Abstract
BACKGROUND AND PURPOSE: To minimize diagnostic confusion, a CSF specimen should be free from traumatically introduced red blood cells (RBCs). The purpose of this research is to determine if patient age, sex, gauge of the lumbar puncture (LP) needle, or the level of LP is associated with an increased risk for traumatic fluoroscopy-guided LP.
MATERIALS AND METHODS: Data were collected retrospectively for consecutive male and female patients of all ages (n = 756) who underwent a fluoroscopy-guided LP for a 2-year period. We defined traumatic LP as a CSF sample with an RBC count more than 500 cells/mm3 without xanthochromia.
RESULTS: Rate of traumatic LP was 13.3%. The rate of traumatic LP at the L4-L5 level (19%) was significantly higher than at the L2-L3 (9%) or L3-L4 level (10%). Patients older than 80 years had higher traumatic LP rates (25.9%) compared with patients between ages 11 and 80 years (12.4%). Sex and gauge of the spinal needle were not associated with increased rate of traumatic LP. Patients younger than 1 year had failed LP rate of 58.8% compared with 3.2% failure rate in older patients.
CONCLUSIONS: Fluoroscopy-guided LP at the L4-L5 level is associated with nearly twice the risk for traumatic puncture compared with the L2-L3 or L3-L4 level. Rates of traumatic result are twice as high in adults older than 80 years compared with younger patients. Failure rates for fluoroscopy-guided LP are low except in children younger than 1 year, in whom failure occurs in most cases.
Fluoroscopy-guided lumbar puncture (LP) is a commonly requested radiologic procedure, usually in the setting of a difficult or failed bedside LP attempt. Failure rates for conventional bedside LP have been estimated to range from 16% to 35%.1 Compared with the conventional bedside approach, there is a higher success rate of obtaining CSF with fluoroscopic guidance because of the ability to visualize important bony landmarks and to avoid any adjacent obstructing degenerative bony changes. In theory, using fluoroscopic guidance should also minimize soft tissue trauma by reducing the number of passes needed to obtain CSF. An ideal CSF specimen should be free from red blood cells (RBCs) introduced as a result of the procedure, particularly in the setting of ruling out subarachnoid hemorrhage (SAH) or in the initial work-up for leukemia. Recent literature suggests that traumatic LP at diagnosis adversely affects the outcome of childhood acute lymphoblastic leukemia.2 A traumatic spinal puncture can also alter the CSF cell count, increase the CSF protein level, and alter culture and cytologic results, thereby creating diagnostic confusion.3 The cause of traumatic puncture is unknown but could result from incidental perforation of the ventral or dorsal epidural venous plexus or cauda equina vessels.4 Conventional bedside LPs have an estimated rate of traumatic puncture ranging from 10.5% to 20% on the basis of chosen CSF RBC cutoff values.5 The rate of traumatic fluoroscopy-guided LP has been estimated to range from 0% to 24%, with rates varying on the basis of the experience and training of the operator.6 The purpose of this research is to determine if the factors of patient age, sex, gauge of the LP needle, or spinal level of LP is associated with an increased risk for traumatic fluoroscopy-guided LP.
Materials and Methods
Data were collected retrospectively for consecutive male and female patients of all ages who had a fluoroscopy-guided LP between January 1, 2005, and December, 31, 2006, at our university, children's hospitals, and private and county hospitals. The most common indications for fluoroscopy-guided LP at our institutions were headache and/or fever, altered mental status, or central nervous system (CNS) malignant tumor/intrathecal therapy for a malignant process. We compiled patient lists electronically by using the Radiology Information System/PACS at the hospitals. We collected the CSF results by using the electronic patient medical record system. Data including the patient's age, sex, gauge of the spinal needle, level of the lumbar spinal puncture, CSF gross appearance, and CSF laboratory analysis results were entered into an Excel (Microsoft, Redmond, Wash) spreadsheet. CSF RBC count in the last available series of tubes was recorded. All fluoroscopy-guided LPs were performed by members of the radiology department including residents (PGY-2–PGY-5), fellows, and staff. All of the fluoroscopy-guided LPs were performed with Quincke-type beveled tip spinal needles. Patients who had a LP without fluoroscopy were excluded. We obtained institutional review board approval for this study.
Data were analyzed with commercially available software (State View; Abacus Concepts, Berkeley, Calif). We assessed statistical significance by calculating χ2 tests of contingency and 95% confidence intervals for each difference. A 2-tailed P value of .05 or less was considered to indicate statistically significant result for the test. There is no well-established guideline regarding what constitutes a traumatic LP; in particular, there is no specific RBC count within the CSF, which is always indicative of a traumatic CSF tap.7 For purposes of stratifying the CSF samples as would be done in clinical practice, we created 3 categories of CSF on the basis of gross appearance and RBC count: 1) CSF RBC count below 500 cells/mm3 was considered nontraumatic, 2) CSF RBC count at or more than 500 cells/mm3 with xanthochromia or when the clinical indication was concern for SAH were considered probable SAH, and 3) all other cases with CSF RBC count at or more than 500 cells/mm3 were considered traumatic.
Results
A total of 756 fluoroscopy-guided LPs performed between January 1, 2005, and December 31, 2006, met the inclusion criteria. There were 334 male and 422 female patients. The average age of the patients was 43.5 years (age age, <1 year to 90 years). The top 3 indications for fluoroscopic LPs were headache and/or fever (52%), altered mental status (19.9%), and malignant tumor involving the CNS (16.6%). Of the 756 cases, 14 had gross descriptions of the CSF obtained but had no CSF laboratory analysis (ie, for some reason, CSF was not sent to the laboratory, was mislabeled, or was not analyzed because of equipment malfunction). Because these cases were not failed attempts but did not include any CSF RBC counts, they were excluded from analysis, leaving 742 as the total number of evaluable cases.
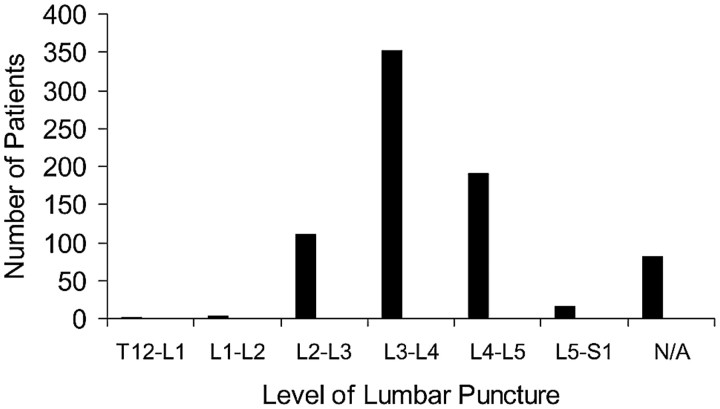
There was an overall 23.7% positive rate of CSF findings (CSF RBC count ≥500 cells/mm3 or xanthochromia or infection). LP levels for all cases are indicated in Fig 1. The most commonly accessed lumbar levels during spinal puncture were L2-L3 (n = 111), L3-L4 (n = 353), and L4-L5 (n = 190). The level of LP was not documented for 80 of the fluoroscopy-guided LP cases. The 2 most common lumbar spinal needle gauge sizes were 20 gauge (n = 249) and 22 gauge (n = 460). Other gauges of lumbar spinal needles including 18-, 19-, 21-, 23-, and 25-gauge needles were not used in sufficient numbers to be able to determine statistical significance. There were 27 cases without documentation of the gauge of the LP needle.
Fig 1.
The x-axis represents the level of the fluoroscopy-guided LP. The y-axis represents the total number of patients who had a spinal puncture at the corresponding level.
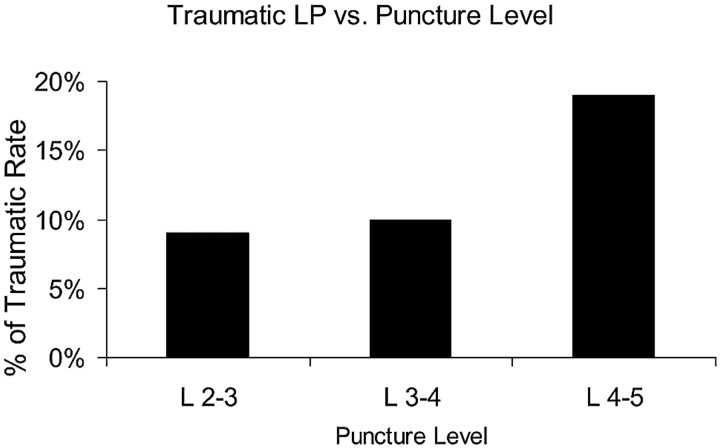
A total of 99 of 742 punctures were traumatic (13.3%). When subgrouped by the 3 most commonly accessed LP levels, traumatic rates were 10 of 111 (9%) at the L2-L3 level, 35 of 353 (10%) at the L3-L4 level, and 36 of 190 (19%) at the L4-L5 level (Fig 2). The higher traumatic LP rate at the L4-L5 level was statistically significantly higher than at the L2-L3 and L3-L4 levels (P < .005).
Fig 2.
The x-axis represents the level of the LP. The y-axis represents the percentage of cases with a traumatic fluoroscopy-guided LP at the corresponding level.
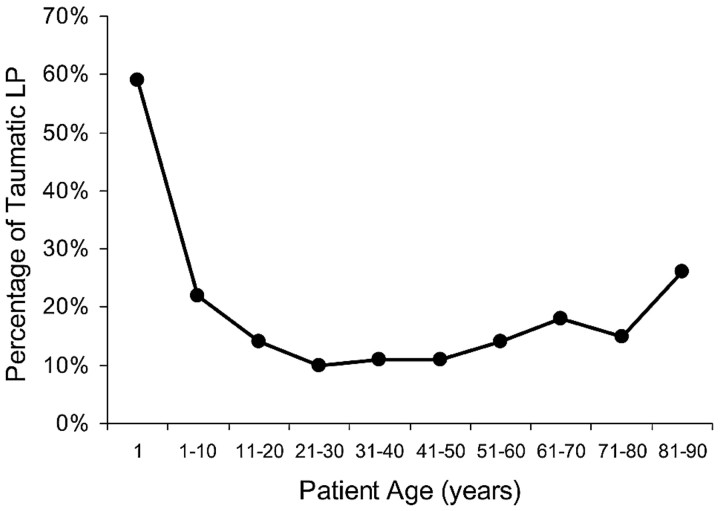
Figure 3 shows average rates of traumatic LP by patient age, with patients subgrouped by decade. Patients older than 80 years had a mean traumatic rate of 25.9% (7/27), which was statistically significantly higher than the average rate of 12.4% (84/678) for patients aged 11 to 80 years (P < .05). However, patients younger than 11 years had an average traumatic rate of 21.6% (8/37), which was not statistically significantly different from the rate for patients between ages 11 and 80 years (P > .10).
Fig 3.
Graph of the percentage of traumatic fluoroscopy-guided LP as a function of age. The x-axis represents the age groups by decade. The y-axis represents the percentage of traumatic spinal punctures for the corresponding age group.
Neither needle gauge (20-gauge vs 22-gauge) nor patient sex was associated with a statistically significant increased risk for traumatic LP (P > .05). There were 33/742 failed fluoroscopy-guided LP attempts (4.4%) in which CSF was not obtained. When subgrouped by patient age, failure rate for patients aged 1 year or older was 58.8% (10/17), compared with failure rate for patients older than 1 year of 3.2% (23/725), with an odds ratio of 47 (95% confidence interval, 16–134). There were 13 of 742 cases ruled as likely SAH (1.8%).
Discussion
Fluoroscopy-guided LP is one of the most common procedures performed by neuroradiologists. A retrospective review of fluoroscopy-guided LPs was undertaken to determine any factors that contribute to a higher rate of traumatic puncture. There have been multiple studies about conventional bedside (non–fluoroscopy-guided) LPs, citing traumatic puncture rates that range from 10.5% to 20% depending on the CSF RBC count cutoff values used and various patient populations studied.5,8 However, there is a paucity of literature regarding similar traumatic rates with fluoroscopy-guided LPs. One study determined the frequency of traumatic fluoroscopy-guided LPs to be from 3.5% to 11.6% on the basis of CSF RBC count cutoff values of more than 1000 cells/mm3 and more than 400 cells/mm3, respectively.6 The 13.3% traumatic rate in our study using a CSF RBC count cutoff value of 500 cells/mm3 as well as xanthochromia and clinical presentation is similar to their 11.6% rate with the CSF RBC count cutoff value of 400 cells/mm3. Further comparison between studies is problematic because different variables were considered for each study. The previous study did not include details about the fluoroscopy-guided LP procedure itself such as needle gauge or level of puncture but, instead, focused primarily on how the level of training among the radiologists (whether residents, fellows, or staff) had no significant effect on the CSF RBC count. In our study, we could not distinguish the level of training among the radiology operators (ie, PGY-2 through PGY-5 radiology residents or neuroradiology fellows or staff).
To stratify and categorize our CSF results, we established criteria to differentiate between nontraumatic and traumatic CSF results. Because there are no well-established guidelines as to what reliably constitutes a traumatic tap, previous studies have used qualitative methods (ie, visual inspection of the CSF for discoloration, clearing in later tubes) or quantitative RBC count levels ranging from 400 to 1000 cells/mm3.4,8 For our study, we created a categorization according to previous publications as well as clinical practice, with the intent to recreate clinical decision making to the greatest extent possible. Toward that end, we defined categories on the basis of CSF appearance and CSF RBC count, with some consideration of clinical scenario as well. Xanthochromia is widely viewed as strongly suggestive of SAH, so we categorized all cases with an RBC count 500 cells/mm3 or more and xanthochromia as probably SAH. For those without xanthochromia but with an RBC count 500 cells/mm3 or more, we considered clinical indication for the study because presentation and clinical scenario typically weigh heavily in interpretation of CSF results (eg, a child with suspected meningitis and multiple LP attempts at bedside with an RBC count 800 cells/mm3 is probably judged to be traumatic, whereas an adult with severe headache and an RBC count 800 cells/mm3 is probably judged to be SAH). Given our categorization, 13 of 742 cases (1.8%) were included in the probable SAH category. If this probable SAH category were not included, we would likely have underestimated the true incidence of SAH in our study population. Conversely, if we used a strict RBC count cutoff value, with higher numbers being considered as SAH, a larger number of our patient population would be classified as having SAH when they were clinically not considered to have this diagnosis. Of the 13 probable SAH cases in our study, 4 patients subsequently underwent diagnostic cerebral angiography, and 1 patient was found to have a right internal carotid terminus aneurysm. Given the small number of patients categorized as having probable SAH, it is unlikely that misclassification of traumatic versus probable SAH would have changed our results significantly.
Our study found that spinal punctures at the L2-L3 or L3-L4 level have less risk for traumatic puncture compared with the L4-L5 level. There does not seem to be a significant difference in the rate of traumatic lumbar spinal puncture between the L2-L3 or L3-L4 level. To our knowledge, no studies have evaluated the association between the level of LP and the rate of traumatic LP. Conventional bedside (non–fluoroscopy-guided) LP techniques recommend spinal punctures at the L3-L4 or L4-L5 interspaces. These levels are below the conus medullaris and are most easily determined by finding the top of the iliac crests and drawing an imaginary line (Tuffier line) across the top, thereby intersecting the L4 spinous process or the L4-L5 interspace.9–11 However, the bedside technique does not allow for confirmation of which level is being punctured. One study found that the intersection of a line joining the iliac crests coincided with the L4 spinous process (or the L4-L5 interspace) 78.6% of the time and the L3 spinous process (or the L3-L4 interspace) 3.7% of the time.12 Our data suggest that fluoroscopy-guided LPs performed at the L4-L5 level have nearly twice (19%) the risk for traumatic puncture as those performed at the L2-L3 (9%) or L3-L4 level (10%). Our study did not investigate the possible causes of this higher rate of traumatic puncture. However, we speculate that 1 factor may be degenerative change of the lumbar spine, which tends to be worse at the lower lumbar spinal levels than at the upper lumbar spinal levels.13,14 Increased incidence of spondylosis at lower lumbar spinal levels could make the fluoroscopy-guided LP procedure more difficult to perform, resulting in extra maneuvering and manipulation of the spinal needle to gain access into the lumbar spinal canal. Another factor may be slightly more abundant epidural fat and areolar tissue (which contains the venous plexus) at the triangular shaped epidural space at the L4-L5 level compared with the ovoid-shaped L2-L3 or L3-L4 level.15 A traumatic puncture could occur if the spinal needle is placed too far laterally or advanced too far anteriorly in the lumbar spinal canal, resulting in injury to the venous epidural veins.16 Another possible contributory factor may be that the L4-L5 level was accessed after failed attempts at other levels. At our institution, residents are generally trained to access the lumbar spinal canal below the L1-L2 level at the lumbar level, which appears most widely patent on fluoroscopic observation. It may be that the L4-L5 level was a second or third option after unsuccessful attempts at higher levels. However, we did not have reliable data from the radiology reports indicating if multiple attempts were made or if other lumbar levels were punctured before successful access.
Another interesting finding was the higher rate of traumatic punctures in older adults (>80 years old). There have been previous studies about geriatric patient populations and the safety of LPs, addressing the rates of postprocedural headaches or back pain.17,18 However, to our knowledge, no studies have evaluated whether there was a higher incidence of traumatic punctures in the geriatric population. In the older adults (>80 years old), the higher rate of traumatic puncture could be the result of a host of factors including advanced osteophytic changes, inability to cooperate with the procedure, or difficulty lying in the prone position. Almost 20% of the fluoroscopic-guided LPs in our study were performed on adult patients with altered mental status as the primary indication for the procedure, perhaps resulting in poor patient cooperation with the spinal puncture procedure. Older adults may also be taking medications that could predispose them to bleeding (ie, aspirin).
Pediatric patients (defined in our study population as ≤10 years old) had a higher rate of traumatic LP, though this finding did not reach statistical significance. A study by Howard et al3 identified risk factors associated with increased rates of traumatic conventional LPs such as African American race, age younger than 1 year, previous traumatic LPs within the past 2 weeks, and previous LP with a platelet count below 50,000. In our youngest population (<1 year old), we found a 25% rate of traumatic fluoroscopy-guided LP. Our failure rate in infants younger than 1 year was 58.8% (10/17) compared with patients older than 1 year, with a failure rate of 3.2% (23/725). We suspect that a higher failure rate in infants younger than 1 year may have been related to traumatic epidural or subdural collections as a result of previous failed conventional bedside LP. In infants, fluoroscopy only aids to confirm the position of the spinal needle tip within the center of the lumbar spinal canal but does little to help guide the spinal needle. The cause of increased rates of traumatic fluoroscopy-guided LP in children and infants is unclear but may be related to overinsertion of the lumbar spinal needle, leading to injury of the venous epidural plexus.19 Because of the high failure rate of fluoroscopy-guided LP in infants younger than 1 year, an alternative diagnostic test (ie, sonography-guided LP) should be considered in cases of failed bedside LP attempt.20
The sex of the patient and the gauge of the beveled-tip spinal needles were not significant factors contributing to an increased rate of traumatic LP. Previous studies have also found that sex had no effect on the proportion of traumatic bedside LPs.3 Only 20-gauge and 22-gauge lumbar spinal needles were used frequently enough to be included in our study. It may be that LPs with 18-gauge or 25-gauge needles may be associated with different risks for traumatic results.
Our study had several limitations. First, it was a retrospective study, so we relied on the accuracy and completeness of electronic medical records (EMR). Although we attempted to collect information about additional patient characteristics, such as body mass index, most cases did not have this information available in the EMR. Even with use of only the key parameters of patient sex, patient age, gauge of the needle, and level of access, multivariate analysis was not meaningful because 98 of our cases had some key data missing. Also, as mentioned above, reliable coagulation status was not available in the EMR for many of the patients in our study. However, it is standard practice at our institution to check coagulation profiles (routine cutoff values of >50,000 for platelet count and <1.5 for international normalized ratio) as well as to verify that the patient is not receiving warfarin (Coumadin), heparin, or enoxaparin (Lovenox) before undergoing fluoroscopy-guided LP. Therefore, it is likely that very few of our patients who underwent fluoroscopy-guided LP had abnormal coagulation profiles at the time of the procedure. Finally, we were unable to confirm the training or experience level of the operators for the LPs done in our study.
Conclusions
Our findings suggest that traumatic fluoroscopy-guided LPs are associated with the lumbar spinal level of puncture as well as the age of the patient. There is a higher rate of traumatic LP at L4-L5 level compared with the L2-L3 or L3-L4 level. There is also a higher rate of traumatic LP for patients older than 80 years. Most fluoroscopy-guided LP attempts in patients younger than 1 year are unsuccessful, and an alternative diagnostic test (ie, sonography-guided LP) should be considered rather than fluoroscopy-guided LP in this age group.
Acknowledgments
We thank Greg Jennings, MD, for assistance in obtaining institutional review board approval for this study as well as Melissa Tseng, MD, for editorial assistance.
Footnotes
Previously presented at: Annual Meeting of the American Society of Neuroradiology, June 3, 2008, New Orleans, La.
References
- 1.Thomas, SR, Jamieson, DR, Muir, KW. Randomised controlled trial of atraumatic versus standard needles for diagnostic lumbar puncture BMJ 2000;321:986–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gajjar A, Harrison PL, Sandlund JT. Traumatic lumbar puncture at diagnosis adversely affects outcome in childhood acute lymphoblastic leukemia. Blood 2000;96:3381–84 [PubMed] [Google Scholar]
- 3.Howard SC, Gajjar AJ, Cheng C, et al. Risk factors for traumatic and blood lumbar puncture in children with acute lymphoblastic leukemia. JAMA 2002;288:2001–07 [DOI] [PubMed] [Google Scholar]
- 4.Shah KH, Edlow JA. Distinguishing traumatic lumbar puncture from true subarachnoid hemorrhage. J Emerg Med 2002;23:67–74 [DOI] [PubMed] [Google Scholar]
- 5.Shah KH, Richard KM, Nicholas S, et al. Incidence of traumatic lumbar puncture. Acad Emerg Med 2003;10:151–54 [DOI] [PubMed] [Google Scholar]
- 6.Eskey CJ, Ogilvy CS. Fluoroscopy-guided lumbar puncture: decreased frequency of traumatic tap and implications for the assessment of CT-negative acute subarachnoid hemorrhage. AJNR Am J Neuroradiol 2001;22:571–76 [PMC free article] [PubMed] [Google Scholar]
- 7.Edlow JA, Bruner KS, Horowitz GL. Xanthochromia. Arch Pathol Lab Med 2002;126:413–15 [DOI] [PubMed] [Google Scholar]
- 8.Tourtellotte WW, Somers JF, Parker JA, et al. A study on traumatic lumbar punctures. Neurology 1958;8:129–34 [DOI] [PubMed] [Google Scholar]
- 9.Gorelick, PB, Biller, J. Lumbar puncture. Technique, indications, and complications. Postgrad Med 1986;79:257. [DOI] [PubMed] [Google Scholar]
- 10.Marton KI, Gean AD. The spinal tap: a new look at an old test. Ann Intern Med 1986;104:840–48 [DOI] [PubMed] [Google Scholar]
- 11.Boon JM, Abrahams PH, Meiring JH, et al. Lumbar puncture: anatomical review of a clinical skill. Clin Anat 2004;117:544–53 [DOI] [PubMed] [Google Scholar]
- 12.Render CA. The reproducibility of the iliac crest as a marker of lumbar spine level. Anaesthesia 1996;51:1070–71 [DOI] [PubMed] [Google Scholar]
- 13.Trouillier H, Birkenmaier C, Kluzik J, et al. Operative treatment for degenerative lumbar spinal canal stenosis. Acta Orthop Belg 2004;70:337–43 [PubMed] [Google Scholar]
- 14.Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg 1991;73:802–08 [PubMed] [Google Scholar]
- 15.Parkin IG, Harrison GR. The topographical anatomy of the lumbar epidural space. J Anat 1985;141:211–17 [PMC free article] [PubMed] [Google Scholar]
- 16.Mehl AL. Interpretation of traumatic lumbar puncture. Clin Pediatr 1986;25:523–26 [DOI] [PubMed] [Google Scholar]
- 17.Hindley NJ, Jobst KA, King E, et al. High acceptability and low morbidity of diagnostic lumbar puncture in elderly subjects of mixed cognitive status. Acta Neurol Scand 1995;91:405–11 [DOI] [PubMed] [Google Scholar]
- 18.Peskind ER, Riekse R, Quinn JF, et al. Safety and acceptability of the research lumbar puncture. Alzheimer Dis Assoc Disord 2005;4:220–25 [DOI] [PubMed] [Google Scholar]
- 19.Craig F, Stroobant J, Winrow A, et al. Depth of insertion of a lumbar puncture needle. Arch Dis Child 1997;77:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coley BD, Shiels WE, Hogan MJ. Diagnostic and interventional ultrasonography in neonatal and infant lumbar puncture. Pediatr Radiol 2001;6:399–402 [DOI] [PubMed] [Google Scholar]