Abstract
BACKGROUND AND PURPOSE: Visualization of the peripheral arteries on single-slab 3D time-of-flight (TOF) MR angiography (MRA) can reflect blood flow velocity. The velocity in the middle cerebral artery (MCA) may correlate with cerebrovascular reactivity (CVR) to acetazolamide, which can be used to assess hemodynamic impairment. The goal of this study was to compare the signal intensity of the MCA on MRA versus CVR quantified by perfusion single-photon emission CT (SPECT).
MATERIALS AND METHODS: The signal intensity of the MCA on single-slab 3D time-of-flight MRA was graded according to the ability to visualize the MCA in 108 cerebral hemispheres of 87 patients with unilateral or bilateral cervical internal carotid artery (ICA) steno-occlusive diseases. SPECT-CVR was also calculated by measuring cerebral blood flow before and after acetazolamide challenge. Ten healthy subjects were studied to obtain control SPECT-CVR values. All subjects provided written informed consent before the study.
RESULTS: CVR was significantly lower in cerebral hemispheres with reduced MCA signal intensity than in those with normal intensity (P < .05). When the reduced signal intensity of the MCA on MRA was defined as abnormal, and when a CVR less than the mean − 2 SD of healthy subjects was defined as reduced, MRA grading resulted in a 86.2% sensitivity and 69.6% specificity, with 51.0% positive-predictive and 93.2% negative-predictive values to detect reduced CVR.
CONCLUSIONS: This simple MRA method can assess hemodynamic impairment with a high negative-predictive value.
As local cerebral perfusion pressure falls, regional cerebral blood flow is maintained by the cerebrovascular autoregulatory mechanisms of precapillary resistance vessel dilation.1,2 Some vasodilatory property of vessels can be detected by measurement of cerebral blood flow at rest and after a vasodilatory stimulus, such as inhalation of carbon dioxide or administration of acetazolamide.3,4 Quantitative assessment of cerebrovascular reactivity (CVR) to acetazolamide can be used to assess the severity of hemodynamic impairment in patients with major cerebral artery occlusive disease.5,6 In fact, recent prospective studies have demonstrated that reduced CVR after acetazolamide administration can predict the risk for stroke recurrence in patients with symptomatic internal carotid artery (ICA) occlusive disease.7–9 Furthermore, patients with reduced CVR are at increased risk for cerebral hyperperfusion after carotid endarterectomy,10,11 which may result in intracerebral hemorrhage or cognitive impairment.12–14
Measurement of cerebral mean transit time is a sensitive method to estimate CVR to acetazolamide.15 Specifically, increased mean transit time values correlate with decreased CVR to acetazolamide, as determined by brain perfusion single-photon emission CT (SPECT).16,17 The cerebral mean transit time is also proportional to blood flow velocity in the middle cerebral artery (MCA).18,19 Thus, the MCA blood flow velocity may identify reduced CVR to acetazolamide.
MR angiography (MRA) with use of 3D time-of-flight (TOF) uses signals generated by the inflow of fresh, unsaturated, and fully magnetized blood spins into the slab.20,21 These spins are gradually saturated during movement within the slab. This saturation effect causes signal intensity loss in the peripheral arteries. The signal intensity loss from the saturation effect is flow velocity dependent and is more pronounced with lower flow velocity,22 particularly in single-slab 3D TOF MRA.21
The goal of our study was to compare the usefulness of MCA signal intensity on single-slab 3D TOF MRA versus CVR to acetazolamide measured by quantitative perfusion SPECT for the detection of cerebral hemodynamic impairment in patients with cervical ICA steno-occlusive diseases.
Materials and Methods
Patients
This study included 87 patients (3 women and 84 men) aged 52 to 81 years (mean age, 68 years) with steno-occlusive diseases in the cervical portion of the ICA. None of the patients had altered level of consciousness, restlessness, dementia, or cardiac failure. All patients underwent MRA at local hospitals with use of a 1.5T or less imager, which revealed preexisting lesions in all cases. Patients diagnosed with Moyamoya disease on MRA were excluded from our study. As per the North American Symptomatic Carotid Endarterectomy Trial,23 cervical MRA at our department with a 3T imager demonstrated more than 70% unilateral ICA stenosis in 60 patients, unilateral ICA occlusion in 6 patients, bilateral ICA stenoses in 19 patients, bilateral ICA occlusions in 1 patient, and unilateral ICA stenosis and contralateral ICA occlusion in 1 patient. Thus, this study included 108 cerebral hemispheres with ipsilateral cervical ICA steno-occlusive diseases and 66 cerebral hemispheres without lesions. Of cerebral hemispheres with lesions, 66 were associated with ischemic symptoms. Furthermore, transient ischemic attacks were present in 36 cerebral hemispheres, including 16 cerebral hemispheres with definite cerebral infarction and 20 cerebral hemispheres without definite cerebral infarction on conventional MR imaging. The remaining 30 cerebral hemispheres had minor complete strokes with definite cerebral infarction on MR imaging.
The study protocol was approved by the local ethics committee. All patients provided written informed consent before the study.
MRA Study
MRA was performed with a 3T imager (Signa Excite HD; GE Healthcare, Milwaukee, Wis). Axial single-slab 3D TOF MRA in the cervical portion was obtained at the carotid bifurcation with use of 5-inch surface coils (TR, 21 ms; TE, 3 ms; variable flip angle, 17–34° in the inferosuperior direction; matrix size, 512 × 256; FOV, 22 cm; section thickness, 1.2 mm; partition size, 60 with zero-fill interpolation [120 sections with 0.6-mm intervals]; NEX, 1; presaturation pulse above the slab, a fat suppression pulse, and a flow compensation; and acquisition time, 4 minutes 26 seconds). Then, axial single-slab 3D TOF MRA of the intracranial arteries, which was parallel to the anterior/posterior commissure line and was covered from the pontomedullary junction to the corpus callosum, was obtained with use of an 8-channel head coil (TR, 30 ms; TE, 3.7 ms; variable flip angles of 17–34° in the inferosuperior direction; matrix size, 512 × 320; FOV, 24 cm; section thickness, 1.0 mm; partition size, 90 with zero-fill interpolation [180 sections with 0.5-mm intervals]; NEX, 1; magnetization transfer pulse and flow compensation; and acquisition time, 6 minutes 22 seconds).
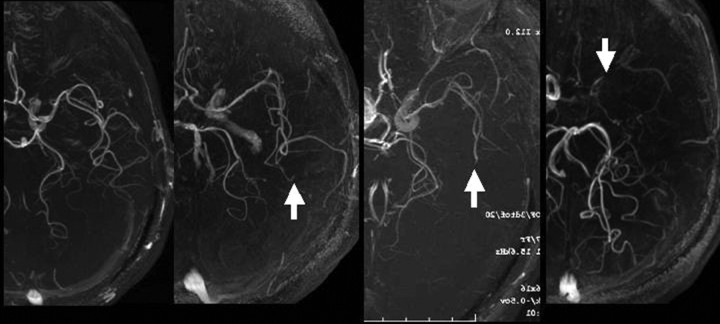
Signal intensity of the MCA in the intracranial MRA was visually classified into the following 4 grades according to the ability to visualize the MCA (Fig 1): all M3 branches of the MCA could be visualized to the cortical surface (grade A), 1 or more M3 branches could not be visualized to the cortical surface (grade B), 1 or more M2 branches could not be visualized along its course (grade C), and the M1 could not be visualized along its course (grade D).
Fig 1.
Degree of visualization of the ipsilateral MCA on brain MRA was graded as follows: all M3 branches of the left MCA could be visualized to the cortical surface (grade A), one M3 branch could not be visualized to the cortical surface (grade B, arrow), one M2 branch could not be visualized along its course (grade C, arrow), and the M1 could not be visualized along its course (grade D, arrow).
To assess interobserver variability of the MRA grading, 2 experienced neuroradiologists independently reviewed all MRA images (first, R.H.; and second, T.I.). In addition, the first observer reviewed the same images a second time, after a 3-month interval to account for the intraobserver variability of the study. Both observers were blinded to all patient information other than the images to be reviewed. Each observer was blind to the other's assessments and to their own previous assessments.
SPECT Study
Brain perfusion was assessed with use of iodine 123 N-isopropyl-p-iodoamphetamine (123I-IMP) and SPECT. We performed SPECT studies using a ring-type SPECT scanner, a Headtome-SET080 (Shimadzu, Kyoto, Japan), which provides 31 tomographic images simultaneously. The spatial resolution of the scanner with a low-energy, all-purpose collimator was 13 mm full width at half maximum (FWHM) at the center of the FOV, and the section thickness was 25 mm FWHM at the FOV center. Image sections were taken at 5-mm center-to-center spacing parallel to the orbitomeatal line. We reconstructed the images using the weighted-filtered backprojection technique, in which the attenuation correction was made by detecting the edge of the object. An attenuation coefficient of 0.065 cm−1, a Butterworth filter (cutoff point, 0.45 cycle/cm; order, 3) and a ramp filter were used for image reconstruction.
The 123I-IMP SPECT studies at the resting state and with acetazolamide challenge were performed as described previously.24 The cerebral blood flow images were calculated according to the 123I-IMP-autoradiography method.24,25 The same standard input function at the resting state was used in the calculation of cerebral blood flow with acetazolamide challenge. The Vd value was assumed to be 35 mL/mL in the calculation of cerebral blood flow images.
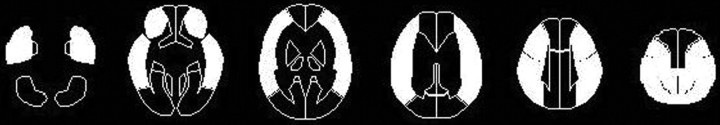
We transformed all SPECT images into the standard brain size and shape by linear and nonlinear transformation using SPM99 for anatomic standardization.26 Thus, the brain images of all subjects had the same anatomic format. Next, 318 constant regions of interest (ROIs) were automatically placed in both the cerebral and cerebellar hemispheres with a 3D stereotaxic ROI template.27 The ROIs were grouped into 10 segments (callosomarginal, pericallosal, precentral, central, parietal, angular, temporal, posterior, hippocampus, and cerebellum) in each hemisphere according to the arterial supply. Only 5 (precentral, central, parietal, angular, and temporal) of these 10 segments were summed and defined as an ROI perfused by the MCA (Fig 2). The mean cerebral blood flow value at the resting state and with acetazolamide challenge was measured in each MCA ROI. Then, CVR to acetazolamide was calculated as follows: CVR (%) = [(acetazolamide challenge cerebral blood flow - resting cerebral blood flow)/resting cerebral blood flow] × 100.
Fig 2.
Diagrams showing the ROIs of a 3D stereotaxic ROI template. The white ROIs (precentral, central, parietal, angular, and temporal segments) indicate territories perfused by the bilateral MCAs.
Using the same method, we studied 10 healthy subjects (8 men and 2 women; age, 35–65 years; mean age, 52.3 years) to obtain control values.24 The control values of CVR were 36.8 ± 9.2%, respectively. When the values of CVR were less than the mean minus 2 SD (ie, 18.4%), they were rated as reduced CVR. All MRA and SPECT studies were performed within 5 days.
Statistical Analysis
To determine the interobserver and intraobserver agreement of the MRA grading, we calculated the proportion of concordant assessments and κ statistics using data determined by the 2 observers. The proportion of concordant assessments was calculated as the number of concordant assessments divided by the total number of assessments. The κ values obtained were interpreted relative to the criteria of Fleiss: values 0.40 or less represent poor agreement, values between 0.40 and 0.75 represent fair to good agreement, and values more than 0.75 represent excellent agreement.28
Descriptive data were expressed as the mean ± SD. The first MRA grading assessments by the first observer were used for all of the following analyses. We examined differences of CVR to acetazolamide among the 4 MRA grades using 1-way analysis of variance followed by Scheffé multiple comparisons. Differences were deemed statistically significance if P was less than .05. We evaluated the incidences of reduced CVR to acetazolamide among the 4 MRA grades using the χ2 test followed by Bonferroni inequality correction. Differences between each MRA grade were deemed statistically significant if P was less than .05/6 = 0.0083. In addition, the grading accuracy of MRA to detect reduced CVR to acetazolamide was assessed with the receiver operating characteristic curve.
Results
The first observer at the first review assessed 108 cerebral hemispheres with lesions among 87 patients as MRA grades of A, (59) B, (26) C (16), and D (7). Interobserver [concordant rate = 0.93, κ = 0.88 (95% CI, 0.76–0.99)] and intraobserver [concordant rate = 0.95, κ = 0.93 (95% CI, 0.80–0.99)] agreements in MRA grading were excellent. Although the concordance rate of cerebral hemispheres with MRA grade A was 1.00 for both the interobserver and intraobserver agreements, the rates for the other MRA grades were less than 0.90, except for the intraobserver agreement in MRA grades C and D (Table). However, none of cerebral hemispheres with MRA grade B, C, or D was subsequently graded as A in any review by either observer. All 66 cerebral hemispheres without lesions among 87 patients were assessed as MRA grades of A in all reviews.
Concordance rates in interobserver and intraobserver agreements of MRA grading in 108 cerebral hemispheres with lesions
| First Review by First Observer |
Second Review by First Observer |
First Review by Second Observer |
|||||
|---|---|---|---|---|---|---|---|
| MRA Grade | Number of Hemispheres | MRA Grades | Number of Hemispheres | Concordance Rate in Intraobserver Agreement | MRA Grade | Number of Hemispheres | Concordance Rate in Interobserver Agreement |
| A | 59 | A | 59 | 1.00 | A | 59 | 1.00 |
| B | 0 | B | 0 | ||||
| C | 0 | C | 0 | ||||
| D | 0 | D | 0 | ||||
| B | 26 | A | 0 | 0.85 | A | 0 | 0.85 |
| B | 22 | B | 22 | ||||
| C | 4 | C | 4 | ||||
| D | 0 | D | 0 | ||||
| C | 16 | A | 0 | 0.94 | A | 0 | 0.88 |
| B | 1 | B | 1 | ||||
| C | 15 | C | 14 | ||||
| D | 0 | D | 1 | ||||
| D | 7 | A | 0 | 1.00 | A | 0 | 0.71 |
| B | 0 | B | 0 | ||||
| C | 0 | C | 2 | ||||
| D | 7 | D | 5 | ||||
Note:—MRA indicates MR angiography.
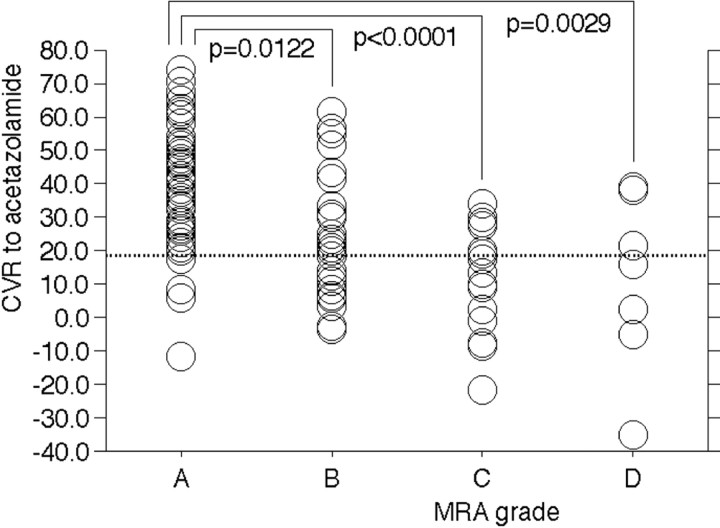
Figure 3 shows CVR to acetazolamide according to each MRA grade in 108 cerebral hemispheres with lesions. CVR was significantly lower in cerebral hemispheres with MRA grade B (24.4 ± 19.0%), C (11.5 ± 15.6%), or D (11.2 ± 26.3%) than in those with MRA grade A (38.4 ± 16.3%). However, there was no difference in hemispheric CVR to acetazolamide when comparing MRA grades B, C, and D. The incidences of reduced CVR to acetazolamide in cerebral hemispheres according to MRA grade were 6.8% (4/59) for A, 42.3% (11/26) for B, 62.5% (10/16) for C, and 57.1% (4/7) for D. Although the incidence was significantly higher in cerebral hemispheres with MRA grade B (P < .0001), C (P < .0001), or D (P = .003) than in those with MRA grade A, there was no difference when comparing MRA grades B, C, and D. None of the 66 cerebral hemispheres without lesions exhibited reduced CVR to acetazolamide.
Fig 3.
Comparison of CVR to acetazolamide among the 4 MRA grades in cerebral hemispheres with lesions.
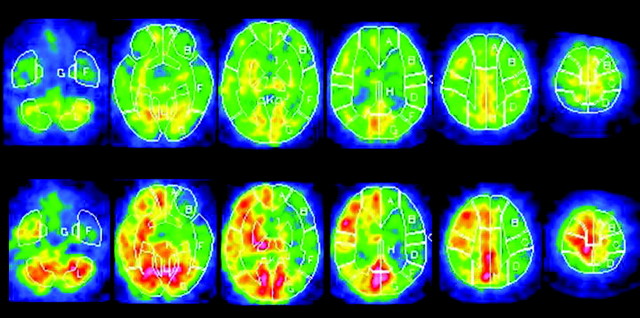
Sensitivity and specificity in the cutoff point lying closest to the left upper corner of the receiver operating characteristic curve in the detection of reduced CVR to acetazolamide in cerebral hemispheres with lesions were 86.2% and 69.6%, respectively, when the cutoff point was set between MRA grades A and B (grade A vs grade B, C, and D). Positive predictive values and negative predictive values were 51.0% and 93.2%, respectively. Figure 4 shows SPECT images in a patient with MRA grade C.
Fig 4.
A 70-year-old man with symptomatic left ICA stenosis (90%) exhibiting MRA grade C (Fig 1). Single-photon emission CT scan shows reduction of resting cerebral blood flow (upper images) and poor acetazolamide-induced increases in perfusion (lower images) in the left MCA territory.
Discussion
Our study demonstrates that a simple MRA method can assess hemodynamic impairment with a high negative predictive value. Although the signal intensity of the MCA in the MRA was visually graded with use of a subjective process, the interobserver and intraobserver agreements of these measurements were excellent.
The 123I-IMP-autoradiography method with SPECT used in our study accurately quantifies CVR and can adequately define subgroups of patients with reduced CVR.24 Furthermore, recent prospective studies have demonstrated that reduced CVR to acetazolamide measured by quantitative brain perfusion SPECT (defined by values less than the mean −2 SD of the CVR or 95% confidence limits obtained in control subjects) can predict the risk for stroke recurrence in patients with symptomatic ICA occlusive disease7,8 and the risk for cerebral hyperperfusion after carotid endarterectomy.11 The same definition of reduced CVR was used in this study.
While single-slab 3D TOF MRA used in our study is less appropriate than multiple overlapping thin-slab acquisition to show intracranial vessels,21 the signal intensity of the arteries displayed by the former method correlates with blood flow velocity.22 As a result, CVR to acetazolamide was significantly lower in cerebral hemispheres with reduced MCA signal intensity than in those with normal MCA signal intensity. Furthermore, the incidence of reduced CVR to acetazolamide was significantly higher in cerebral hemispheres with reduced MCA signal intensity than in those with normal MCA signal intensity.
Our study also determined the threshold of MRA grading required to detect hemispheres with reduced CVR. Assuming the SPECT-CVR as the true determinant of hemodynamic impairment, MRA grading was associated with a 93.2% negative predictive value for the detection of reduced CVR in cerebral hemispheres with lesions. The interobserver and intraobserver variability for MRA grading had a concordance rate of 1.00 in cerebral hemispheres with normal MCA signal intensity, and none of the cerebral hemispheres determined as having reduced MCA signal intensity were subsequently graded as having normal MCA signal intensity at any other time. In addition, all cerebral hemispheres without lesions and without reduced CVR to acetazolamide were assessed as MRA grades of A in all reviews. These properties support the usefulness of this MRA method as a screening test for hemispheric hemodynamic impairment.
Our study possessed several limitations. When the ICA is chronically occluded, the collateral blood flows via the anterior or posterior communicating arteries, or the leptomeningeal anastomosis often perfuses the occluded area, thereby maintaining normal hemodynamics. By contrast, longer paths of collateral blood flow result in higher saturation of inflowing spins. As a result, collateral blood flow signals are lost, and the affected MCA is not displayed in single-slab 3D TOF MRA. In addition, when 1 or more MCA branches are not visualized in the MRA, they may simply be occluded, and the degree of the visualization may not reflect the velocity of inflowing blood relevant to ICA steno-occlusive diseases. These phenomena may account for the low positive predictive value for the detection of reduced hemispheric CVR with use of the present method.
In our study, we used axial single-slab 3D TOF MRA of the intracranial arteries that was parallel to the anterior/posterior commissure line. Although the MRA sequence and method are unique, they are easily reproducible on most clinical 1.5T MR imagers. Thus, the present MRA methods may not be limited to particular institutions.
In contrast to the 3T MR imaging model used in our study, most institutions commonly use a 1.5T MR imager. Although use of a 1.5T MR imager for 3D TOF MRA results in inferior depiction of intracranial arteries because of lower spatial resolution and lower signal-to-noise ratio with decreased T1 relaxation time,29,30 MRA imaging is more sensitive and specific for decreased velocity of inflowing blood with a 1.5T MR imager than with a 3T MR imager. Thus, the use of a 1.5T MR imager may be suitable for the detection of hemodynamic impairment in conjunction with this method.
Although SPECT with an acetazolamide challenge is a reliable method to identify patients with hemodynamic impairment,7,8,10,11,24 the clinical use of SPECT is precluded by its high cost and limited availability. In addition, acetazolamide is associated with a variety of frequent adverse effects, including metabolic acidosis, hypokalemia, numbness of the extremities, headache, tinnitus, gastrointestinal tract disturbances, and Stevens-Johnson syndrome.31,32 Recent studies have demonstrated that the perfusion-weighted MR imaging-cerebral blood volume method also can identify patients with hemodynamic impairment.33,34 However, gadolinium-based contrast agents may be associated with the development of nephrogenic systemic fibrosis in the setting of renal insufficiency.35 By contrast, the present MRA method does not require administration of radioisotope or contrast agents, and its short scanning time is well suited for clinical screening tests.
Conclusions
Our study demonstrates that a simple MRA method can assess hemodynamic impairment with a high negative predictive value. When patients are diagnosed with hemodynamic impairment in the affected cerebral hemisphere by the present method, results should be confirmed with the use of positron- emission tomography, SPECT with an acetazolamide challenge, or perfusion-weighted MR imaging. Regardless, use of the present method as a screening test will eliminate the need for follow-up studies in nearly half of patients (45% in our study). However, the present method also failed to detect hemodynamic impairment in 7% of patients. Additional investigation regarding the relationship between the MRA grading system and the risk for stroke recurrence in patients with symptomatic ICA occlusive disease or the risk for cerebral hyperperfusion after carotid endarterectomy will be beneficial to determine the effect of false-negative results in the clinical setting.
References
- 1.Gibbs JM, Wise RJ, Leenders KL, et al. Evaluation of cerebral perfusion reserve in patients with carotid-artery occlusion. Lancet 1984;1:310–14 [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Raichle ME. Positron emission tomography and its application to the study of cerebrovascular disease in man. Stroke 1985;16:361–76 [DOI] [PubMed] [Google Scholar]
- 3.Ringelstein EB, Grosse W, Matentzoglu S, et al. Non-invasive assessment of the cerebral vasomotor reactivity by means of transcranial Doppler sonography during hyper- and hypocapnea. Klin Wochenschr 1986;64:194–95 [DOI] [PubMed] [Google Scholar]
- 4.Ringelstein EB, Van Eyck S, Mertens I. Evaluation of cerebral vasomotor reactivity by various vasodilating stimuli: comparison of CO2 to acetazolamide. J Cereb Blood Flow Metab 1992;12:162–68 [DOI] [PubMed] [Google Scholar]
- 5.Nemoto EM, Yonas H, Kuwabara H, et al. Identification of hemodynamic compromise by cerebrovascular reserve and oxygen extraction fraction in occlusive vascular disease. J Cereb Blood Flow Metab 2004;24:1081–89 [DOI] [PubMed] [Google Scholar]
- 6.Yamauchi H, Okazawa H, Kishibe Y, et al. Oxygen extraction fraction and acetazolamide reactivity in symptomatic carotid artery disease. J Neurol Neurosurg Psychiatry 2004;75:33–37 [PMC free article] [PubMed] [Google Scholar]
- 7.Kuroda S, Houkin K, Kamiyama H, et al. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it? Stroke 2001;32:2110–16 [DOI] [PubMed] [Google Scholar]
- 8.Ogasawara K, Ogawa A, Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: a xenon-133 single-photon emission computed tomography study. Stroke 2002;33:1857–62 [DOI] [PubMed] [Google Scholar]
- 9.Yonas H, Smith HA, Durham SR, et al. Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg 1993;79:483–89 [DOI] [PubMed] [Google Scholar]
- 10.Hosoda K, Kawaguchi T, Shibata Y, et al. Cerebral vasoreactivity and internal carotid artery flow help to identify patients at risk for hyperperfusion after carotid endarterectomy. Stroke 2001;32:1567–73 [DOI] [PubMed] [Google Scholar]
- 11.Ogasawara K, Yukawa H, Kobayashi M, et al. Prediction and monitoring of cerebral hyperperfusion after carotid endarterectomy by using single-photon emission computerized tomography scanning. J Neurosurg 2003;99:504–10 [DOI] [PubMed] [Google Scholar]
- 12.Piepgras DG, Morgan MK, Sundt TM Jr, et al. Intracerebral hemorrhage after carotid endarterectomy. J Neurosurg 1988;68:532–36 [DOI] [PubMed] [Google Scholar]
- 13.Ogasawara K, Yamadate K, Kobayashi M, et al. Postoperative cerebral hyperperfusion associated with impaired cognitive function in patients undergoing carotid endarterectomy. J Neurosurg 2005;102:38–44 [DOI] [PubMed] [Google Scholar]
- 14.Ogasawara K, Sakai N, Kuroiwa T, et al. Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg 2007;107:1130–36 [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Lee SJ, Shin T, et al. Correlative assessment of hemodynamic parameters obtained with T2*-weighted perfusion MR imaging and SPECT in symptomatic carotid artery occlusion. AJNR Am J Neuroradiol 2000;21:1450–56 [PMC free article] [PubMed] [Google Scholar]
- 16.Kikuchi K, Murase K, Miki H, et al. Quantitative evaluation of mean transit times obtained with dynamic susceptibility contrast-enhanced MR imaging and with (133)Xe SPECT in occlusive cerebrovascular disease. AJR Am J Roentgenol 2002;179:229–35 [DOI] [PubMed] [Google Scholar]
- 17.Hirano T, Minematsu K, Hasegawa Y, et al. Acetazolamide reactivity on 123I-IMP single photon emission computed tomography in patients with major cerebral artery occlusive disease: correlation with positron emission tomography parameters. J Cereb Blood Flow Metab 1994;14:763–70 [DOI] [PubMed] [Google Scholar]
- 18.Wardlaw JM, Dennis MS, Merrick MV, et al. Relationship between absolute mean cerebral transit time and absolute mean flow velocity on transcranial Doppler ultrasound after ischemic stroke. J Neuroimaging 2002;12:104–11 [DOI] [PubMed] [Google Scholar]
- 19.Naylor AR, Merrick MV, Slattery JM, et al. Parametric imaging of cerebral vascular reserve: 2. Reproducibility, response to CO2 and correlation with middle cerebral artery velocities. Eur J Nucl Med 1991;18:259–64 [DOI] [PubMed] [Google Scholar]
- 20.Marchal G, Bosmans H, Van Fraeyenhoven L, et al. Intracranial vascular lesions: optimization and clinical evaluation of three-dimensional time-of flight MR angiography. Radiology 1990;175:443–48 [DOI] [PubMed] [Google Scholar]
- 21.Davis WL, Blatter DD, Harnsberger HR, et al. Intracranial MR angiography: comparison of single-volume three-dimensional time-of-flight and multiple overlapping thin slab acquisition techniques. AJR Am J Roentgenol 1994;163:915–20 [DOI] [PubMed] [Google Scholar]
- 22.Kodama T, Watanabe K. Influence of imaging parameters, flow velocity, and pulsatile flow on three-dimensional time-of-flight MR angiography: experimental studies. Eur J Radiol 1997;26:83–91 [DOI] [PubMed] [Google Scholar]
- 23.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis N Engl J Med 1991;325:445–53 [DOI] [PubMed] [Google Scholar]
- 24.Ogasawara K, Ito H, Sasoh M, et al. Quantitative measurement of regional cerebrovascular reactivity to acetazolamide using 123I-N-isopropyl-p-iodoamphetamine autoradiography with SPECT: validation study using H215O with PET. J Nucl Med 2003;44:520–25 [PubMed] [Google Scholar]
- 25.Iida H, Itoh H, Nakazawa M, et al. Quantitative mapping of regional cerebral blood flow using iodine-123-IMP and SPECT. J Nucl Med 1994;35:2019–30 [PubMed] [Google Scholar]
- 26.Friston KJ, Frith CD, Liddle PF, et al. The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab 1990;10:458–66 [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi R, Matsuda H, Yoshioka K, et al. Cerebral blood flow SPET in transient global amnesia with automated ROI analysis by 3DSRT. Eur J Nucl Med Mol Imaging 2004;31:578–89 [DOI] [PubMed] [Google Scholar]
- 28.Fleiss JL. Statistical Methods for Rates and Proportions. New York: Wiley;1981
- 29.Bernstein MA, Huston J 3rd, Lin C, et al. High-resolution intracranial and cervical MRA at 3.0T: technical considerations and initial experience. Magn Reson Med 2001;46:955–62 [DOI] [PubMed] [Google Scholar]
- 30.Thomas SD, Al-Kwifi O, Emery DJ, et al. Application of magnetization transfer at 3.0 T in three-dimensional time-of-flight magnetic resonance angiography of the intracranial arteries. J Magn Reson Imaging 2002;15:479–83 [DOI] [PubMed] [Google Scholar]
- 31.Derick RJ. Carbonic anhydrase inhibitors. In: Mauger TF, Craig EL, eds. Hevener's Ocular Pharmacology. 6th ed. St Louis: CV Mosby;1994. :56–60
- 32.Ogasawara K, Tomitsuka N, Kobayashi M, et al. Stevens-Johnson syndrome associated with intravenous acetazolamide administration for evaluation of cerebrovascular reactivity. Case report. Neurol Med Chir (Tokyo) 2006;46:161–63 [DOI] [PubMed] [Google Scholar]
- 33.Kikuchi K, Murase K, Miki H, et al. Measurement of cerebral hemodynamics with perfusion-weighted MR imaging: comparison with pre- and post-acetazolamide 133Xe-SPECT in occlusive carotid disease. AJNR Am J Neuroradiol 2001;22:248–54 [PMC free article] [PubMed] [Google Scholar]
- 34.Endo H, Inoue T, Ogasawara K, et al. Quantitative assessment of cerebral hemodynamics using perfusion-weighted magnetic resonance imaging in patients with major cerebral artery occlusive disease: comparison with positron emission tomography. Stroke 2006;37:388–92 [DOI] [PubMed] [Google Scholar]
- 35.Wiginton CD, Kelly B, Oto A, et al. Gadolinium-based contrast exposure, nephrogenic systemic fibrosis, and gadolinium detection in tissue. AJR Am J Roentgenol 2008;190:1060–68 [DOI] [PubMed] [Google Scholar]