Abstract
SUMMARY: In complex regional pain syndrome (CRPS), functional imaging studies gave evidence for an important role of the central nervous system (CNS) in the pathogenesis of the disease. Especially, reorganization in central somatosensory and motor networks was demonstrated, leading to an altered central processing of tactile and nociceptive stimuli, as well as to an altered cerebral organization of movement. These findings may explain a number of clinical signs and symptoms occurring in the course of the disease and seem to be closely related to chronic pain in CRPS. Neurorehabilitative strategies, which target cortical areas and aim to restore impaired sensorimotor function in patients with CRPS, therefore, may be effective not only in restoring impaired function but also in pain reduction. This article reviews findings of functional imaging studies, which have been conducted to clarify CNS involvement in the course of CRPS.
Complex regional pain syndromes (CRPSs) are painful disorders that may develop as a disproportionate consequence of a trauma, typically affecting a limb.1 Clinically, they are characterized by pain and related sensory abnormalities, edema, autonomic dysfunction, motor symptoms, and trophic changes.2,3 The diagnostic criteria of CRPS were developed in a consensus conference on the basis of clinical signs and symptoms.4 These criteria are the following: 1) Syndrome develops after initiating noxious event including the absence (CRPS I, formerly reflex sympathetic dystrophy) or presence (CRPS II, formerly causalgia) of a peripheral nerve lesion; 2) spontaneous pain or allodynia/hyperalgesia occurs and is not limited to the territory of a single peripheral nerve and is disproportionate to the inciting event; 3) there is or has been evidence of edema, skin blood flow abnormality, or abnormal sudomotor activity in the region of the pain since the inciting event; and 4) the diagnosis is excluded by the existence of conditions that would otherwise account for the degree of pain and dysfunction. Revisions to these classic criteria have been proposed to increase specificity while preserving high sensitivity and might be included by the International Association for the Study of Pain in future revisions of their formal taxonomy and diagnostic criteria for pain states.5,6
Although numerous pathophysiologic mechanisms contributing to clinical signs and symptoms in CRPS have been identified, a pathophysiologic explanation of the whole disease is still lacking.7 Peripheral mechanisms that contribute to the pathogenesis of CRPS include neurogenic inflammation,8 peripheral sensitization,9 and sympathicoafferent coupling.10 On the other hand, on the basis of extensive evidence from clinical observations and experimentation on humans, the hypothesis has been put forward that CRPS is a disease primarily of the central nervous system (CNS), involving changes in central sympathetic, somatosensory, and motor systems.11 For example, Wasner et al12 have shown that thermoregulatory reflexes are disturbed in the distal parts of the affected extremity in patients with CRPS I, which has been attributed to central changes reflected by alterations in the activity in cutaneous vasoconstrictor neurons. Besides, Rommel et al13,14 have demonstrated that up to 50% of patients with chronic CRPS I develop hypoesthesia on the entire half of the body or in the upper quadrant ipsilateral to the affected limb. The anatomic distribution of these changes suggests that they might be due to changes in central processing of tactile stimuli, presumably at a thalamic or cortical level. Additionally, in many patients, motor symptoms occur, including muscle weakness, tremor, dystonia, and a neglectlike syndrome.15,16 These motor changes are unlikely to be related to a peripheral process but are supposed to be the result of specific alterations of the central motor system induced by the disease.1,11,17,18
During the past few years, increasing evidence for the hypothesis of CRPS as a CNS disease has come from a number of studies involving functional imaging methods. With functional MR imaging (fMRI), single-photon emission CT (SPECT), and mapping techniques based on electroencephalography (EEG), magnetoencephalography (MEG), and transcranial magnetic stimulation (TMS), alterations of central somatosensory and motor processing have been detected mainly in patients with CRPS I. In this review, we give an overview of these findings of CNS involvement in patients with CRPS, focused on the central somatosensory and motor system, and discuss them with respect to possible therapeutic implications.
Somatosensory System
In a pioneering study, Fukumoto et al in 199919 reported an alteration of contralateral thalamic perfusion in patients with CRPS I as revealed by iodine-123-labeled iodoamphetamine SPECT. This was the first functional imaging study providing evidence for an important role of the CNS in the pathogenesis of CRPS. This study was followed by a number of studies dealing with an altered central somatosensory network in CRPS. To examine possible alterations in the central processing of tactile stimuli, Juottonen et al20 used MEG to assess somatosensory-evoked fields in response to tactile stimulation applied to the tip of the thumb, the index finger, and the little finger in patients with CRPS I of the hand. They found a significantly stronger response in the contralateral primary somatosensory cortex (S1) and a nonsignificant tendency toward a stronger response in the contralateral secondary somatosensory cortex (S2) after stimulation of the index finger of the affected hand compared with the unaffected hand. In addition, the distance between the S1 representations of the thumb and little finger of the affected hand was significantly shorter compared with the representations of the thumb and little finger of the unaffected hand. However, an individual correlation between pain intensity and the amount of cortical alteration could not be established. These findings were interpreted with respect to an overlapping or smearing of cortical finger representations due to chronic noxious input or to simultaneous finger movements as a consequence of disturbed motor coordination.
Similar overlapping or smeared cortical finger representation previously had been observed in patients with painful focal dystonia.21 This reorganization of the cortical finger representations in S1 might explain the phenomenon of referred sensations, which has been described in patients with CRPS.22,23 The results by Juottonen et al20 were reproduced in another MEG study conducted by Maihöfner et al, 24 who also observed an increased strength of magnetic fields and a reduced distance between thumb and little finger representation after tactile stimulation in S1 contralateral to the affected hand. However, the study of Maihöfner et al even extended these findings, showing a shift of the cortical S1 representation of the affected hand toward the lip representation and establishing a correlation between the amount of cortical reorganization and the intensity of CRPS pain and the extent of mechanical hyperalgesia. In contrast to the previous MEG study, Maihöfner et al used the McGill Pain Questionnaire to assess sensory, affective, and evaluative components of chronic pain,25 instead of rating acute pain alone, which was not related to cortical reorganization. Other clinical features such as motor and autonomic symptoms, including misuse of the affected hand, did not correlate with the amount of cortical reorganization, emphasizing the impact of pain and hyperalgesia for these cortical phenomena. However, given the fact that pain-related cortical reorganization was also observed in other chronic pain syndromes,26-29 Maihöfner et al concluded that the observed cortical reorganization in S1 might not be CRPS-specific but might explain the complex sensory features occurring in CRPS.
Most interesting, Maihöfner et al30 conducted a follow-up study in their patients with CRPS in order to get information about the time course of S1 reorganization at least 1 year after therapy. They found a reversal of cortical reorganization coincident with clinical improvement. The only factor that predicted this reduction of cortical reorganization was the reduction of pain, putting additional emphasis on the relationship between S1 reorganization and chronic pain.
Using a different methodologic approach, Pleger et al31 used EEG to localize dipole sources of somatosensory-evoked potentials (SEP) after electrical stimulation of the median and ulnar nerve in patients with CRPS I. Results were similar to the results observed in the MEG studies after tactile finger stimulation: After electrical stimulation of the median and ulnar nerve, they found a significantly smaller distance between dipole sources of the N20 SEP component, which are generated in area 3b of S1, after stimulation of the nerves at the affected hand compared with the unaffected hand. This indicated a smaller S1 representation of the CRPS-affected hand and probably a higher overlap of the cortical somatosensory representation of the median and ulnar nerve. Similar to the findings in the MEG studies, the amount of cortical reorganization significantly correlated with the degree of chronic CRPS pain but failed to correlate with current pain intensity or with the degree of immobilization.
fMRI during electrical stimulation of the index finger was combined with assessment of 2-point-discrimination thresholds as a marker for tactile perception, to get more information about possible cortical reorganization not only in S1 but also in S2 and to assess possible perceptual consequences of S1 and S2 reorganization in patients with CRPS.32 In this study, cortical signals within S1 and S2 were significantly reduced contralateral to the CRPS-affected index finger compared with the ipsilateral side and with the representation in healthy controls. In addition, correlation analysis revealed a significant correlation between mean sustained pain intensity, 2-point-discrimination threshold, and reduction in signal-intensity strength in the contralateral S1 and S2 after stimulation of the CRPS-affected index finger (Fig 1). Hence, low pain levels were associated with small side-to-side differences, whereas patients with a distinctive hemispheric and discriminative asymmetry reported the highest pain levels. Again, neither reduced use nor current pain intensity significantly correlated with the cortical changes in S1 and S2. It was concluded that the observed changes in S2 might arise from a reduced forward propagation of inputs generated in corresponding S1 maps, given the fact that the activation level of S2 depends on either direct or indirect inputs from S1.33 Besides, this study demonstrated the functional relevance of cortical S1 and S2 reorganization with respect to the central processing of tactile stimuli. As a possible explanation, it was suggested that ongoing painful inputs might lead to an enhanced activation level of neurons responding to nociceptive inputs at the expense of neurons involved in tactile perception.
Fig 1.
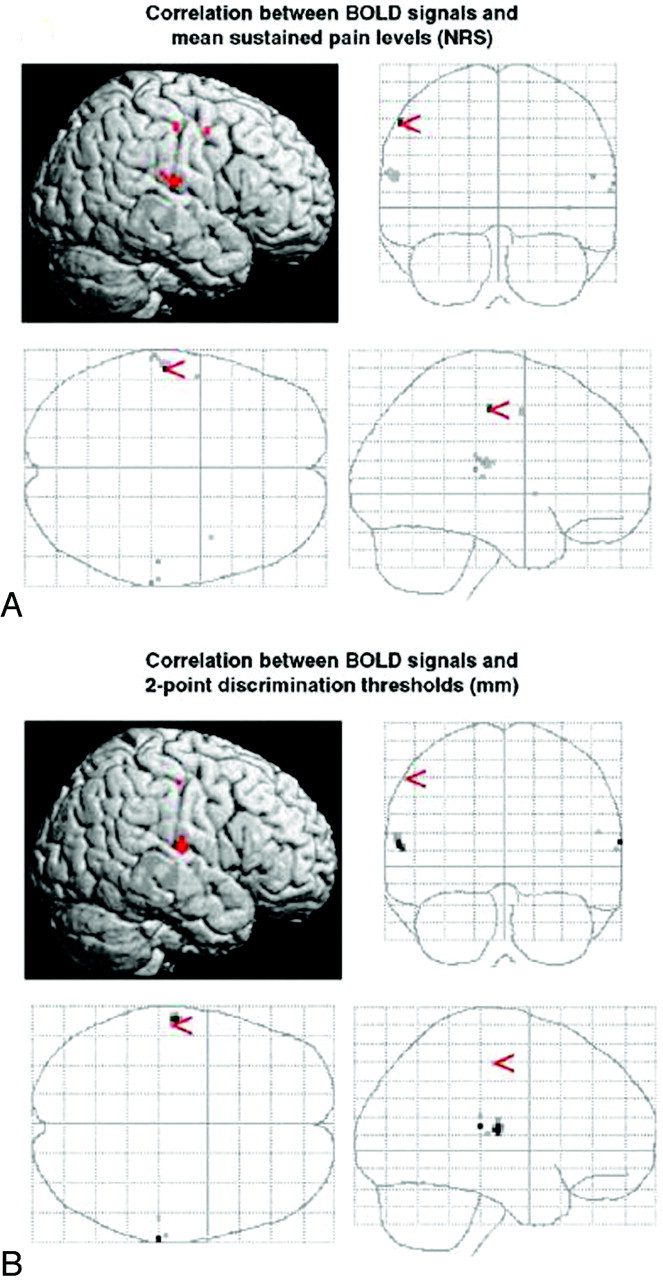
Correlation analysis. BOLD contrast negatively correlates to mean sustained pain levels (A) and 2-point-discrimination thresholds (B). Pain-related BOLD contrast is found in Brodmann area (BA) 1 of the S1 (A), whereas perception-related BOLD contrast is found in BA 2 of S1 (B). Both analyses reveal BOLD contrast localized in the same region of S2. NRS indicates numeric rating scale. Reprinted with permission of Elsevier from Pleger et al.32
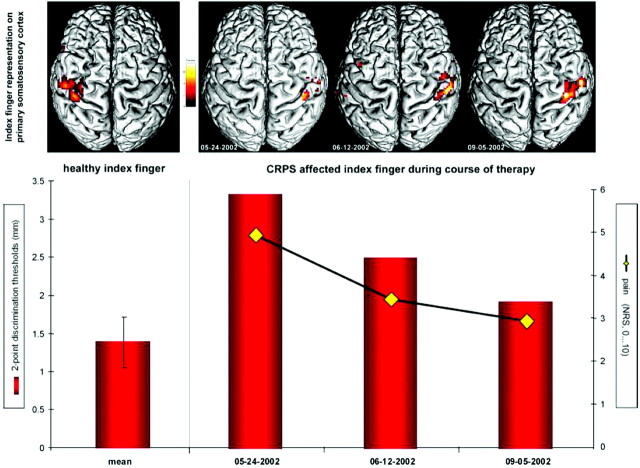
In 6 of these patients with CRPS who were treated by antinociceptive drug therapy accompanied by a pain-adapted sensorimotor training program for 1–6 months, a follow-up study was conducted, and the time course of S1 and S2 activation after stimulation of the index finger was assessed by fMRI as well as pain intensity and 2-point-discrimination performance.34 The treatment led to a persistent decrease in pain intensity, which was accompanied by a restoration of the impaired tactile discrimination and an increase in cortical map size of the affected index finger in contralateral S1 and S2 (Fig 2). It was suggested that the rehabilitative training might strengthen sensory and proprioceptive feedback mechanisms that compete with nociceptive inputs and interact with relays of pain processing and cortical maps in contralateral S1 and S2.
Fig 2.
Changes in S1 representation, pain intensity, and discrimination ability during the course of therapy in a single patient. BOLD contrast received from cortical maps on S1 contralateral to the healthy (left image) and to the CRPS-affected side (right, images of 3 consecutive measurements). Contrast maps are shown from above, projected on the individual rendered T1-weighted MR imaging dataset. The diagram below shows 2-point-discrimination thresholds of the healthy index finger (left column) and of the CRPS-affected index finger (right, columns indicate values of 3 consecutive measurements) as well as the intensity of CRPS pain (yellow rhombus, 3 consecutive evaluations). NRS indicates numeric rating scale. Reprinted with permission of Wiley-Liss from Pleger et al.34
The above-mentioned studies were mainly designed to examine brain regions activated by either natural or electrical stimulation of fast-conduction Aβ fibers and, therefore, involved in the processing of tactile stimuli. In another study, Maihöfner et al35 used fMRI to examine the cortical network involved in the processing of pin-prick hyperalgesia in patients with CRPS I, because pin-pricking is known to activate Aδ fibers.36,37 Compared with nonpainful mechanical stimulation, during pin-prick hyperalgesia, there was a significantly increased activation of the S1 cortex contralaterally, S2 cortex bilaterally, insular cortex bilaterally, parietal association cortex (PA) contralaterally, inferior parietal lobule (IPL) bilaterally, superior, middle, and inferior frontal cortex bilaterally, and the anterior cingulate cortex bilaterally. It was concluded that the cortical network underlying pin-prick hyperalgesia comprises areas involved in nociceptive, motor, and attention processing, which might account for a number of clinical symptoms occurring in CRPS.
In another fMRI study conducted in patients with CRPS I, the cortical network underlying dynamic-mechanical allodynia was assessed.38 Dynamic mechanical allodynia is characterized by pain elicited by usually nonpainful tactile stimuli and is mediated by Aβ fibers, which get access to the nociceptive system due to central sensitization.39 Similar to pin-prick hyperalgesia, mechanical allodynia led to widespread cortical activations, including brain regions involved in motor and cognitive processing. In contrast, deactivations were detected in the visual, vestibular, and temporal cortices, suggesting a shift of activation from tonically active sensory systems into somatosensory-related brain areas. However, it remained an open question if these findings were CRPS-specific or if they were found in allodynia in general, because similar patterns of cortical activation during allodynia testing in a heterogeneous group of patients with neuropathic pain were observed with respect to the S2/insular cortices.40
Recently, fMRI was used to assess patterns of CNS activation following mechanical and thermal stimuli for the first time in children with CRPS of the lower limb.41 In contrast to adults, CRPS symptoms in pediatric patients often clinically resolve within several months to 2 years,42 which offered the possibility to study children during an active period of pain and after symptomatic recovery. During active pain, stimuli to the affected limb produced a greater level of positive blood oxygen level–dependent (BOLD) activations than stimuli to the unaffected limb, similar to the responses seen in adults,38 suggesting similar underlying pathophysiologic mechanisms. Most interesting, in the asymptomatic state, there were still significant differences in the CNS responses after stimulation of the affected and the unaffected limbs, especially involving regions in the frontal and parietal lobes, insula, and basal ganglia (ie, in regions involved in cognitive and affective function). However, the functional relevance of this finding (eg, with respect to pain processing) in later life remained unclear.
Motor System
Using TMS mapping of the motor cortex (MC) representation of the long extensor muscles of the fingers, Krause et al43 found a significant interhemispheric asymmetry between the MC representation of the affected and the unaffected hands with respect to the size, cumulative motor-evoked potentials amplitude, and volume of the motor output map.43 The MC representation corresponding to the unaffected hand was significantly larger compared with the representation corresponding to the affected hand. These findings resembled the representational asymmetries in S120,24,31,32 and might be explained by the close anatomic and functional relationship between MC and S1.44-47 The MC representational asymmetry was discussed with respect to chronic pain, though no direct relationship between the amount of interhemispheric asymmetry and pain intensity could be established.
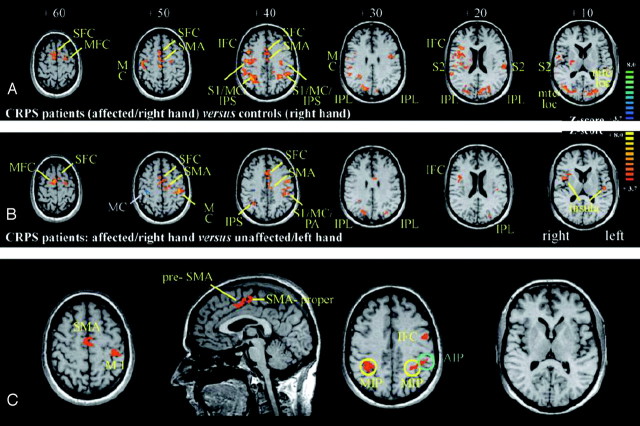
To assess possible alterations of the central motor system more closely, Maihöfner et al48 used fMRI to investigate cerebral activations during motor performance (finger tapping) in patients with CRPS I of the upper extremity. During finger tapping on the CRPS affected side, markedly larger brain activations were detected compared with those in healthy control subjects but also compared with the patient's unaffected side (Fig 3A, -B). The greater response during finger tapping on the CRPS-affected side involved the classic motor areas (MC), supplementary motor cortices (SMA), and intraparietal sulci (IPS) bilaterally, but also the bilateral S1, S2, IPL, and the superior frontal cortices as well as the ipsilateral middle and inferior frontal cortices. In addition, the individual degree of motor impairment as assessed by the individual tapping frequencies, significantly correlated with contralateral MC and bilateral SMA activations but also with bilateral activity in the medial area and left hemispheric activity in the anterior area of IPS, as well as with activity within the contralateral inferior frontal cortex (Fig 3C). The larger brain activation during finger tapping in CRPS was discussed with respect to defective inhibitory mechanisms at the level of the MC, which already had been demonstrated in patients with CRPS I by using MEG and paired-pulse TMS in previous studies.18,20,49,50 The authors concluded that substantial adaptive changes within the CNS might contribute to motor symptoms in CRPS.
Fig 3.
A, Contrast map of the general linear model (GLM) calculated by subtracting the tapping condition of controls (right hand) from the tapping condition in patients (CRPS-affected side [ie, right hand]). Brain sections are referred to by the superior-inferior level in millimeters relative to a line through the anterior and posterior commissure. Z-score indicates level of significance. B, Contrast map of the GLM calculated by subtracting the tapping condition on the unaffected side (ie, left hand) from the affected side (ie, right hand) in patients with CRPS. Brain sections are referred to by the superior-inferior level in millimeters relative to a line through the anterior and posterior commissure. Z-score indicates level of significance. C, Implementation of the individual degrees of motor impairment (z-values of tapping frequencies [ie, values of the individual maximum tapping frequencies normalized to the mean maximum tapping frequencies of controls]) as regressors in the GLM model for the condition “tapping on the CRPS-affected side.” With this approach, brain areas correlating with the degree of motor impairment are isolated. Key areas of activations are indicated according to the following abbreviations: SFC indicates superior frontal cortex; MFC, middle frontal cortex; IFC, inferior frontal cortex; AIP, anterior intraparietal area, MIP, medial intraparietal area; mtc, middle temporal cortex; loc, lateral occipital complex. Reprinted with permission of Oxford University Press from Maihöfner et al.48
Whereas patients with CRPS with dystonia were excluded in the study by Maihöfner et al48 to achieve a homogeneous patient group, patients with CRPS I with dystonia were explicitly examined by fMRI in a recent study by Gieteling et al.51 The distribution of cerebral activations was assessed during both motor execution and imaging of movement of affected as well as unaffected limbs and compared with the activation pattern in healthy controls. There were no differences between patients and controls when they executed movements or when they imagined moving their unaffected hand. In contrast, compared with controls, imagined movement of the affected hand in patients with CRPS led to less activation in the ipsilateral prefrontal and premotor cortices and the anterior part of the insular cortex. In the contralateral hemisphere, the postcentral gyrus and the inferior parietal cortex were less activated. The authors concluded that patients with CRPS I and dystonia displayed areas with decreased activation during imaging of movements that are involved in planning of movement, multimodal sensorimotor integration, autonomic function, and pain. It was suggested that chronic pain might alter the cerebral organization of movement by functional interaction between these regions, which might explain the occurrence of motor symptoms like dystonia in CRPS.
Conclusions
During the last decade, functional imaging studies provided increasing evidence for an important role of the CNS in the pathogenesis of CRPS. Especially, reorganization in central somatosensory and motor networks was demonstrated, leading to an altered central processing of tactile and nociceptive stimuli, as well as to an altered cerebral organization of movement. In a number of studies, typical clinical CRPS symptoms could be directly linked to this CNS reorganization, such as impaired tactile perception (hypoesthesia) in the absence of peripheral nerve lesions, dystonia, or reduced finger-tapping frequency as a marker of motor impairment.32,48,51 Many studies provided evidence for a close relationship between chronic pain and CNS reorganization in somatosensory and motor networks in CRPS. It can be hypothesized that persistent nociceptive CNS inputs, probably due to peripheral mechanisms such as neurogenic inflammation, interfere with central networks of tactile perception and motor control, therefore inducing plastic changes in these networks. An alternative but not mutually exclusive hypothesis is that the disturbance of cortical representations of movement and tactile perception itself promotes pain perception, being at least in part cause and not only consequence of chronic pain in patients with CRPS. The latter hypothesis is supported by the fact that neurorehabilitative strategies, which target cortical areas and aim to restore impaired sensorimotor function in patients with CRPS, have proved to be effective not only in restoring impaired function, but also in pain reduction.52,53 Again functional imaging techniques might be a useful tool to accompany such therapy studies,34 to help in developing optimized therapies to restore the alterations occurring in somatosensory and motor network in patients with CRPS.
References
- 1.Jänig W, Baron R. Complex regional pain syndrome: mystery explained? Lancet Neurol 2003;2:687–97 [DOI] [PubMed] [Google Scholar]
- 2.Wasner G, Backonja MM, Baron R. Traumatic neuralgias: complex regional pain syndromes (reflex sympathetic dystrophy and causalgia)—clinical characteristics, pathophysiological mechanisms and therapy. Neurol Clin 1998;16:851–68 [DOI] [PubMed] [Google Scholar]
- 3.Baron R, Wasner G. Complex regional pain syndromes. Curr Pain Headache Rep 2001;5:114–23 [DOI] [PubMed] [Google Scholar]
- 4.Stanton-Hicks M, Jänig W, Hassenbusch S, et al. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain 1995;63:127–33 [DOI] [PubMed] [Google Scholar]
- 5.Harden RN, Bruehl S, Stanton-Hicks M, et al. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med 2007;8:326–31 [DOI] [PubMed] [Google Scholar]
- 6.Bruehl S, Harden RN, Galer BS, et al. External validation of IASP diagnostic criteria for complex regional pain syndrome and proposed research diagnostic criteria: International Association for the Study of Pain. Pain 1999;81:147–54 [DOI] [PubMed] [Google Scholar]
- 7.Birklein F, Handwerker HO. Complex regional pain syndrome: how to resolve the complexity? Pain 2001;94:1–6 [DOI] [PubMed] [Google Scholar]
- 8.Weber M, Birklein F, Neundörfer B, et al. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain 2001;91:251–57 [DOI] [PubMed] [Google Scholar]
- 9.Sieweke N, Birklein F, Riedl B, et al. Patterns of hyperalgesia in complex regional pain syndrome. Pain 1999;80:171–77 [DOI] [PubMed] [Google Scholar]
- 10.Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science 1991;251:1608–10 [DOI] [PubMed] [Google Scholar]
- 11.Jänig W, Baron R. Complex regional pain syndrome is a disease of the central nervous system. Clin Auton Res 2002;12:150–64 [DOI] [PubMed] [Google Scholar]
- 12.Wasner G, Schattschneider J, Heckmann K, et al. Vascular abnormalities in reflex sympathetic dystrophy (CRPS I): mechanisms and diagnostic value. Brain 2001;124:587–99 [DOI] [PubMed] [Google Scholar]
- 13.Rommel O, Gehling M, Dertwinkel R, et al. Hemisensory impairment in patients with complex regional pain syndrome. Pain 1999;80:95–101 [DOI] [PubMed] [Google Scholar]
- 14.Rommel O, Malin JP, Zenz M, et al. Quantitative sensory testing, neurophysiological and psychological examination in patients with complex regional pain syndrome and hemisensory deficits. Pain 2001;93:279–93 [DOI] [PubMed] [Google Scholar]
- 15.Schwartzman RJ, Kerrigan J The movement disorder of reflex sympathetic dystrophy. Neurology 1990;40:57–61 [DOI] [PubMed] [Google Scholar]
- 16.Frettlöh J, Huppe M, Maier C. Severity and specificity of neglect-like symptoms in patients with complex regional pain syndrome (CRPS) compared to chronic limb pain of other origins. Pain 2006;124:184–89 [DOI] [PubMed] [Google Scholar]
- 17.van Hilten JJ, van De Beek WJ, Vein AA, et al. Clinical aspects of multifocal or generalized tonic dystonia in reflex sympathetic dystrophy. Neurology 2001;56:1762–65 [DOI] [PubMed] [Google Scholar]
- 18.Schwenkreis P, Janssen F, Rommel O, et al. Bilateral motor cortex disinhibition in complex regional pain syndrome (CRPS) type I of the hand. Neurology 2003;61:515–19 [DOI] [PubMed] [Google Scholar]
- 19.Fukumoto M, Ushida T, Zinchuk VS, et al. Contralateral thalamic perfusion in patients with reflex sympathetic dystrophy syndrome. Lancet 1999;354:1790–91 [DOI] [PubMed] [Google Scholar]
- 20.Juottonen K, Gockel M, Silen T, et al. Altered central sensorimotor processing in patients with complex regional pain syndrome. Pain 2002;98:315–23 [DOI] [PubMed] [Google Scholar]
- 21.Elbert T, Candia V, Altenmüller E, et al. Alteration of digital representations in somatosensory cortex in focal hand dystonia. Neuroreport 1998;9:3571–75 [DOI] [PubMed] [Google Scholar]
- 22.Maihöfner C, Neundorfer B, Birklein F, et al. Mislocalization of tactile stimulation in patients with complex regional pain syndrome. J Neurol 2006;253:772–79 [DOI] [PubMed] [Google Scholar]
- 23.McCabe CS, Haigh RC, Halligan PW, et al. Referred sensations in patients with complex regional pain syndrome type 1. Rheumatology (Oxford) 2003;42:1067–73. Epub 2003 Apr 16 [DOI] [PubMed] [Google Scholar]
- 24.Maihöfner C, Handwerker HO, Neundorfer B, et al. Patterns of cortical reorganization in complex regional pain syndrome. Neurology 2003;61:1707–15 [DOI] [PubMed] [Google Scholar]
- 25.Stein C, Mendl G. The German counterpart to McGill Pain Questionnaire. Pain 1988;32:251–55 [DOI] [PubMed] [Google Scholar]
- 26.Flor H, Braun C, Elbert T, et al. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett 1997;224:5–8 [DOI] [PubMed] [Google Scholar]
- 27.Flor H, Elbert T, Knecht S, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 1995;375:482–84 [DOI] [PubMed] [Google Scholar]
- 28.Tecchio F, Padua L, Aprile I, et al. Carpal tunnel syndrome modifies sensory hand cortical somatotopy: a MEG study. Hum Brain Mapp 2002;17:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maihöfner C, Neundorfer B, Stefan H, et al. Cortical processing of brush-evoked allodynia. Neuroreport 2003;14:785–89 [DOI] [PubMed] [Google Scholar]
- 30.Maihöfner C, Handwerker HO, Neundorfer B, et al. Cortical reorganization during recovery from complex regional pain syndrome. Neurology 2004;63:693–701 [DOI] [PubMed] [Google Scholar]
- 31.Pleger B, Tegenthoff M, Schwenkreis P, et al. Mean sustained pain levels are linked to hemispherical side-to-side differences of primary somatosensory cortex in the Complex Regional Pain Syndrome I. Exp Brain Res 2004;155:115–19 [DOI] [PubMed] [Google Scholar]
- 32.Pleger B, Ragert P, Schwenkreis P, et al. Patterns of cortical reorganization parallel impaired tactile discrimination and pain intensity in complex regional pain syndrome. Neuroimage 2006;32:503–10 [DOI] [PubMed] [Google Scholar]
- 33.Garraghty PE, Pons TP, Kaas JH. Ablations of areas 3b (SI proper) and 3a of somatosensory cortex in marmosets deactivate the second and parietal ventral somatosensory areas. Somatosens Mot Res 1990;7:125–35 [DOI] [PubMed] [Google Scholar]
- 34.Pleger B, Tegenthoff M, Ragert P, et al. Sensorimotor retuning in complex regional pain syndrome parallels pain reduction. Ann Neurol 2005;57:425–29 [DOI] [PubMed] [Google Scholar]
- 35.Maihöfner C, Forster C, Birklein F, et al. Brain processing during mechanical hyperalgesia in complex regional pain syndrome: a functional MRI study. Pain 2005;114:93–103 [DOI] [PubMed] [Google Scholar]
- 36.Baumgartner U, Magerl W, Klein T, et al. Neurogenic hyperalgesia versus painful hypoalgesia: two distinct mechanisms of neuropathic pain. Pain 2002;96:141–51 [DOI] [PubMed] [Google Scholar]
- 37.Ziegler EA, Magerl W, Meyer RA, et al. Secondary hyperalgesia to punctate mechanical stimuli: central sensitization to A-fibre nociceptor input. Brain 1999;122 (pt 12):2245–57 [DOI] [PubMed] [Google Scholar]
- 38.Maihöfner C, Handwerker HO, Birklein F. Functional imaging of allodynia in complex regional pain syndrome. Neurology 2006;66:711–17 [DOI] [PubMed] [Google Scholar]
- 39.Torebjörk HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol 1992;448:765–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peyron R, Schneider F, Faillenot I, et al. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology 2004;63:1838–46 [DOI] [PubMed] [Google Scholar]
- 41.Lebel A, Becerra L, Wallin D, et al. fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain 2008;131:1854–79 [DOI] [PubMed] [Google Scholar]
- 42.Low AK, Ward K, Wines AP. Pediatric complex regional pain syndrome. J Pediatr Orthop 2007;27:567–72 [DOI] [PubMed] [Google Scholar]
- 43.Krause P, Förderreuther S, Straube A. TMS motor cortical brain mapping in patients with complex regional pain syndrome type I. Clin Neurophysiol 2006;117:169–76 [DOI] [PubMed] [Google Scholar]
- 44.Lemon RN. Functional properties of monkey motor cortex neurones receiving afferent input from the hand and fingers. J Physiol 1981;311:497–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porter LL. Patterns of connectivity in the cat sensory-motor cortex: a light and electron microscope analysis of the projection arising from area 3a. J Comp Neurol 1991;312:404–14 [DOI] [PubMed] [Google Scholar]
- 46.Porter LL. Morphological characterization of a cortico-cortical relay in the cat sensorimotor cortex. Cereb Cortex 1997;7:100–09 [DOI] [PubMed] [Google Scholar]
- 47.Kaneko T, Caria MA, Asanuma H. Information processing within the motor cortex. II. Intracortical connections between neurons receiving somatosensory cortical input and motor output neurons of the cortex. J Comp Neurol 1994;345:172–84 [DOI] [PubMed] [Google Scholar]
- 48.Maihöfner C, Baron R, DeCol R, et al. The motor system shows adaptive changes in complex regional pain syndrome. Brain 2007;130:2671–87 [DOI] [PubMed] [Google Scholar]
- 49.Schwenkreis P, Maier C, Tegenthoff M. Motor cortex disinhibition in complex regional pain syndrome (CRPS): a unilateral or bilateral phenomenon? Pain 2005;115:219–20; author reply 20–21 [DOI] [PubMed] [Google Scholar]
- 50.Eisenberg E, Chistyakov AV, Yudashkin M, et al. Evidence for cortical hyperexcitability of the affected limb representation area in CRPS: a psychophysical and transcranial magnetic stimulation study. Pain 2005;113:99–105 [DOI] [PubMed] [Google Scholar]
- 51.Gieteling EW, van Rijn MA, de Jong BM, et al. Cerebral activation during motor imagery in complex regional pain syndrome type 1 with dystonia. Pain 2008;134:302–09 [DOI] [PubMed] [Google Scholar]
- 52.McCabe CS, Haigh RC, Ring EF, et al. A controlled pilot study of the utility of mirror visual feedback in the treatment of complex regional pain syndrome (type 1). Rheumatology (Oxford)2003;42:97–101 [DOI] [PubMed] [Google Scholar]
- 53.Moseley GL, Zalucki NM, Wiech K. Tactile discrimination, but not tactile stimulation alone, reduces chronic limb pain. Pain 2008;137:600–08 [DOI] [PubMed] [Google Scholar]