Abstract
SUMMARY: Late infantile GM1 gangliosidosis is a rare lysosomal disorder characterized by mental deterioration and progressive spastic, cerebellar, and extrapyramidal signs, without facial dysmorphisms and organomegaly. Neuroimaging findings have been reported in only a few cases. Here we report on predominant globus pallidus MR signal-intensity abnormalities in 2 patients with the late infantile form of GM1 gangliosidosis.
GM1 gangliosidosis is a rare metabolic disorder due to deficiency of the lysosomal enzyme β-galactosidase, resulting in accumulation of GM1 gangliosides and other glycoconjugates in the brain and visceral organs. There are 3 clinical forms correlating with the degree of residual activity of the mutant enzyme. The infantile form (type 1) is a severe degenerative encephalopathy presenting between birth and 6 months with coarse facial features, skeletal dysostosis, and hepatosplenomegaly, leading to death within the first 2 years of life. Patients with the late infantile or juvenile form (type 2) present after 1 year of age with motor delay in the absence of dysmorphisms and organomegaly; later, mental deterioration and spastic, cerebellar, and extrapyramidal signs dominate the neurologic picture, probably as a consequence of predominant basal ganglia storage of gangliosides.1 The adult form (type 3) has a slowly progressive course and predominant extrapyramidal features without visceral or skeletal changes.2 Reports on the neuroimaging features of the very rare late infantile GM1 gangliosidosis are scant and have mainly included brain atrophy and white matter and basal ganglia abnormalities.3,4 We report 2 young patients in whom MR imaging predominantly showed evidence of globus pallidum paramagnetic ion accumulation.
Case Reports
Case 1
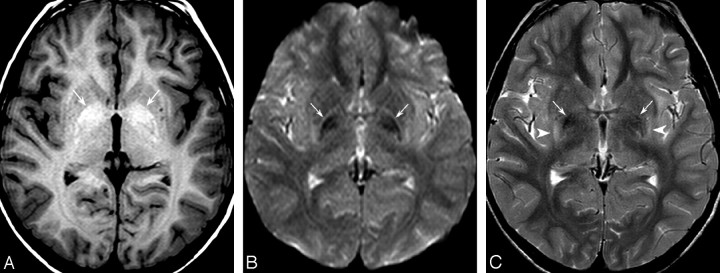
This 8-year-old boy presented with developmental delay and recent swallowing difficulties. He was born at term from consanguineous parents. Family history was negative. He started to walk at 2 years of age and had language delay. At 8 years, he had a generalized seizure. Findings of the physical examination were normal with no dysmorphic features or hepatosplenomegaly. Neurologic examination revealed extrapyramidal rigidity and dystonic postures affecting particularly the upper limbs. Speech was severely impaired because of oromandibular dystonia. He was dysmetric and had an ataxic gait. Reflexes were normal. Routine blood tests, α-fetoprotein, vitamin E, copper, and ceruloplasmin titers were negative. Metabolic work-up, including blood and urine chromatography of amino acids and urinary organic acids, and peroxysomal function tests, were unremarkable. Fundoscopy findings, visual and brain stem auditory evoked potentials, and nerve conduction velocity were normal, whereas electromyography disclosed mild neurogenic abnormalities. Electroencephalography showed nonspecific bilateral occipitotemporal slow waves. Lysosomal enzyme assay in cultured skin fibroblasts revealed a β-galactosidase activity of 5.8 nmol/mg/h (normal mean, 145 ± 32 nmol/mg/h). Spine x-rays revealed a short anteroposterior diameter of the vertebral bodies and anterior hypoplasia (hook-shaped) at the thoracolumbar level; MR imaging showed bilateral hyperintensity of the globus pallidum on T1-weighted images (Fig 1A), with corresponding low signal intensity on gradient-echo T2*-weighted images and on both b=0 (Fig 1B) and b=1000 diffusion-weighted images and, less conspicuously, on T2-weighted (Fig 1C) and fluid-attenuated inversion recovery (FLAIR) images, consistent with susceptibility effects from paramagnetic ion deposition. The latter 2 sequences also revealed high signal intensity of the posterior putamen bilaterally.
Fig 1.
1.5T MR images in an 8-year-old boy with GM1 gangliosidosis. A, Spin-echo T1-weighted image (TR/TE/NEX, 655 ms/15 ms/2) shows abnormal hyperintensity of the globus pallidum bilaterally (arrows). B, B0 diffusion-weighted image (TR/TE/NEX, 3072 ms/70 ms/1) confirms profound hypointensity of both pallida (arrows), consistent with paramagnetic effects. C, Axial fast spin-echo T2-weighted image (TR/TE/NEX, 4161 ms/100 ms/2) shows pallidal hypointensity (arrows) associated with hyperintensity of the posterior putamen bilaterally (arrowheads). Notice normal white matter signal intensity.
Case 2
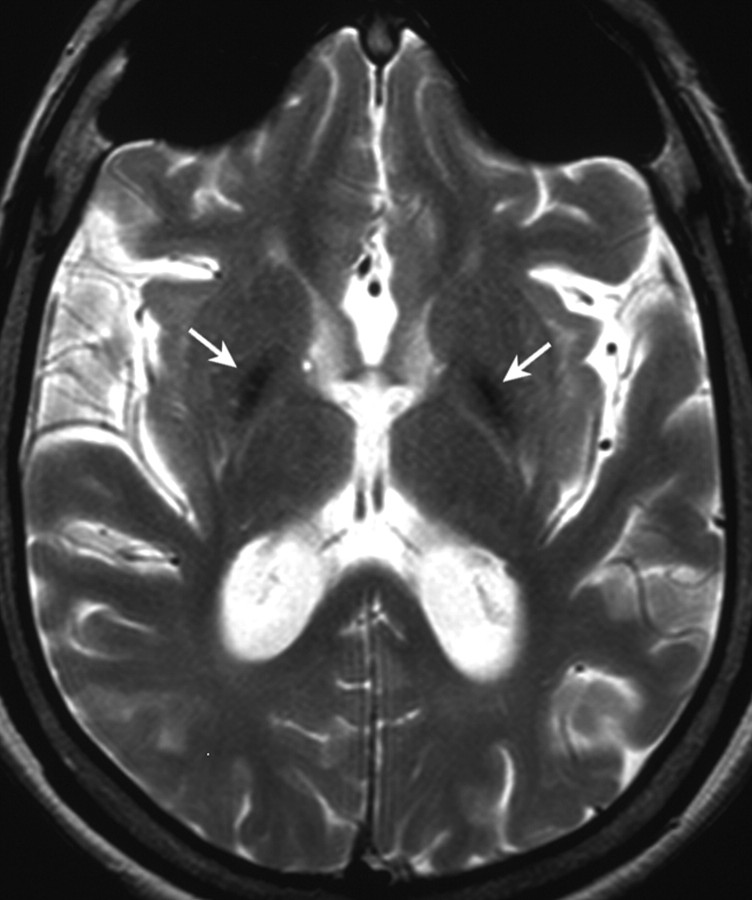
This 20-year-old girl was diagnosed with GM1 gangliosidosis at 3 years of age by β-galactosidase assay on skin fibroblasts, which resulted in 63 nmol/mg/h (normal, 117–408 nmol/mg/h). Parents were nonconsanguineous, and the family history was negative for neurological disorders. She was born at term after a normal pregnancy. She presented with psychomotor delay from 2 years of age; flattened vertebral bodies with a hook-shaped deformity at the thoracolumbar junction were first detected at the age of 3. The subsequent clinical course was characterized by progressive spastic-dystonic tetraparesis. At 19 years of age, she was readmitted because of swallowing difficulties, worsening of dystonic postures, and marked dysarthria with loss of phonatory competences. Brain stem auditory evoked potentials and sensory conduction velocity were normal, whereas motor conduction velocity was slightly reduced. MR imaging showed generalized brain atrophy and atrophic T2 hyperintense putamina. Both globi pallidi showed profound signal-intensity hypointensity on T2-weighted (Fig 2) and FLAIR images and on b=0 and b=1000 diffusion-weighted images, consistent with paramagnetic ion depositions.
Fig 2.
Axial T2-weighted image from patient 2 shows hypointense pallida (arrows) and diffuse cortical atrophy.
Discussion
Iron accumulation within the extrapyramidal nuclei is a physiologic phenomenon of aging. During the first 10 years of life, these nuclei are isointense with cortical gray matter in all MR images. In most patients by 25 years of age, the globus pallidum, followed by the red nucleus and pars reticulate of the substantia nigra, become hypointense relative to cortical gray matter on long TR/TE sequences, whereas the dentate nucleus decreases in signal intensity more slowly and inconsistently. Hypointensity of the striate nucleus is typical of elderly individuals and may equal that of the globus pallidum in the eighth decade.5 Shortening of both T1 and T2 relaxation times is due to iron deposition and probably arises from proton diffusion through local areas of magnetic inhomogeneity, due to the iron-containing moieties; the magnitude of this effect is dependent on several factors, including concentration of paramagnetic substances, signal intensity–to-noise ratio, and field strength.
Paramagnetic ion (ie, iron, calcium, and copper) deposition is a common phenomenon in several neurodegenerative diseases. In disorders such as Alzheimer disease, Parkinson disease, and multiple sclerosis, iron deposition plays a causal role in neurodegeneration, probably through increased oxidative stress. Furthermore, neurodegeneration with brain iron accumulation (NBIA) is a group of infantile-, juvenile-, and adult-onset genetic disorders characterized by iron deposition and associated with neuronal death, which include pantothenate kinase−associated neurodegeneration (formerly known as Hallervorden-Spatz disease), hereditary ferritinopathy, infantile neuroaxonal dystrophy, and aceruloplasminemia. Dramatic evidence of focal brain iron accumulation in the extrapyramidal nuclei with profound hypointensity on long TR/TE sequences is usually the first indication of NBIA; the globus pallidum is consistently involved, whereas specific signal-intensity abnormalities, such as the “eye-of-the-tiger” sign or additional nucleus involvement, allow phenotypic differentiation among the various disorders and may be helpful for clinical and subsequent molecular diagnosis.6 Globus pallidum T2 hypointensity is also a known feature of β-thalassemia major and human immunodeficiency virus, in which it is caused by iron deposition, and of Wilson disease, in which it reflects the paramagnetic properties of copper. However, a large subgroup of patients with imaging findings of extrapyramidal paramagnetic ion deposition still remains without a defined diagnosis.
To our knowledge, MR imaging findings in the different forms of GM1 gangliosidosis have only rarely been reported, with most articles focusing on thalamic and white matter abnormalities. Hyperattenuation of the thalami on CT with corresponding low signal intensity on T2-weighted images is the most consistently reported finding in the late infantile form3,7; this finding has been related to intracytoplasmic accumulation of hydrophobic ganglioside, resulting in lower water content of the involved structures.8 High T2-weighted signal intensity of the supratentorial white matter, consistent with abnormal myelination, has also been reported.3,4,7 We did not find similar abnormalities in our patients, in whom the thalami and the white matter showed normal signal intensity. Instead, both our patients presented with predominant abnormal signal intensities in the globus pallidum, consistent with paramagnetic ion deposition, and additional putaminal hyperintensities that might have suggested Wilson disease as a presumptive diagnosis.
In the pediatric age group, evidence of T1 and T2 shortening in the extrapyramidal nuclei is a consistently abnormal finding that should prompt further investigations aimed at revealing an underlying neurodegenerative disorder. Identification of additional diseases characterized by such findings may broaden the phenotypic spectrum, improve the diagnosis, and inform the genetic counseling. Our observations suggest that GM1 gangliosidosis should be included among these disorders.
In conclusion, we have highlighted predominant globus pallidus MR signal intensity abnormalities in patients with GM1 gangliosidosis. We suggest that this disease should be included in the differential diagnosis of young patients presenting with neuroimaging evidence of extrapyramidal nucleus paramagnetic ion deposition. We await further reports on larger series to shed light on the full neuroimaging spectrum of this disorder.
References
- 1.Suzuki Y, Sakuraba H, Oshima A. Beta-galactosidase deficiency (beta-galactosidosis): GM1 gangliosidosis and Morquio B disease. In: Scriver CR, Beaudet AL, Sly WS, et al., eds. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill;1995. :1785–2823
- 2.Yoshida K, Oshima A, Sakuraba H, et al. GM1 gangliosidosis in adults: clinical and molecular analysis of 16 Japanese patients. Ann Neurol 1992;31:328–32 [DOI] [PubMed] [Google Scholar]
- 3.Chen CY, Zimmermann RA, Lee CC, et al. Neuroimaging findings in late infantile GM1 gangliosidosis. AJNR Am J Neuroradiol 1998;19:1628–30 [PMC free article] [PubMed] [Google Scholar]
- 4.Guguraj A, Sztriha L, Hertecant J, et al. Magnetic resonance imaging findings and novel mutations in GM1 gangliosidosis. J Child Neurol 2005;20:57–60 [DOI] [PubMed] [Google Scholar]
- 5.Aoki S, Okada Y, Nishimura K, et al. Normal deposition of brain iron in childhood and adolescence: MR imaging at 1.5 T. Radiology 1989;172:381–85 [DOI] [PubMed] [Google Scholar]
- 6.McNeill A, Birchall D, Hayflick SJ, et al. T2* and FSE MRI distinguishes four subtypes of neurodegeneration with brain iron accumulation. Neurology 2008;70:1614–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erol I, Alehan F, Pourbagher MA, et al. Neuroimaging findings in infantile GM1 gangliosidosis. Eur J Pediatr Neurol 2006;10:245–48 [DOI] [PubMed] [Google Scholar]
- 8.Al-Essa M, Bakheet SM, Patay ZJ, et al. FDG PET, MRI and clinical observations in a patient with infantile GM1 gangliosidosis. Brain Dev 1999;21:559–62 [DOI] [PubMed] [Google Scholar]