Abstract
BACKGROUND AND PURPOSE: Hemorrhage is known to occur in posterior reversible encephalopathy syndrome (PRES), but the characteristics have not been analyzed in detail. The purpose of this study was to evaluate the imaging and clinical features of hemorrhage in PRES.
MATERIALS AND METHODS: Retrospective assessment of 151 patients with PRES was performed, and 23 patients were identified who had intracranial hemorrhage at toxicity. Hemorrhage types were identified and tabulated, including minute focal hemorrhages (<5 mm), sulcal subarachnoid hemorrhage, and focal hematoma. Clinical features of hemorrhage and nonhemorrhage PRES groups were evaluated, including toxicity blood pressure, coagulation profile/platelet counts, coagulation-altering medication, and clinical conditions associated with PRES. Toxicity mean arterial pressure (MAP) groups were defined as normal (<106 mm Hg), mildly hypertensive (106–116 mm Hg), or severely hypertensive (>116 mm Hg).
RESULTS: The overall incidence of hemorrhage was 15.2%, with borderline statistical significance noted between the observed clinical associations (P = .07). Hemorrhage was significantly more common (P = .02) after allogeneic bone marrow transplantation (allo-BMT) than after solid-organ transplantation. The 3 hemorrhage types were noted with equal frequency. A single hemorrhage type was found in 16 patients, with multiple types noted in 7. Patients undergoing therapeutic anticoagulation were statistically more likely to develop hemorrhage (P = .04). No difference in hemorrhage incidence was found among the 3 blood pressure subgroups (range, 14.9%–15.9%).
CONCLUSIONS: Three distinct types of hemorrhage (minute hemorrhage, sulcal subarachnoid hemorrhage, hematoma) were identified in PRES with equal frequency. The greatest hemorrhage frequency was seen after allo-BMT and in patients undergoing therapeutic anticoagulation. Hemorrhage rate was independent of the toxicity blood pressure.
Posterior reversible encephalopathy syndrome (PRES) is characterized by a variety of symptoms ranging from headache, altered mental status, and visual loss to seizures and loss of consciousness, accompanied by a typical CT or MR imaging pattern.1–8 The imaging appearance was originally noted in patients with preeclampsia/eclampsia, after transplantation (solid-organ transplantation or allogeneic bone marrow transplantation [allo-BMT]), or in the setting of severe hypertension.9–23 The wider spectrum of patients who develop this toxicity was highlighted by Hinchey et al in 1996,24 and the term “PRES” was used to characterize the typical and unique imaging appearance. The mechanism of PRES is not yet determined; however, it is thought to be related to endothelial cell dysfunction/injury leading to blood-brain barrier leakage, with resultant cortical and subcortical vasogenic edema.4,17,25–29
Intracranial hemorrhage is known to occur in PRES; however, no study has comprehensively focused on this subgroup of patients with PRES. The purpose of this study was to assess the clinical and imaging features of patients with PRES and hemorrhage.
Materials and Methods
The radiology report data base at our institution was searched from January 1998 to August 2007 for any patients in whom “PRES” or “posterior reversible encephalopathy” was noted on brain MR imaging. Additional searches were performed for other conditions known to lead to or be associated with PRES, including the following: cyclosporine neurotoxicity, Tacrolimus (FK-506) neurotoxicity, systemic lupus erythematosus, scleroderma, and hypertensive encephalopathy.
The scope of the imaging features seen in PRES has been described previously.1,3,5,30 Brain CT/MR imaging studies were reviewed in the identified patients for features consistent with the characteristics of cyclosporine/Tacrolimus (FK-506) neurotoxic syndrome, eclampsia, or PRES. Cases were included by consensus agreement between 2 experienced neuroradiologists. Criteria for confirmation of PRES included complete or partial expression of the typical PRES pattern, reversibility on follow-up imaging, and a presentation consistent with a clinical neurotoxic syndrome, such as headache, mental status change, or seizure.
A total of 151 patients were identified who had significant clinical neurotoxicity and brain imaging features consistent with PRES. In 23 patients, findings consistent with intracranial hemorrhage of any size were identified, and these patients represented the focus of this report. Institutional review board approval was obtained for this retrospective study.
The clinical inpatient and outpatient records of these patients were comprehensively reviewed for the following: symptoms at presentation, blood pressure at symptomatic toxicity, coagulation measures, and clinical associations, with specific attention paid to identifying clinical features leading to and surrounding the development of PRES. Known associations, including transplant or cyclosporine/Tacrolimus (FK-506) neurotoxic syndrome, infection/sepsis/shock, autoimmune disease, chemotherapy, and eclampsia, were documented. When >1 clinical association was present, the clinically dominant association was used for tabulation. Baseline and toxicity blood pressures were acquired, and mean arterial pressure (MAP = 2/3 diastolic pressure + 1/3 systolic pressure) was calculated at toxicity. Blood pressure was graded at toxicity as 1) normal (MAP ≤ 105 mm Hg), 2), slightly elevated (MAP = 106–115 mm Hg), and 3) significant hypertension (MAP ≥ 116 mm Hg).
Coagulation State
Coagulation state was categorized into 4 subtypes: 1) normal coagulation; 2) intrinsic coagulopathy: intrinsic thrombocytopenia or independent non-medication-induced elevated international normalized ratio (INR), prothrombin time (PT), or partial thromboplastin time (PTT); 3) subtherapeutic anticoagulation: on oral or intravenous medication that may alter platelet function or the coagulation cascade without identifiable change in INR, PT, PTT (ie, aspirin, clopidogrel, subtherapeutic warfarin); 4) therapeutic anticoagulation: on heparin/warfarin with elevated INR, PT, PTT, or thrombocytopenia and platelet/coagulation-altering medications (ie, aspirin, heparin, warfarin). Institutional limits of normal included the following: 1) platelet count <125 × 103 μL−1, INR < 1.3, PT < 14.0 seconds, PTT < 35 seconds.
Imaging Evaluation
CT studies were obtained with 2.5- to 5-mm section thickness through the posterior fossa along with 5- to 10-mm section thickness through the supratentorial hemispheres, depending on the study date. Contrast material, when used, consisted of intravenous 150 cc iothalamate meglumine (Conray 60; Mallinckrodt, St. Louis Mo), iohexol 300 (Omnipaque; GE Healthcare, Milwaukee Wis), or 125 cc ioversol 350 (Optiray; Mallinckrodt).
MR imaging, when used, was performed at 1.5T and included sagittal and axial T1-weighted images (TR/TE/section/NEX, 600 ms/minimum/5 mm/1) with 5-mm section thickness, spin-echo or fast spin-echo axial proton-density images (TR/TE/section/NEX, 2000–2500 ms/minimum/5/1), T2-weighted images (TR/TEeff/section/NEX, 2500–3000 ms/84–102 ms/5/1), and T2* multiplanar gradient-echo images (TR/TE/section/NEX, 800 ms/25 ms/5/.75; flip angle, 20°). Historically, contrast-enhanced T1-weighted images were obtained with 0.1-mmol/kg gadolinium dimeglumine (Magnavist; Berlex Laboratories, Wayne, NJ), gadopentatate (Prohance; Bracco Diagnostics, Princeton NJ), or gadobenate dimeglumine (Multihance; Bracco Diagnostics) by using typical T1-weighted parameters as described above. Currently, gadolinium contrast dose is administered, depending on renal function, by using a calculated glomerular filtration rate (GFR) including the following: GFR ≥ 30, full dose; GFR 15–29, half dose; GFR < 15, dialysis after gadolinium administration. Fluid-attenuated inversion recovery (FLAIR) images (TR/TE/TI, 9000–10,000 ms/149 ms/2200 ms), and diffusion-weighted imaging sequences (single-shot echo-planar; TR/TE/section/matrix, 10,000 ms/minimum/5 mm/128) were also available in most patients.
CT/MR Imaging Identification of Hemorrhage
The CT/MR imaging studies were further reviewed for evidence of intracranial hemorrhage. Three types of hemorrhage were noted and tabulated including the following: 1) parenchymal hematoma, 2) subarachnoid blood in cortical sulci, and 3) minute hemorrhages (< 5 mm) in the brain parenchyma. In accordance with standard criteria for CT interpretation, hyperattenuated substrate in either the brain parenchyma or cortical subarachnoid space was considered consistent with hemorrhage. By MR imaging, identification of blood byproducts on standard T1, T2, and FLAIR imaging was dependent on the imaging sequence assessed and expected hemoglobin state. Minute hemorrhages were described when foci of dephasing were <5 mm, consistent with new blood by-products, typically identified and confirmed on the multi-planar gradient recall sequence.
Statistical Assessment
SAS software (Version 9.1) was used for all analyses (SAS/STAT User's Guide, 2004; SAS Institute, Cary, NC). All statistical tests were 2-tailed, and statistical significance was set at P < .05. χ2 tests were used to evaluate differences among the proportions of patients with hemorrhage/hemorrhage type according to toxicity blood pressure, clinical association, and coagulation state. When expected frequencies were small, exact tests were used for differences between proportions. The comparisons between coagulation states of the proportion of patients with hemorrhage, adjusting for blood pressure, were conducted by using a logistic regression model.
Results
The results are summarized in Tables 1–4. Intracranial hemorrhage was found in 23 of 151 (15.2%) patients identified with PRES. Seven patients were men and 16 were women (age range, 24–79 years; average, 46 years). Among the 23 patients, confusion or moderate-to-severe altered mentation was the presenting symptom in 8 (35%), with seizures (frequently accompanied by or preceded by headache, vision change, or altered mentation), in 15 (65%).
Table 1:
Hemorrhage in PRES: relationship to clinical associations and blood pressure at toxicity
| Toxicity Association | Total No. Pts with PRES | Patients with PRES and Hemorrhage |
|||
|---|---|---|---|---|---|
| No. Hemorrhage (%) | Blood Pressure in Pts with PRES Hemorrhage |
||||
| Normotensive (MAP ≤ 105) | Mild HTN (MAP, 106–115) | Severe HTN (MAP ≥ 116) | |||
| Immunosuppression‡ | 50 | 11 (22.0) | 3 | 1 | 7 |
| Solid-organ transplant | 34 | 4 (11.8) | 2 | 1 | 1 |
| Allo-BMT | 15 | 7 (46.7) | 1 | 0 | 6 |
| Other* | 1 | 0 (0.0) | – | – | – |
| Infection/sepsis/shock | 52 | 6 (11.5) | 4 | 1 | 1 |
| Autoimmune | 12 | 2 (16.7) | 0 | 0 | 2 |
| Chemotherapy | 10 | 1 (10.0) | 0 | 1 | 0 |
| Eclampsia† | 18 | 1 (5.6) | 0 | 0 | 1 |
| Unknown | 9 | 2 (22.2) | 0 | 0 | 2 |
| Total | 151 | 23 (15.2) | 7 | 3 | 13 |
Note:—Pts indicates patients; CsA, cyclosporine; HTN, hypertension; –, none; PRES, posterior reversible encephalopathy syndrome; MAP, mean arterial pressure; FK-506, tacrolimus; Allo-BMT, allogeneic bone marrow transplantation.
A single patient with psoriasis on cyclosporine.
Patients with eclampsia and delayed eclampsia.
Transplant, on cyclosporine, or tacrolimus (FK-506).
Table 4:
Coagulation state and blood pressures at toxicity in 150 patients with PRES: 23 with hemorrhage, 127 without hemorrhage
| Blood Pressure at Toxicity | No. Pts | Normal Coagulation Platelets, Coagulation Parameters, and No Medications (No. Pts with PRES) |
Abnormal Coagulation |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Intrinsic Coagulopathy Thrombocytopenia or Abnormal PT, PTT, INR (No. Pts with PRES) |
Subtherapeutic, on Medications Affecting Platelet Function or Coagulation (No. Pts with PRES) |
Therapeutic, on Medication Affecting Platelet Function or Coagulation (No. Pts with PRES) |
|||||||
| W/O Hem | With Hem | W/O Hem | With Hem | W/O Hem | With Hem | W/O Hem | With Hem | ||
| Normotensive (MAP ≤ 105) | 44 | 15 | 3 | 17 | 1 | 3 | 1 | 2 | 2 |
| Mild HTN (MAP, 106–115) | 20 | 8 | 2 | 5 | 1 | 3 | 0 | 0 | 1 |
| Severe HTN (MAP ≥ 116) | 86 | 45 | 5 | 15 | 6 | 10 | 0 | 4 | 1 |
| Total | 150 | 68 | 10 | 37 | 8 | 16 | 1 | 6 | 4 |
Note:—W/O indicates without; Hem, hemorrhage; PT, prothrombin time; PTT, partial thromboplastin time; INR, international normalized ratio.
In the overall data base of 151 patients with PRES, 44 (29.1%) were normotensive, 20 (13.2%) were mildly hypertensive, and 87 (57.6%) were severely hypertensive at toxicity. Clinical associations included the following: posttransplantation/immunosuppression, 50 (33.1%); infection/sepsis/shock, 52 (34.4%); eclampsia/delayed eclampsia, 18 (11.9%); autoimmune disease, 12 (7.9%); postcancer chemotherapy, 10 (6.6%); and nonspecific associations including isolated hypertension, 9 (5.9%).
The frequency of hemorrhage in the typical conditions associated with PRES is summarized in Table 1. Overall, hemorrhage was identified most frequently in patients on immune suppression (22%) and least frequently in patients with eclampsia/delayed eclampsia (5%). When the frequency of hemorrhage in PRES was analyzed considering all tabulated individual clinical associations (ie, solid-organ transplantation, allo-BMT, isolated cyclosporine toxicity, eclampsia, infection/sepsis/shock, autoimmume disease, postcancer chemotherapy, unknown), a borderline statistical significance in the rate of hemorrhage (P = .07) was identified. The rate of hemorrhage was much greater in patients after allo-BMT (46.6%) compared with those with solid-organ transplantation (11.7%), and this specific difference was statistically significant (P = .02).
Hemorrhage was identified with similar frequency in all blood pressure groups, including 7 of 44 (15.9%) normotensive patients, 3 of 20 (15%) mildly hypertensive patients, and 13 of 87 (14.9%) severely hypertensive patients (Table 3). Toxicity blood pressures varied among the different clinical association categories, but the differences were not statistically significant (Table 1).
Table 3:
Hemorrhage subtype in PRES: relationship to blood pressure at toxicity
| Blood Pressure at Toxicity | Total No. Pts with PRES | Patients with PRES and Hemorrhage |
||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | Hemorrhage Subtype |
|||||||
| Minute | Sulcal | Focal | Hematoma + Sulcal | Hematoma + Minute | Sulcal + Minute | |||
| Normotensive (MAP ≤ 105) | 44 | 7 (15.9) | 2 | 2 | 0 | 0 | 3 | 0 |
| Mildly hypertensive (MAP = 106–115) | 20 | 3 (15) | 2 | 0 | 1 | 0 | 0 | 0 |
| Severe hypertension (MAP ≥ 116) | 87 | 13 (14.9) | 2 | 3 | 4 | 1 | 2 | 1 |
| Total | 151 | 23 (15.2) | 6 | 5 | 5 | 1 | 5 | 1 |
The rate of hemorrhage after allo-BMT was high relative to other PRES-associated conditions. Hemorrhage in this clinical group was primarily identified in the severely hypertensive subset. When the patients with allo-BMT were excluded, the incidence of hemorrhage in all blood pressure groups changed to 6 of 37 (16.2%) in normotensive patients, 3 of 19 (15.7%) in mildly hypertensive patients, and 7 of 80 (8.8%) in severely hypertensive patients. These differences were not statistically significant (P = .34).
In 16 of 23 (70%) patients with PRES and hemorrhage, neurologic recovery was complete. Average time to resolution of the clinical symptoms that accompanied PRES neurotoxicity was 6.6 days (range, 2–16 days), with average discharge in 15 days (range, 3–76 days). Discharge delay was typically related to the patient's primary clinical problem and not neurologic sequelae from PRES. Seven of 23 (30%) patients had a poor clinical outcome (Table 2). Six patients died after developing toxicity and PRES with hemorrhage (average survival, 17 days; range, 1–64 days). Four patients recovered or stabilized neurologically but subsequently died from multiorgan dysfunction (MOD) related to their systemic toxicity. One patient developed a hematoma within PRES edema on day 4 and died of herniation and MOD on day 6. A second patient in coma from deep thalamic-striatal minute hemorrhages related to PRES died on day 64 of MOD. In 1 patient with a focal hematoma and minute hemorrhages, a permanent neurologic deficit was present at discharge (hemiparesis, facial droop, hemianopsia).
Table 2:
Hemorrhage subtypes in PRES: relationship to clinical associations and adverse clinical outcomes
| Toxicity Association | Total No. Pts with PRES | Patients with PRES and Hemorrhage |
|||
|---|---|---|---|---|---|
| No. Pts with Hemorrhage | Hemorrhage Subtypes (No. Pts) |
||||
| Minute | Sulcal | Hematoma | |||
| Immunosuppression‡ | 50 | 11 | 5 | 4 | 3 |
| Solid-organ transplant | 34 | 4 | 4 (1D) | 1 | 0 |
| Allo-BMT | 15 | 7 | 1 (1D) | 3 (1D) | 3 (2D) |
| Other† | 1 | 0 | 0 | 0 | 0 |
| Infection/sepsis/shock | 52 | 6 | 4 | 1 | 4 (1D) |
| Autoimmune | 12 | 2 | 2* | 0 | 2* |
| Chemotherapy | 10 | 1 | 1 | 0 | 0 |
| Eclampsia | 18 | 1 | 0 | 1 | 1 |
| Unknown | 9 | 2 | 0 | 1 | 1 |
| Total | 151 | 23 | 12 | 7 | 11 |
Note:—D indicates that the patient died.
A single patient with autoimmune disease and both minute hemorrhage and focal hematoma who sustained a permanent deficit (homonymous hemianopsia, hemiparesis, and facial droop).
A single patient with psoriasis on cyclosporine.
Transplant, on cyclosporine, or tacrolimus (FK-506).
Imaging Features
The imaging features of hemorrhage in the 23 patients with PRES are summarized in Tables 2 and 3 and Figs 1–3. Both CT and MR imaging were available at toxicity in 16 patients, with 4 undergoing CT only and 3, MR only.
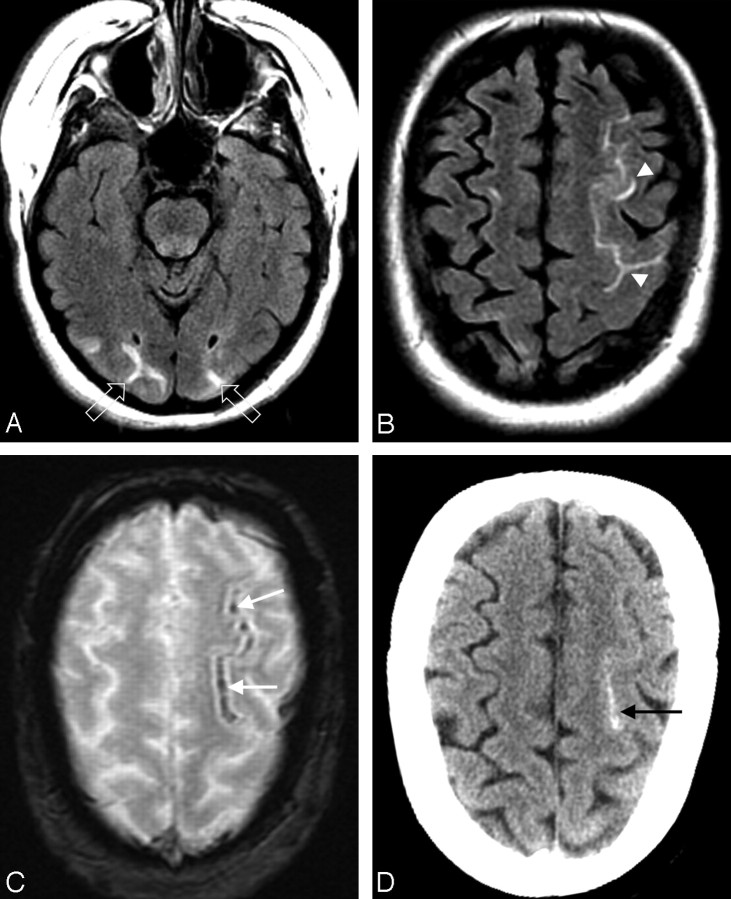
Fig 1.
A 27-year-old man with necrotic pneumonia and lung abscess. A, MR FLAIR image demonstrates PRES vasogenic edema in the parietal and frontal lobes (arrows). B, Gradient image demonstrates minute hemorrhages in the left frontal lobe (arrows).
Fig 2.
A 50-year-old woman with fever and severe hypertension. A and B, FLAIR MR image demonstrates sulcal signal abnormality and PRES vasogenic edema in the left frontal lobe (arrowheads) and edema in the occipital lobes bilaterally (open arrows). C, Gradient MR image demonstrates linear low signal intensity consistent with sulcal subarachnoid hemorrhage (arrows). D, CT image demonstrates high attenuation consistent with the MR imaging appearance, further confirming the sulcal subarachnoid hemorrhage (arrow).
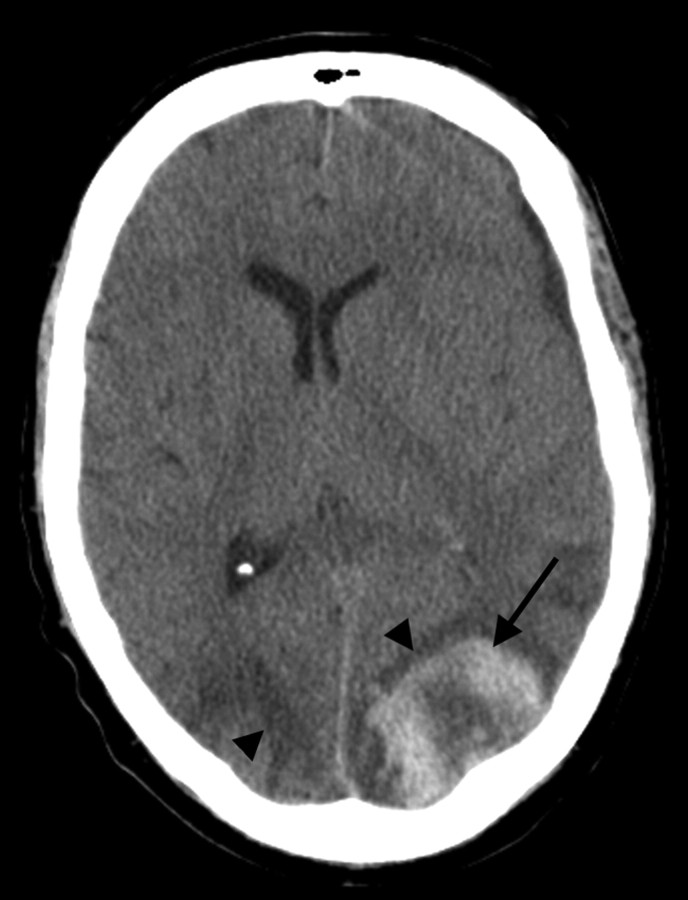
Fig 3.
A 50-year-old man status post allo-BMT for acute myelogenous leukemia. CT scan demonstrates PRES vasogenic edema in the parietal region bilaterally (arrowheads), along with an acute hematoma in the left parietal lobe (arrow).
In 16 patients, a single hemorrhage type was identified (minute, 6 patients; sulcal, 5 patients; focal hematoma, 5 patients), with combined hemorrhage types identified in 7 patients. Although the numbers are small, an increased incidence in focal hematoma was identified in immunosuppressed patients after allo-BMT (P = .02) compared with patients after solid-organ transplantation. Other than this observation, no significant difference was noted between the hemorrhage types and associated clinical conditions in patients with PRES. In addition, no significant difference was noted between the hemorrhage type and blood pressure in patients with PRES neurotoxicity.
In patients with focal hematoma, the hematoma was present at initial imaging in 9 of 11 patients, with delayed hematoma development identified on follow-up imaging in 2 patients (day 2 MR imaging, 1 patient; day 3 CT, 1 patient). Sulcal hemorrhage was identified on the initial imaging study in all 7 patients. In 3 of 12 patients with minute hemorrhage, MR imaging only was performed at toxicity, and findings in all 3 patients were positive. Minute hemorrhages were seen on initial CT scans in 2 of the remaining 9 patients but were identified only on follow-up MR imaging in 7 of 9 patients (day 1 MR imaging, 2 patients; day 2 MR imaging, 5 patients).
Coagulation State
Coagulation data were available in 150 of 151 patients with PRES. The impact of coagulation state on hemorrhage in PRES is summarized in Table 4. In patients with hemorrhage, 10 of 23 (43%) had normal coagulation, with 13 of 23 (57%) categorized in 1 of the 3 abnormal groups. In the 127 patients without hemorrhage, 68 (53.4%) had normal coagulation, with 59 (46.5%) in 1 of the 3 abnormal coagulation groups. The overall difference in coagulation state between the hemorrhage and nonhemorrhage PRES patients was not statistically significant.
Therapeutic anticoagulation was present in 4 of 23 (17.4%) patients with PRES and hemorrhage, compared with 6 of 127 (6.7%) patients without hemorrhage (Table 4). This difference was statistically significant when compared to patients with normal coagulation (P = .04) and borderline in significance when stratified for blood pressure (P = .06). Patients in this group underwent the following treatments: heparin drip with elevated PTT (4 patients), warfarin with elevated INR (2 patients), heparin drip with thrombocytopenia (1 patient), and baby aspirin with thrombocytopenia (3 patients).
Intrinsic coagulopathy was present in 8 of 23 (34.8%) patients with hemorrhage and 37 of 127 (29.1%) patients without hemorrhage. All patients with thrombocytopenia in the hemorrhage group had platelet counts below 80 × 103 μL−1. Subtherapeutic anticoagulation was present in 1 normotensive patient with hemorrhage (on clopidogrel at toxicity; platelet count/coagulation levels, normal) and 16 patients without hemorrhage. These coagulation states were not statistically significant when compared with those in patients with normal coagulation.
Discussion
The mechanism behind PRES remains controversial and unproven. Severe hypertension leading to a blood pressure that exceeds the upper limits of autoregulation, with subsequent forced hyperperfusion, has been suggested. Conversely, vasculopathy and hypoperfusion have been reported, and the PRES imaging appearance resembles a watershed distribution.18,31–35 Potential etiologic factors include endothelial injury/dysfunction with alteration of the blood-brain barrier, as is present in eclampsia, or other causes, such as conditioning regimens before transplantation, effects of graft-versus-host disease, or the results of the immunosuppressive drugs cyclosporine and tacrolimus.
Prior studies have demonstrated parenchymal hematoma or sulcal subarachnoid hemorrhage in 5%–17% of patients with PRES,5,8,36 but the mechanism behind hemorrhage in PRES is equally unclear. Doss-Esper et al37 have proposed 2 hypotheses: 1) nonaneurysmal subarachnoid (sulcal) hemorrhage due to rupture of pial vessels in the face of severe hypertension and impaired cerebral autoregulation, and 2) postischemic reperfusion injury leading to multifocal brain hemorrhages.
Although the incidence of hemorrhage in our PRES population (15.2%) is similar to that in previous reports,5,8,36 our data demonstrate a number of new and important observations.
Associated Clinical Conditions
There was a borderline difference in the incidence of hemorrhage in PRES between the different toxicity-associated conditions (P = .07), with the highest rate seen in the setting of immunosuppression (22%) and the lowest rate, in eclampsia (5.5%). PRES-related hemorrhage was statistically more common (P = .02) in the allo-BMT subgroup (46.6%) compared with patients with solid-organ transplants (11.7%), and this difference likely accounts for the overall high rate in the immunosuppression group.
The reason for the high rate of PRES-related hemorrhage in the allo-BMT subgroup is not clear. The incidence of PRES or cyclosporine toxicity after allo-BMT is known to be significant.16,38,39 Allo-BMT is also known to be a complex clinical state with multiple systemic challenges: graft-versus-host effects, veno-occlusive disease, bone marrow transplant thrombotic microangiopathy, and opportunistic infection. The immunosuppression drugs cyclosporine and tacrolimus are typically administered at higher dose levels after allo-BMT for control of graft-versus-host disease, and these drugs can potentially augment toxicity through systemic effects.
Cyclosporine and tacrolimus can cause direct endothelial injury, and the immunosuppressive drugs inhibit T-cell function, with a resultant altered immune response and increased risk of infection.40–44 Cyclosporine is also known to induce vasoconstriction through endothelial cell production and release of endothelin and systemic sympathetic stimulation.43,45–47 Systemic/renal vasoconstriction with resultant hypertension could lead to reduced cerebral blood flow due to autoregulatory vasoconstriction. In addition, these drugs could reduce cerebral blood flow directly, secondary to cerebral vasoconstriction. The combined effects might lead to increased but complex cerebral vascular instability, with an increased risk of hemorrhage.
Presence of Hypertension
Our data fail to demonstrate a relationship between PRES-related hemorrhage and moderate or severe hypertension at toxicity. No statistical difference in the incidence of hemorrhage was appreciated when the toxicity association was evaluated relative to blood pressure category (Table 1) or hemorrhage type (Table 2) in the overall population. Therefore, in the overall population, blood pressure does not appear to affect the incidence of hemorrhage in PRES, and hemorrhage type appears to occur with similar frequency among the different PRES toxicity-associated conditions.
Of interest, although not statistically significant, the incidence of hemorrhage was markedly less in the severely hypertensive subset (8.8%) when the unique allo-BMT subgroup was excluded. The extent of PRES vasogenic edema has previously been found to be less in severely hypertensive patients who developed PRES in association with infection/sepsis/shock.48 Similarly, the extent of PRES vasogenic edema was less in patients after liver transplantation (typically lower MAP at toxicity) compared with PRES after kidney transplantation (typically high MAP at toxicity).49 These observations in patients with PRES who present with severe hypertension (less vasogenic edema, no increase in hemorrhage rate) bring into question whether severe hypertension is a direct cause of PRES neurotoxicity. The role of hypertension in PRES may be more complex than previously considered.
Also of interest, hemorrhage was equally common (15.9%) in the normotensive patients who developed PRES. While controversial, hypoperfusion has been demonstrated in PRES.32,34,35 Vasoconstriction of medium and large size vessels has been documented in patients with PRES and single-photon emission tomography, MR perfusion, and CT perfusion have demonstrated hypoperfusion.31,32,34,35,50 Vasoconstriction coupled with blood pressure instability could predispose to reperfusion injury as occurs in patients following subarachnoid hemorrhage.51 Our observations might be consistent with the previously stated theory of postischemic reperfusion as a potential cause of hemorrhage in PRES.37 In addition, lymphocyte trafficking has been noted in PRES,4 and recently, direct histologic evidence of endothelial activation, T-cell dysfunction, and brain hypoxemia has been demonstrated with upregulated endothelial/cellular vascular endothelial growth factor in reversible encephalopathy posttransplantation.52
Hemorrhage may be seen in the setting of reperfusion injury, as can develop after stroke thrombolysis or spontaneous embolus recanalization.51,53–56 Small areas of focal petechial hemorrhage into an infarct bed are common in embolic infarction (hemorrhagic infarction).55,56 In thrombolysis-related reperfusion injury, both small petechial hemorrhages and focal hematoma can be observed.53,54 The mechanism of reperfusion injury is complex and appears to involve elements of both ischemic capillary injury and immune cellular reaction.51 Similar to the observations in hemorrhagic transformation of infarction, in which poor outcome is generally related to the development of a significant focal hematoma,53,55 clinical outcome in most of our patients with PRES was not affected by the identification of hemorrhage. Regions of both infarction and hemorrhage are also known to develop in the setting of cerebral vasoconstriction or vasospasm.55,57–60 In aneurysmal subarachnoid hemorrhage, early vasospasm can occur with infarction and both petechial blood and focal hematoma formation.55 In the reversible cerebral vasoconstriction syndrome, watershed infarction and focal brain hematomas have been observed, potentially due to reperfusion.60 In addition, similar to PRES, isolated sulcal subarachnoid hemorrhage has been seen in reversible cerebral vasoconstriction syndrome.59
Hemorrhage has also been observed in the potentially related postinfectious or immune-triggered leukoencephalopathies. Although not typically seen on imaging studies in patients with multiple sclerosis, evidence of vein wall damage (intramural fibrinoid, collagenized thickening), recent or old hemorrhage (hemosiderin), and thrombosed vessels has been noted at histopathology in addition to the typical observations of perivenous immune cell response and demyelination.61 Hemorrhage has occasionally been seen in acute disseminated encephalomyelitis (ADEM),62 a leukoencephalopathy typically identified in children after nonspecific infection, viral illness, or vaccination.63–65 Hemorrhage is the hallmark of acute hemorrhagic leukoencephalitis (AHL), often considered a severe hemorrhagic form of ADEM.65,66
Histologically, ADEM demonstrates a predominantly lymphocytic/macrophage perivascular immune cell reaction and infiltrate, characteristic sleeve-like demyelination, and occasional minute perivascular hemorrhage.62,67,68 In contrast, AHL typically demonstrates a prominent perivascular neutrophilic infiltrate, with accompanying necrotizing vasculitis and more extensive petechial hemorrhages, with a characteristic perivascular ball or ring appearance.67–70 MR imaging in ADEM has demonstrated lesions in the thalami, basal ganglia, and brain stem in addition to typical periventricular and hemispheric white matter involvement.63–65 In patients with AHL, MR imaging has demonstrated both minute focal hemorrhages and larger focal hematomas in the hemispheres, frontal lobes, deep white matter, and thalami.65,66,71–74 Of note, similar thalamic lesions and histologic pattern have been reported in patients with influenza-related encephalopathy/encephalitis; PRES with evidence of vasculopathy has recently been identified in association with influenza A.75
Coagulation and Hemorrhage in PRES
Patients with therapeutic anticoagulation were statistically more likely to develop PRES-related hemorrhage (P = .04). This group included those on therapeutic anticoagulation or those taking aspirin in the face of thrombocytopenia. Of interest, patients with intrinsic coagulopathy (intrinsic thrombocytopenia or abnormal coagulation) or subtherapeutic anticoagulation were not statistically more likely to develop PRES-related hemorrhage. These coagulation-challenged states do not appear to present a greater risk of hemorrhage compared with the PRES population with normal coagulation.
Imaging Features of Hemorrhage in PRES
Three distinct types of hemorrhage were noted in our patients, including focal minute hemorrhages, larger more typical focal hematomas, and sulcal-based subarachnoid hemorrhage. These hemorrhages were seen both alone and in combination. In an isolated comparison between solid-organ transplantation and allo-BMT, there was a statistically increased observation of focal hematoma in the allo-BMT group (P = .02). Other than this observation, there was no statistical difference in the incidence of the 3 hemorrhage subtypes, either as related to blood pressure category (Table 3) or toxicity association (Table 2). Therefore, neither blood pressure at toxicity nor associated clinical condition appears to be related to the development of the different hemorrhage types in our PRES population.
In 2 patients with focal hematoma, initial CT findings were negative, with hemorrhage found on follow-up CT. These represent delayed hemorrhage and may suggest that close follow-up studies may be warranted in PRES. In 7 patients with minute hemorrhages, initial CT findings were negative, with small foci of blood seen on follow-up MR imaging performed the same day in 2 patients or the following day in 5 patients. Whether these represent improved recognition of microhemorrhage due to increased sensitivity of MR imaging or delayed hemorrhage is uncertain.
Routine gradient-echo sequences were typically included in our MR imaging protocols for early detection of blood byproducts. Currently, even more advanced susceptibility-weighted sequences are being developed that appear to be 3–6 times more sensitive than conventional T2*-weighted gradient-echo sequences in detecting subtle minute hemorrhages.76,77 Use of these sequences could, in the future, increase the detection of minute hemorrhage in PRES and other conditions, including stroke and trauma.
Due to the retrospective nature of our work, several caveats are important to note. Similar to other large PRES studies, a portion of our population did not undergo MR imaging; this omission could reduce minute hemorrhage detection. In addition, repeat MR imaging was not routinely performed in all patients, clearly limiting detection of delayed hemorrhage. Therefore, the true incidence of hemorrhage in PRES, in particular minute hemorrhage, might be larger than we have identified. In addition, our experience is heavily influenced by organ and bone marrow transplantation, due to the practice patterns at our institution. This could influence both hemorrhage frequency and type encountered.
Conclusions
The incidence of hemorrhage in our patients with PRES parallels that in previous reports (15.2%), with 3 types of hemorrhage noted, minute, sulcal subarachnoid, and focal hematoma. A borderline difference in the hemorrhage rate was seen among the various clinical conditions associated with PRES (P = .07), the greatest noted after transplantation and the least in association with eclampsia or delayed eclampsia. In patients with transplants, the frequency of hemorrhage was significantly greater (P = .02) after allo-BMT (46.6%) compared with solid-organ transplantation (11.7%) and the presence of focal hematoma was statistically more common in the allo-BMT group (P = .02). The incidence of hemorrhage was also greater (P = .04) in patients therapeutically anticoagulated at neurotoxicity.
No difference in the incidence of the 3 hemorrhage types (minute, subarachnoid, and hematoma) was seen with respect to the associated clinical condition or presence of hypertension at toxicity. Severe hypertension at toxicity did not influence the frequency of hemorrhage in PRES in the overall population, but when the unique allo-BMT subgroup was excluded, the incidence of hemorrhage was less (8.8%) in the severely hypertensive subset.
References
- 1.Gocmen R, Ozgen B, Oguz KK. Widening the spectrum of PRES: series from a tertiary care center. Eur J Radiol 2007;62:454–59. Epub 2007 Jan 10 [DOI] [PubMed] [Google Scholar]
- 2.Ahn KJ, You WJ, Jeong SL, et al. Atypical manifestations of reversible posterior leukoencephalopathy syndrome: findings on diffusion imaging and ADC mapping. Neuroradiology 2004;46:978–83 [DOI] [PubMed] [Google Scholar]
- 3.Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome (PRES). AJNR Am J Neuroradiol 2007;28:1320–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartynski WS. Posterior reversible encephalopathy syndrome. Part 1. Fundamental imaging and clinical features. AJNR Am J Neuroradiol 2008;29:1036–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKinney AM, Short J, Truwit CL, et al. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol 2007;189:904–12 [DOI] [PubMed] [Google Scholar]
- 6.Gijtenbeek JM, van den Bent MJ, Vecht CJ. Cyclosporine neurotoxicity: a review. J Neurol 1999;246:339–46 [DOI] [PubMed] [Google Scholar]
- 7.Garg RK. Posterior leukoencephalopathy syndrome. Postgrad Med J 2001;77:24–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee VH, Wijdicks EF, Manno EM, et al. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol 2008;65:205–10 [DOI] [PubMed] [Google Scholar]
- 9.Colosimo C Jr, Fileni A, Moschini M, et al. CT findings in eclampsia. Neuroradiology 1985;27:313–17 [DOI] [PubMed] [Google Scholar]
- 10.Fisher M, Maister B, Jacobs R. Hypertensive encephalopathy: diffuse reversible white matter CT abnormalities. Ann Neurol 1985;18:268–70 [DOI] [PubMed] [Google Scholar]
- 11.Lewis LK, Hinshaw DB Jr, Will AD, et al. CT and angiographic correlation of severe neurological disease in toxemia of pregnancy. Neuroradiology 1988;30:59–64 [DOI] [PubMed] [Google Scholar]
- 12.Naheedy MH, Biller J, Schiffer M, et al. Toxemia of pregnancy: cerebral CT findings. J Comput Assist Tomogr 1985;9:497–501 [DOI] [PubMed] [Google Scholar]
- 13.de Groen PC, Aksamit AJ, Rakela J, et al. Central nervous system toxicity after liver transplantation: the role of cyclosporine and cholesterol. N Engl J Med 1987;317:861–66 [DOI] [PubMed] [Google Scholar]
- 14.Ghalie R, Fitzsimmons WE, Bennett D, et al. Cortical blindness: a rare complication of cyclosporine therapy. Bone Marrow Transplant 1990;6:147–49 [PubMed] [Google Scholar]
- 15.Rail DL, Perkin GD. Computerized tomographic appearance of hypertensive encephalopathy. Arch Neurol 1980;37:310–11 [DOI] [PubMed] [Google Scholar]
- 16.Reece DE, Frei-Lahr DA, Shepherd JD, et al. Neurologic complications in allogeneic bone marrow transplant patients receiving cyclosporin. Bone Marrow Transplant 1991;8:393–401 [PubMed] [Google Scholar]
- 17.Trommer BL, Homer D, Mikhael MA. Cerebral vasospasm and eclampsia. Stroke 1988;19:326–29 [DOI] [PubMed] [Google Scholar]
- 18.Truwit CL, Denaro CP, Lake JR, et al. MR imaging of reversible cyclosporin A-induced neurotoxicity. AJNR Am J Neuroradiol 1991;12:651–59 [PMC free article] [PubMed] [Google Scholar]
- 19.Waldron RL 2nd, Abbott DC, Vellody D. Computed tomography in preeclampsia-eclampsia syndrome. AJNR Am J Neuroradiol 1985;6:442–43 [PMC free article] [PubMed] [Google Scholar]
- 20.Weingarten KL, Zimmerman RD, Pinto RS, et al. Computed tomographic changes of hypertensive encephalopathy. AJNR Am J Neuroradiol 1985;6:395–98 [PMC free article] [PubMed] [Google Scholar]
- 21.Will AD, Lewis KL, Hinshaw DB Jr, et al. Cerebral vasoconstriction in toxemia. Neurology 1987;37:1555–57 [DOI] [PubMed] [Google Scholar]
- 22.Wilson SE, de Groen PC, Aksamit AJ, et al. Cyclosporin A-induced reversible cortical blindness. J Clin Neuroophthalmol 1988;8:215–20 [PubMed] [Google Scholar]
- 23.Raroque HG Jr, Orrison WW, Rosenberg GA. Neurologic involvement in toxemia of pregnancy: reversible MRI lesions. Neurology 1990;40:167–69 [DOI] [PubMed] [Google Scholar]
- 24.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494–500 [DOI] [PubMed] [Google Scholar]
- 25.Coughlin WF, McMurdo SK, Reeves T. MR imaging of postpartum cortical blindness. J Comput Assist Tomogr 1989;13:572–76 [DOI] [PubMed] [Google Scholar]
- 26.Toole JF. Lacunar syndromes and hypertensive encephalopathy. In: Toole JF, ed. Cerebrovascular Disorders. 5th ed. New York: Raven;1999. :342–55
- 27.Bartynski WS, Zeigler ZR, Shadduck RK, et al. Variable incidence of cyclosporine and FK-506 neurotoxicity in hematopoietic malignancies and marrow conditions after allogeneic bone marrow transplantation. Neurocrit Care 2005;3:33–45 [DOI] [PubMed] [Google Scholar]
- 28.Bartynski WS. Posterior reversible encephalopathy syndrome. Part 2. Controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol 2008;29:1043–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strandgaard S, Olesen J, Skinhoj E, et al. Autoregulation of brain circulation in severe arterial hypertension. BMJ 1973;1:507–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitaguchi H, Tomimoto H, Miki Y, et al. A brainstem variant of reversible posterior leukoencephalopathy syndrome. Neuroradiology 2005;47:652–56 [DOI] [PubMed] [Google Scholar]
- 31.Brubaker LM, Smith JK, Lee YZ, et al. Hemodynamic and permeability changes in posterior reversible encephalopathy syndrome measured by dynamic susceptibility perfusion-weighted MR imaging. AJNR Am J Neuroradiol 2005;26:825–30 [PMC free article] [PubMed] [Google Scholar]
- 32.Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 2008;29:447–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartynski WS, Grabb BC, Zeigler Z, et al. Watershed imaging features and clinical vascular injury in cyclosporin A neurotoxicity. J Comput Assist Tomogr 1997;21:872–80 [DOI] [PubMed] [Google Scholar]
- 34.Engelter ST, Petrella JR, Alberts MJ, et al. Assessment of cerebral microcirculation in a patient with hypertensive encephalopathy using MR perfusion imaging. AJR Am J Roentgenol 1999;173:1491–93 [DOI] [PubMed] [Google Scholar]
- 35.Naidu K, Moodley J, Corr P, et al. Single photon emission and cerebral computerised tomographic scan and transcranial Doppler sonographic findings in eclampsia. Br J Obstet Gynaecol 1997;104:1165–72 [DOI] [PubMed] [Google Scholar]
- 36.Singhal AB. Postpartum angiopathy with reversible posterior leukoencephalopathy. Arch Neurol 2004;61:411–16 [DOI] [PubMed] [Google Scholar]
- 37.Doss-Esper CE, Singhal AB, Smith MS, et al. Reversible posterior leukoencephalopathy, cerebral vasoconstriction, and strokes after intravenous immune globulin therapy in Guillain-Barre syndrome. J Neuroimaging 2005;15:188–92 [DOI] [PubMed] [Google Scholar]
- 38.Bartynski WS, Zeigler ZR, Shadduck RK, et al. Pretransplantation conditioning influence on the occurrence of cyclosporine or FK-506 neurotoxicity in allogeneic bone marrow transplantation. AJNR Am J Neuroradiol 2004;25:261–69 [PMC free article] [PubMed] [Google Scholar]
- 39.Zimmer WE, Hourihane JM, Wang HZ, et al. The effect of human leukocyte antigen disparity on cyclosporine neurotoxicity after allogeneic bone marrow transplantation. AJNR Am J Neuroradiol 1998;19:601–08, discussion 609–10 [PMC free article] [PubMed] [Google Scholar]
- 40.Zoja C, Furci L, Ghilardi F, et al. Cyclosporin-induced endothelial cell injury. Lab Invest 1986;55:455–62 [PubMed] [Google Scholar]
- 41.Kochi S, Takanaga H, Matsuo H, et al. Effect of cyclosporin A or tacrolimus on the function of blood-brain barrier cells. Eur J Pharmacol 1999;372:287–95 [DOI] [PubMed] [Google Scholar]
- 42.Kochi S, Takanaga H, Matsuo H, et al. Induction of apoptosis in mouse brain capillary endothelial cells by cyclosporin A and tacrolimus. Life Sci 2000;66:2255–60 [DOI] [PubMed] [Google Scholar]
- 43.Ramanathan V, Helderman JH. Cyclosporine formulations. In: Sayegh M, Remuzzi G, eds. Current and Future Immunosuppressive Therapies Following Transplantation. the Netherlands: Kluwer Academic;2001. :111–21
- 44.Shapiro R. Tacrolimus. In: Sayegh M, Remuzzi G, eds. Current and Future Immunosuppressive Therapies Following Transplantation. the Netherlands: Kluwer Academic;2001. :123–42
- 45.Benigni A, Morigi M, Perico N, et al. The acute effect of FK506 and cyclosporine on endothelial cell function and renal vascular resistance. Transplantation 1992;54:775–80 [DOI] [PubMed] [Google Scholar]
- 46.Marsen TA, Weber F, Egink G, et al. Cyclosporin A induces prepro endothelin-1 gene transcription in human endothelial cells. Eur J Pharmacol 1999;379:97–106 [DOI] [PubMed] [Google Scholar]
- 47.Kon V, Sugiura M, Inagami T, et al. Role of endothelin in cyclosporine-induced glomerular dysfunction. Kidney Int 1990;37:1487–91 [DOI] [PubMed] [Google Scholar]
- 48.Bartynski WS, Boardman JF, Zeigler ZR, et al. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol 2006;27:2179–90 [PMC free article] [PubMed] [Google Scholar]
- 49.Bartynski WS, Tan HP, Boardman JF, et al. Posterior reversible encephalopathy syndrome after solid organ transplantation. AJNR Am J Neuroradiol 2008;29:924–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casey SO, McKinney A, Teksam M, et al. CT perfusion imaging in the management of posterior reversible encephalopathy. Neuroradiology 2004;46:272–76 [DOI] [PubMed] [Google Scholar]
- 51.Pan J, Konstas AA, Bateman B, et al. Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies Neuroradiology 2007;49:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horbinski C, Bartynski WS, Carson-Walter E, et al. Reversible encephalopathy after cardiac transplantation: histologic evidence of endothelial activation, T-cell specific trafficking, and vascular endothelial growth factor expression. AJNR Am J Neuroradiol 2009;30:588–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome—results of a prospective multicenter study. Stroke 2008;39:2249–56 [DOI] [PubMed] [Google Scholar]
- 54.Thanvi BR, Treadwell S, Robinson T. Haemorrhagic transformation in acute ischaemic stroke following thrombolysis therapy: classification, pathogenesis and risk factors. Postgrad Med J 2008;84:361–67 [DOI] [PubMed] [Google Scholar]
- 55.Teal PA, Pessin MS. Hemorrhagic transformation: the spectrum of ischemia-related brain hemorrhage. Neurosurg Clin N Am 1992;3:601–10 [PubMed] [Google Scholar]
- 56.Ferrer I, Kaste M, Kalimo H. Vascular diseases. In: Love S, Louis DN, Ellison DW, eds. Greenfield's Neuropathology. 8th ed. London, UK: Hodder Arnold;2008. :121–240
- 57.Rabinstein AA, Weigand S, Atkinson JL, Wijdicks EF. Patterns of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke 2005;36:992–97 [DOI] [PubMed] [Google Scholar]
- 58.Weidauer S, Lanfermann H, Raabe A, et al. Impairment of cerebral perfusion and infarct patterns attributable to vasospasm after aneurysmal subarachnoid hemorrhage: a prospective MRI and DSA study. Stroke 2007;38:1831–36 [DOI] [PubMed] [Google Scholar]
- 59.Edlow BL, Kasner SE, Hurst RW, et al. Reversible cerebral vasoconstriction syndrome associated with subarachnoid hemorrhage. Neurocrit Care 2007;7:203–10 [DOI] [PubMed] [Google Scholar]
- 60.Singhal AB. Cerebral vasoconstriction syndromes. Top Stroke Rehabil 2004;11:1–6 [DOI] [PubMed] [Google Scholar]
- 61.Adams CW. Perivascular iron deposition and other vascular damage in multiple sclerosis. J Neurol Neurosurg Psychiatry 1988;51:260–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prineas JW, McDonald WI, Franklin RJM. Demyelinating diseases. In: Graham DI, Lantos PL, eds. Greenfield's Neuropathology. 7th ed. London, UK: Arnold;2002. :471–550
- 63.Dale RC. Acute disseminated encephalomyelitis. Semin Pediatr Infect Dis 2003;14:90–95 [DOI] [PubMed] [Google Scholar]
- 64.Hynson JL, Kornberg AJ, Coleman LT, et al. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology 2001;56:1308–12 [DOI] [PubMed] [Google Scholar]
- 65.Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology 2002;59:1224–31 [DOI] [PubMed] [Google Scholar]
- 66.Lee HY, Chang KH, Kim JH, et al. Serial MR imaging findings of acute hemorrhagic leukoencephalitis: a case report. AJNR Am J Neuroradiol 2005;26:1996–99 [PMC free article] [PubMed] [Google Scholar]
- 67.Hart MN, Earle KM. Haemorrhagic and perivenous encephalitis: a clinical-pathological review of 38 cases. J Neurol Neurosurg Psychiatry 1975;38:585–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sobel R, Moore GRW. Demyelinating disease. In: Love S, Louis DN, Ellison DW, eds. Greenfield's Neuropathology. 8th ed. London, UK: Hodder Arnold;2008. :1513–608
- 69.Kuperan S, Ostrow P, Landi MK, et al. Acute hemorrhagic leukoencephalitis vs ADEM: FLAIR MRI and neuropathology findings. Neurology 2003;60:721–22 [DOI] [PubMed] [Google Scholar]
- 70.Vartanian TK, Monte S. Case records of the Massachusetts General Hospital: weekly clinicopathological exercises—case 1–1999. A 53-year-old man with fever and rapid neurologic deterioration. N Engl J Med 1999;340:127–35 [DOI] [PubMed] [Google Scholar]
- 71.Gibbs WN, Kreidie MA, Kim RC, et al. Acute hemorrhagic leukoencephalitis: neuroimaging features and neuropathologic diagnosis. J Comput Assist Tomogr 2005;29:689–93 [DOI] [PubMed] [Google Scholar]
- 72.Klein CJ, Wijdicks EF, Earnest FT. Full recovery after acute hemorrhagic leukoencephalitis (Hurst's disease). J Neurol 2000;247:977–79 [DOI] [PubMed] [Google Scholar]
- 73.Mader I, Wolff M, Niemann G, et al. Acute haemorrhagic encephalomyelitis (AHEM): MRI findings. Neuropediatrics 2004;35:143–46 [DOI] [PubMed] [Google Scholar]
- 74.Markus R, Brew BJ, Turner J, et al. Successful outcome with aggressive treatment of acute haemorrhagic leukoencephalitis. J Neurol Neurosurg Psychiatry 1997;63:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bartynski WS, Upadhyaya A, Boardman JF. Posterior reversible encephalopathy syndrome (PRES) and cerebral vasculopathy associated with influenza A infection: report of a case and review of the literature. J Comput Assist Tomogr 2009. , In press [DOI] [PubMed]
- 76.Haacke EM, Mittal S, Wu Z, et al. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol 2009;30:19–30. Epub 2009 Jan 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mittal S, Wu Z, Neelavalli J, et al. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol 2009;30:232–52. Epub 2009 Jan 8 [DOI] [PMC free article] [PubMed] [Google Scholar]