aneurysms were not evaluated in the International Subarachnoid Aneurysm Trial; therefore, we cannot extrapolate the results in favor of coiling to patients with these extremely small aneurysms. Endovascular treatment of these aneurysms is considered to be technically challenging, and high complication rates have been reported.2,3 Very few series in the English literature focus on the outcome of embolization of very small aneurysms. Suzuki et al4 reported on the endovascular treatment of aneurysms less than 3 mm in diameter, with a favorable neck-to-fundus ratio. We report consecutive cases of endovascular coil occlusion of very small cerebral aneurysms with at least one of the aneurysmal dimensions being less than 2 mm. We included cases with an unfavorable neck-to-fundus ratio in the study. We report our experience and discuss the technical issues in the diagnosis and treatment in this subset of very small aneurysms.
Materials and Methods
We retrospectively reviewed the aneurysms treated by the endovascular method in our center. The Institutional Review Board gave approval for the study. In this period, 129 aneurysms were treated in our institution in 120 patients, with most (n = 110) of the patients presenting with a subarachnoid hemorrhage (SAH). Whereas 119 aneurysms were treated with embolization, 10 were operated on. In our study, we included ruptured saccular aneurysms 2 mm or smaller, which measured less than 2 mm in at least 1 of the dimensions. Aneurysms with an unfavorable neck-to-fundus ratio were also included in the study. Patients with dissecting aneurysms or suspected pseudoaneurysms were excluded from the study. Seven patients fulfilled the above-mentioned criteria and were retrospectively analyzed. There were 3 men and 4 women with age ranging from 18 to 66 years (mean age, 40.5 years). The locations of the aneurysms were the posterior cerebral (n = 1), anterior communicating (n = 3), middle cerebral (n = 1), posterior communicating (n = 1), and anterior choroidal (n = 1) arteries. SAH was documented by CT scan in all of the patients. The World Federation of Neurological Surgeons (WFNS) classification was grade II in 3 patients, grade III in 3 patients, and grade IV in 1 patient. Five patients were treated within 3 weeks of SAH. One patient (Case 2) with an anterior communicating arterial aneurysm had a large intraventricular bleed at the time of presentation. She was treated with extraventricular drainage at another institution and made gradual recovery. More than a month after the bleed, she was referred to our hospital for treatment. Another patient (Case 1), a foreign national, had 2 episodes of SAH. Results of digital subtraction angiography (DSA) done in this patient's native country were considered to be negative. Hydrocephalus and bed sores along with systemic infection developed, and she came to our institution 6 months after the second episode. CT angiography (CTA) had been performed in 3 patients and detected the aneurysm in 2 of the patients. All of the procedures were performed on a single-plane angiography unit (dFA Axiom Artis; Siemens, Erlangen, Germany).
The embolization procedure was performed with the patient under general anesthesia. DSA was performed followed by 3DRA. The results on DSA were negative in 2 patients in whom the aneurysm was detected after 3DRA. Guided by the 3D images, we obtained additional DSA images to evaluate the morphologic features of the aneurysm. The measurement of the aneurysmal size was made on the 3D angiograms. Under roadmap guidance, a microcatheter (Excelsior SL 10; Boston Scientific, Natick, Mass) was carefully guided over a microguidewire (Agility 10; Cordis, Miami Lakes, Fla; Transcend 14 Soft Tip, Boston Scientific) into the aneurysm. A wire was never introduced into the aneurysm, and the catheter was shaped so that it pointed toward the aneurysm. The tip of the catheter was kept at the neck of the aneurysm, and, if needed, the catheter was navigated farther into the aneurysm over the first coil loop. Another angiogram was performed at this stage to evaluate aneurysmal opacification. In some cases, balloon assistance was used, particularly when the aneurysm was less than 2 mm in 2 of the dimensions so as to prevent prolapse of the coil loop. The balloon could have also proved to be useful in the event of aneurysmal rupture. The shortest available length of a soft coil was used. In one case, with the aneurysmal dimension less than 2 mm in all 3 planes, an attempt was made to make the loop smaller than 2 mm by giving a careful twist to the coil loop.
The coil placement was done very slowly, and subtle microcatheter manipulation was used to avoid tension build-up in the aneurysm and to allow the coil loop to form inside the aneurysm. If balloon assistance was used, the balloon inflation was varied during coil placement to allow for microcatheter movement. High magnification was useful to observe the coil movement inside the aneurysm. At the end of coil placement, the microcatheter was slowly withdrawn so as to give the coil enough space in the aneurysm. If the tip of the coil was seen projecting outside of the aneurysm, further manipulation was not attempted. Even if minimal contrast filling was seen, no additional coil placement was attempted.
Angiographic CT (DynaCT; Siemens, Erlangen, Germany) was done to exclude any hemorrhage. Thereafter, the microcatheter and the balloon were withdrawn. Low-molecular-weight heparin was given for 24 to 48 hours whenever the coil loop was seen to project into the parent vessel.
Results
Coil placement was successful in 6 cases, whereas in 1 case spontaneous thrombosis of the aneurysm occurred during the procedure, and the coil had to be withdrawn. Complete immediate aneurysmal occlusion was seen in 5 cases, with minimal residual filling in 2 cases. The residual filling, if seen, was seen in the center of the 2-mm coil loop. In 2 cases, minimal projection of the coil tip was seen into the parent artery before detachment and was considered to be acceptable. In 1 case, the coil tip prolapsed into the parent artery after detachment. No untoward consequences of coil prolapse were seen in these cases. In 1 patient, considerable resistance was experienced during coil placement, and it could not be completely inserted into the aneurysm. The coil was withdrawn, and repeated angiograms showed thrombosis of the aneurysm. Thromboembolic occlusion of a cortical branch of the anterior cerebral artery was seen in this case, probably because of displacement of the clot during coil manipulation in the thrombosed aneurysm. Retrograde filling of the artery was seen through the leptomeningeal collateral artery, and the patient did not have any clinical sequelae. All of the patients had unchanged clinical status after embolization. Six patients had a modified Rankin score (mRS) of 1, whereas 1 patient had a score of 3. Clinical follow-up was available in all patients except for the patient from another country. The follow-up ranged from 7 to 24 months, with a mean follow-up of 17.8 months and cumulative follow-up of 107 months. All of the patients with available follow-up are independent in day-to-day activities with an mRS of 0 or 1.
Follow-up DSA in 3 patients and MR angiography in 1 patient revealed complete occlusion of the aneurysm. Change in the configuration of the coil was seen in 2 patients. Minimal aneurysmal filling seen in 1 of the patients had completely disappeared.
Representative Cases
Case 1.
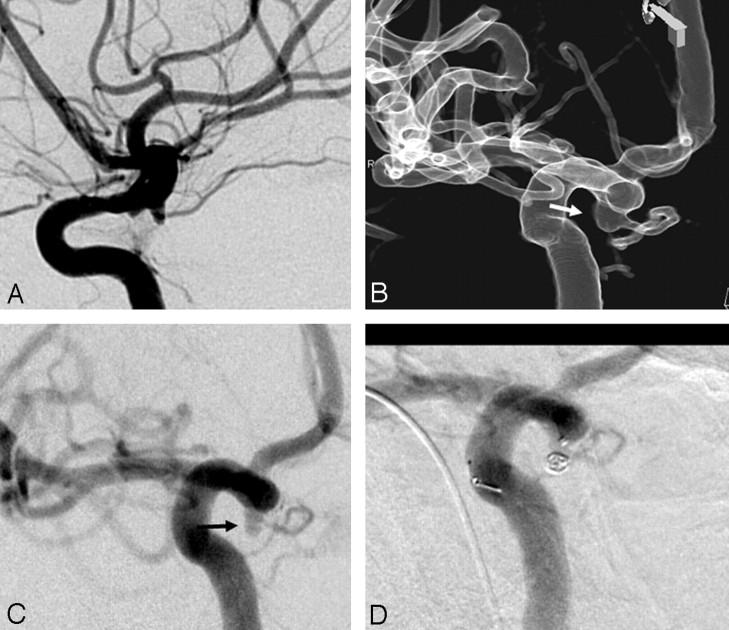
A 57-year-old woman presented with SAH 1 year previously. Results on DSA performed at another institution were reported to be normal, and she was treated conservatively. She had another hemorrhage and was referred to our institution. She had WFNS grade IV with marked hydrocephalus, bed sores, and systemic infections. Results on DSA revealed a small bulge at the origin of the right anterior choroidal artery (AchoA; Fig 1A). The 3DRA profiled the aneurysm in relationship with the AchoA (Fig 1B). The DSA in the same angulation revealed that the aneurysm had a narrow neck, which was not apparent in the 3D images (Fig 1C). The aneurysm measured 2 × 2 × 1.7 mm. Coil embolization was performed with a Trufill DCS Orbit 2 mm × 1.5 cm coil (Cordis), resulting in complete aneurysmal occlusion (Fig 1D). The patient made considerable neurologic recovery after treatment of her infections and bed sores and after undergoing ventriculoperitoneal shunt placement. She was a foreign national and has not returned for follow-up.
Fig 1.
A, ICA angiogram (lateral view). B, A 3D image profiling the aneurysm. C, DSA in the same angulation as the 3D image shows a very small aneurysm (arrow) and its relationship with the anterior choroidal artery (arrowhead). D, Postembolization DSA.
Case 2.
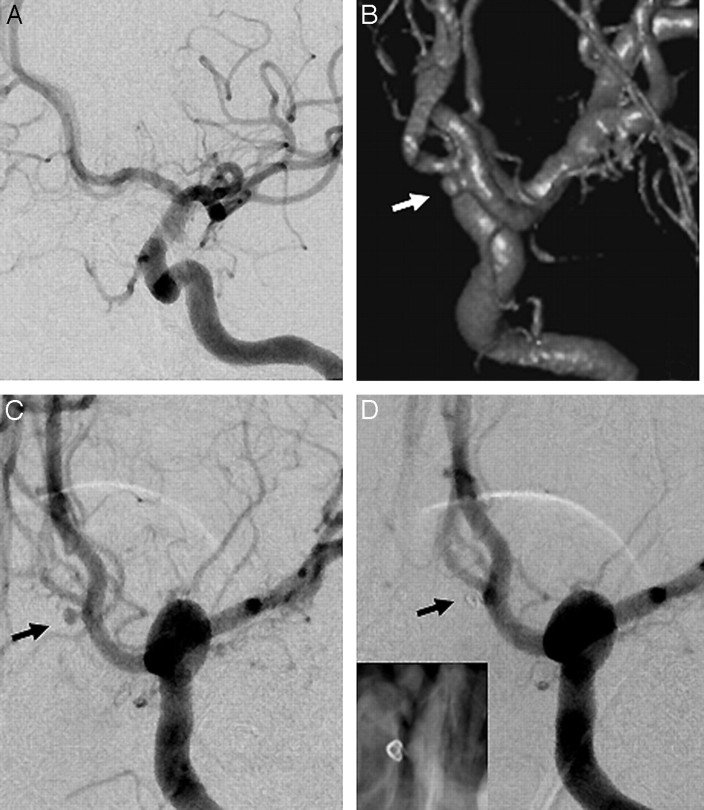
A 30-year-old woman presented with SAH 1 month previously. She was treated with ventricular drainage and slowly made an almost complete recovery. Results on DSA at another institution revealed a doubtful small aneurysm (Fig. 2A) near the junction of the anterior communicating artery and left anterior cerebral artery (ACA). CTA confirmed an aneurysm in the A1 segment of the left ACA. The 3DRA images clearly showed the aneurysm involving the posteromedial wall in the A1 segment of the left ACA (Fig 2B). DSA images in angulations shown by 3D images clearly profiled the aneurysm measuring 2 × 2 × 1.6 mm (Fig 2C). Embolization with a Guglielmi detachable coil (10 UltraSoft 2 mm × 1 cm; Boston Scientific) resulted in complete immediate thrombosis despite the appearance of “loose packing” (Fig 2D). MR angiography at 6-month follow-up revealed persistent occlusion of the aneurysm. At 24-month clinical follow-up, mRS was 0.
Fig 2.
A, DSA image shows a doubtful left anterior communicating aneurysm. B, 3DRA image clearly shows the aneurysm involving the posteromedial wall in the A1 segment of the left ACA (arrow). C, DSA image profiling the aneurysm. D, Postembolization DSA. Inset shows the coil loop.
Case 3.
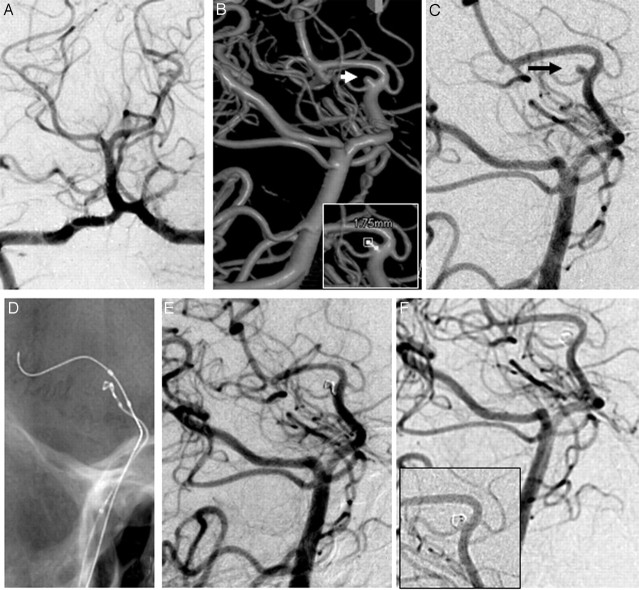
A 25-year-old woman presented with a 1-day-old SAH (WFNS grade I) in the left perimesencephalic cistern. Results on CTA were reported as normal. DSA was considered to be unremarkable (Fig 3A). 3DRA (Fig 2B) and DSA (Fig 3C) images in angulations as guided by 3D images revealed a very small (1.8 × 1.7 × 1.7 mm) aneurysm in the left posterior cerebral artery arising at the origin of the posterior choroidal artery. Balloon-assisted coil embolization was performed (Fig 3D,E) with a HyperSoft 2-mm × 1-cm coil (MicroPlex; MicroVention, Aliso Viejo, Calif). The coil loop was twisted to make it smaller than 2 mm. Postembolization DSA showed almost complete thrombosis of the aneurysm, with the coil end projecting into the parent vessel (Fig 3F). She was given low-molecular-weight heparin for 24 hours and made an uneventful recovery. Follow-up DSA after 6 months revealed a completely thrombosed aneurysm with change in the configuration of the coil (Fig 3G). The tip of the coil had now completely gone into the aneurysm. At 23-month clinical follow-up, mRS was 0.
Fig 3.
A, DSA image (anteroposterior view). B, 3D image shows a small aneurysm at the origin of the posterior choroidal artery. C, DSA in the same angulation as the 3D image. D, Coil embolization with balloon assistance. E, Postembolization DSA. F, Follow-up DSA (note the coil artifact in the inset image).
Case 4.
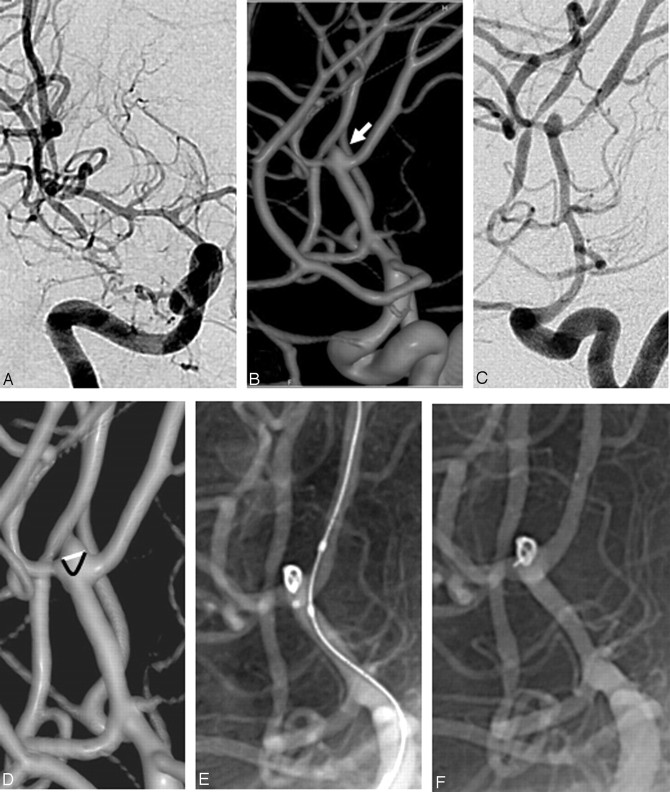
A 16-year-old boy presented with a 3-day-old SAH (WFNS grade II). DSA revealed vasospasm along with a doubtful aneurysm in the right middle cerebral artery (MCA) bifurcation (Fig 4A). 3DRA revealed a very small (2.2 × 2 × 1.6 mm) MCA bifurcation aneurysm with a broad neck (Fig 4B). The neck-to-fundus measurement was 1.6 mm, and it was decided to accept the coil bulge into the MCA bifurcation as illustrated in Fig 4D. We considered that the coil projecting into the vessel would reconstruct the MCA bifurcation, and this was considered to be the “true neck” of the aneurysm. Intra-arterial vasodilation was performed by injecting 1 mg of nimodipine (Nimodip; USV, Mumbai, India) through the guiding catheter for 30 minutes. Thereafter, balloon-assisted coil embolization was performed with a Trufill DCS Orbit 2-mm × 1.5-cm coil (Cordis), resulting in an almost complete occlusion of the aneurysm (Fig 4E). After detachment, the tip of the coil prolapsed into the upper division of the MCA (Fig 4E). No thrombus formation was seen, and the patient was extubated in intact neurologic condition. Low-molecular-weight heparin was given for 24 hours followed by low-dose aspirin (75 mg once a day) for 1 week. At 6-month follow-up, mRS was 0.
Fig 4.
A, DSA image (anteroposterior view). B, 3D image showing the aneurysm (arrow). C, DSA in the same angulation as the 3D image. D, 3D image, with the white line indicating the conventional concept of the aneurysmal neck and the black line indicating the aneurysmal neck considered by us, to allow a coil bulge so as to reconstruct the bifurcation. E, Angiogram before coil detachment shows a well-placed coil with the microcatheter tip outside the aneurysm. F, Angiogram after coil detachment.
Discussion
The International Study of Unruptured Intracranial Aneurysms demonstrated that in the absence of additional previously ruptured aneurysms, small aneurysms involving the anterior circulation only carry a 0.1% per-year risk for rupture.5 However, many experienced neurosurgeons and endovascular therapists report that most ruptured aneurysms encountered in practice are small.6 As seen in our study, aneurysms smaller than 2 mm can also result in an SAH and constituted 7% of ruptured aneurysms in our short experience. The incidence may have been higher in our study because some of the patients were referred after negative results on DSA or CTA.
In our study, 3DRA was most accurate in detection, analysis, and planning for endovascular therapy. CTA and DSA may not detect some of these aneurysms. Lack of sensitivity and interobserver variation of CTA in the detection of very small aneurysms has been seen in previous studies.7 This is related to the lower resolution of CTA compared with DSA.8 In our series, of the 3 cases in which CTA was performed, results were negative in 1 case, underlining the higher accuracy of 3DRA. 3DRA and DSA are of higher resolution than CTA and are better in the detection of aneurysms and in defining the morphologic features of the aneurysm. The relationship with adjacent small vessels (as in Case 1) is also likely to be more accurately delineated by conventional angiography. Work-up of SAH with CTA versus conventional angiography is a frequently debated topic and, as pointed out by Kallmes et al,8 CTA can result in false-negative results, and 3DRA probably remains the most accurate study in these patients. Van Rooij et al9 have also reported that very small aneurysms can be commonly detected by 3DRA despite negative findings on DSA. These very small aneurysms can be interpreted as the loop of the vessel or can be overlapped by normal arteries in conventional DSA (Case 3), and 3DRA is useful in these circumstances. However, in some of the cases, the true aneurysm morphology, particularly the neck size, was clearly delineated by the DSA performed according to the angulations shown by the 3D images (Case 1). The reason may be inadequate filling of the aneurysm or of higher resolution of conventional DSA compared with 3D imaging, which may be crucial in the assessment of very small aneurysms.
Surgical treatment of very small aneurysms poses a unique challenge because they are often thin-walled and may be too small to accept a clip without narrowing or tearing the parent vessel. In such cases, wrapping of the artery with muscle, Surgicel (Ethicon, Somerville, NJ), or muslin gauze, coating of the aneurysm with vinyl polymers or cyanoacrylate adhesives, and direct coagulation of the aneurysm have been variably described as treatment options.10-12 In a similar fashion, very small aneurysm sizes may limit endovascular options. Small size makes for challenging aneurysm catheterization, the risk for perforation by microcatheters that load and spring forward, and difficulty placing multiple coils.13 The small size of the aneurysm may be associated with a higher risk for rupture during embolization.2,3 Review of the English-language literature revealed only 1 study by Suzuki et al,4 who reported on endovascular treatment of very small aneurysms. However, they studied aneurysms of at least 3 mm with a favorable fundus-to-neck ratio of 1.5. Alternative endovascular options described include stent-graft placement, placing 2 overlapping stents, and use of liquid embolic agents.14 Stent grafts are usable for the intracranial internal carotid artery and for the V4 segment. The stiffness of the stent and the high expansion pressures are the 2 major drawbacks. By placing 1 stent inside the other, stent permeability can be reduced, which may result in significant hemodynamic changes with accelerated aneurysmal thrombosis.14,15 Deployment of multiple stents, however, may require several treatment sessions to allow for the integration of the stents into the vessel wall from session to session.14 In addition, the patient has to be prescribed antiplatelet devices for these stents, which can be a problem in cases with ruptured aneurysms, particularly because the timeframe for aneurysmal thrombosis is unpredictable. A regular microcatheter can block aneurysmal inflow in aneurysms with a very narrow neck. This may allow the occlusion of the aneurysm with an appropriate amount of highly concentrated, rapidly polymerizing glue. However, polymer emboli may result from excessive or rapid glue injection.14 We prefer to perform coil embolization in small aneurysms. Coil embolization is a tested method with favorable long-term results in the prevention of rebleeding. The technique also avoids the technical problems associated with other endovascular options. We studied cases of 2-mm aneurysms with 1 of the dimensions being less than 2 mm. The smallest coil available during the period of study was 2-mm diameter, and the treatment dynamics change considerably in such cases compared with aneurysms larger than 2 mm. We observed that the embolization technique may need modification depending on whether 1 (Cases 1, 2) or more (Case 3) dimensions were less than 2 mm.
A major limitation of the endovascular treatment of small aneurysms was the possibility of intraoperative rupture. Nguyen et al3 reported a fivefold increase in the incidence of rupture during endovascular treatment of aneurysms that were ≤3 mm compared with larger aneurysms. To prevent this complication, careful microcatheter placement at the neck of the aneurysm and use of the soft coil loop to enter the aneurysm were useful. The coil chosen was of shortest length of soft type to avoid excessive manipulation and tension build-up in the aneurysm. In fact, even if the coil tip was seen to protrude in the parent vessel, it was considered to be acceptable; therefore, forceful or repeated manipulations were avoided (Case 3).
Balloon placement can be useful to control hemorrhage in the event of rupture. Nguyen et al3 also observed that among cases with procedure-related rupture, inflation of a compliant balloon was associated with better outcome. However, manipulation of the balloon had to be done with care so as to prevent sudden movement of the microcatheter tip. The balloon inflation was partial and varied during the coil placement so as to allow for microcatheter movement, except when the coil tip or loop tended to prolapse out of the aneurysm. Lim et al16 have studied the structural limitations of currently available microcatheters and coils for endovascular coiling of very small aneurysms. They observed that the lengths of the detachment zone, which is known to be a stiff segment, of the currently available coils were approximately 0.5 to 0.8 mm, and the distance between the distal end of the distal markers of the microcatheters and the detachment zone of the coil ranges from approximately 1.2 to 2.8 mm. Therefore, to prevent rupture of very small aneurysms during coiling, the distal marker of the selected microcatheter preferably should be located near the aneurysmal neck. At the end of coil placement, slow withdrawal of the microcatheter can help in avoiding any potential injury from the relatively stiff detachment zone. Refinement of currently available devices may be essential to achieve safer coiling of very small aneurysms.
Another issue is retention of the coils in such small aneurysms. Balloon assistance was of considerable help, particularly when the aneurysm was smaller than 2 mm so as to retain the coil loop inside the aneurysm (Case 3). If only 1 dimension of the aneurysm were less than 2 mm, we believed that the aneurysm could accommodate the coil. However, in cases of 2 or all 3 dimensions being less than 2 mm, the coil loop did not form inside the aneurysm; therefore, twisting the coil loop to make it smaller than 2 mm or balloon assistance to form the coil loop was useful (Case 3). In cases of bifurcation aneurysm, the coil could be allowed to bulge into the parent artery so as to reconstruct the arterial bifurcation (Case 4). As seen in our study, these aneurysms did not have to be packed densely so as to occlude them. Placement of 1 or 2 coil loops resulted in thrombosis of the aneurysm despite apparent loose packing (Case 2) and was considered to be favorable to other endovascular alternatives, such as stent placement. Goddard et al13 also reported on the use of a single coil in the treatment of small aneurysms with reasonable long-term stability in their series. Because modern technology is helping us to detect and treat such small ruptured aneurysms, we perhaps need coils smaller than 2 mm in diameter. In fact a coil of 1.5-mm diameter has been recently introduced (Axium; ev3, Irvine, Calif) and may help us to treat such very small aneurysms.
Drawbacks of our study included a small sample size and lack of angiographic follow-up in some of the cases. Some of these aneurysms may be partially thrombosed, and this may explain the change in coil configuration as seen in follow-up angiography (Case 3). Partially thrombosed aneurysms are more likely to recur, and perhaps a long-term follow-up is needed to be certain of the durability of coil occlusion in these cases. In general, the mean age of 40.5 years in our series was lower than most series of aneurysms. This may have biased the results in favor of a positive outcome because anatomic features such as atherosclerosis or extreme tortuosity were absent in the younger age group, favoring precise catheter manipulation. The difficulty of the cases and the chance of complication or rupture are probably much higher in patients who are older and harbor these comorbidities. In view of technical issues in endovascular treatment, clipping remains a reasonable treatment option in these cases. In general, a head-to-head comparison of clipping and coiling would need to be performed before we can routinely recommend coiling of the aneurysms.
Conclusions
Very small (2 mm or smaller) saccular aneurysms can be treated by endovascular coil embolization. 3DRA is most useful in the detection and treatment planning in these cases. Use of short, soft coils and balloon assistance is also useful. In view of the possibility of intraoperative rupture and coil prolapse, careful consideration of the technical issues in the treatment of very small aneurysms is useful to achieve success while avoiding complications.
Footnotes
Previously presented as a short lecture at: Vascular Leaders Summit 2008 in Bangkok, Thailand, June 20–23, 2008.
References
- 1.Molyneux AJ, Kerr RS, Yu LM, et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005;366:809–17 [DOI] [PubMed] [Google Scholar]
- 2.Sluzewski M, Bosch JA, van Rooij WJ, et al. Rupture of intracranial aneurysms during treatment with Guglielmi detachable coils: incidence, outcome, and risk factors. J Neurosurg 2001;94:238–40 [DOI] [PubMed] [Google Scholar]
- 3.Nguyen TN, Raymond J, Guilbert F, et al. Association of endovascular therapy of very small ruptured aneurysms with higher rates of procedure-related rupture. J Neurosurg 2008;108:1088–92 [DOI] [PubMed] [Google Scholar]
- 4.Suzuki S, Kurata A, Ohmomo T, et al. Endovascular surgery for very small ruptured intracranial aneurysms. Technical note. J Neurosurg 2006;105:777–80 [DOI] [PubMed] [Google Scholar]
- 5.Wiebers DO, Whisnant JP, Huston J 3rd, et al. International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003;362:103–10 [DOI] [PubMed] [Google Scholar]
- 6.Weir B, Disney L, Karrison T. Sizes of ruptured and unruptured aneurysms in relation to their sites and ages of the patient. J Neurosurg 2002;96:64–70 [DOI] [PubMed] [Google Scholar]
- 7.Lubicz B, Levivier M, François O, et al. Sixty-four-row multisection CT angiography for detection and evaluation of ruptured intracranial aneurysms: interobserver and intertechnique reproducibility. AJNR Am J Neuroradiol 2007;28:1949–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kallmes DF, Layton K, Marx WF, et al. Death by nondiagnosis: why emergent CT angiography should not be done for patients with subarachnoid hemorrhage. AJNR Am J Neuroradiol 2007;28:1837–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Rooij WJ, Sprengers ME, de Gast AN, et al. 3D rotational angiography: the new gold standard in the detection of additional intracranial aneurysms. AJNR Am J Neuroradiol 2008;29:976–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujiwara S, Fugii K, Nishio S, et al. Long-term results of wrapping of intracranial ruptured aneurysms. Acta Neurochir (Wien) 1990;103:27–29 [DOI] [PubMed] [Google Scholar]
- 11.Yashon D, White RJ, Arias BA, et al. Cyanoacrylate encasement of intracranial aneurysms: technical note. J Neurosurg 1971;34:709–13 [DOI] [PubMed] [Google Scholar]
- 12.Nussbaum ES, Erickson DL. The fate of intracranial microaneurysms treated with bipolar electrocoagulation and parent vessel reinforcement. Neurosurgery 1999;45:1172–75 [DOI] [PubMed] [Google Scholar]
- 13.Goddard JK, Moran CJ, Cross DT 3rd, et al. Absent relationship between the coil-embolization ratio in small aneurysms treated with a single detachable coil and outcomes. AJNR Am J Neuroradiol 2005;26:1916–20 [PMC free article] [PubMed] [Google Scholar]
- 14.Henkes H, Reinartz J, Preiss H, et al. Endovascular treatment of small intracranial aneurysms: three aternatives to coil occlusion. Minim Invasive Neurosurg 2006;49:65–69 [DOI] [PubMed] [Google Scholar]
- 15.Doerfler A, Wanke I, Egelhof T, et al. Double-stent method: therapeutic alternative for small wide-necked aneurysms. Technical note. J Neurosurg 2004;100:150–54 [DOI] [PubMed] [Google Scholar]
- 16.Lim YC, Kim BM, Shin YS, et al. Structural limitations of currently available microcatheters and coils for endovascular coiling of very small aneurysms. Neuroradiology 2008;50:423–27 [DOI] [PubMed] [Google Scholar]