Abstract
SUMMARY: We report a case of oligodendroglial hyperplasia detected by using high-resolution high-field MR imaging. This disorder is considered part of the spectrum of cortical migrational abnormalities and is found with increased incidence in patients with epilepsy. Surgery offers the best chance for cure in patients with medically refractory partial complex epilepsy. Accurate localization and detection of the full lesion extent by using a high-resolution imaging technique such as 3T MR imaging is important to surgical success. Detection of subtle dysplastic lesions such as oligodendroglial hyperplasia may be clinically relevant.
We report a case of oligodendroglial hyperplasia detected by using high-resolution high-field MR imaging. This disorder is considered in the spectrum of cortical migrational abnormalities and is found with increased incidence in patients with epilepsy. Because it may be amenable to surgical resection and possible cure, its detection by using 3T MR imaging is clinically important.
Case Report
A 21-year-old man presented with a 10-year history of partial complex epilepsy that had been refractory to multiple antiepileptic medications. At the time of surgical consultation, these included oxcarbazepine (Trileptal), levetiracetam (Keppra), and topiramate (Topamax). His seizures occurred with a frequency of approximately 2–3 times per week.
Semiologic and electroencephalographic (EEG) findings both supported a right frontal lobe epileptogenic focus. An interictal single-photon emission CT study suggested a right frontal lobe focus that correlated with a right frontal lobe focus also suspected on video EEG monitoring. Findings of a 1.5T MR imaging study at our institution revealed no abnormality. Subsequent 3T findings revealed an abnormality corresponding to the right frontal lobe electrophysiologic focus (Figs 1 and 2). The decision was made with the family for the patient to undergo surgical resection, given the good location and limited size of the anatomic abnormality. A subdural electrode grid was placed, and surgical resection of the right frontal lesion was performed without complication.
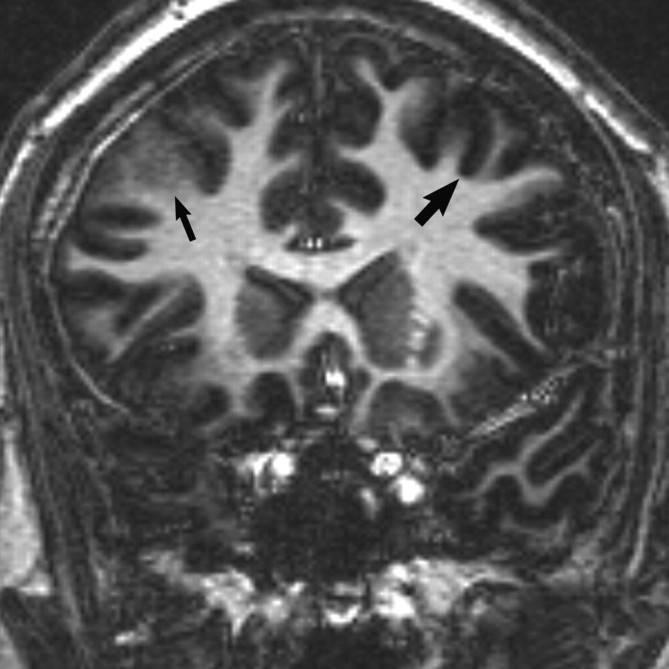
Fig 1.
Coronal 3D T1 echo-spoiled gradient-echo image demonstrates an area of cortical thickening and indistinctness involving the right frontal lobe (thin arrow). Compare with the normal left frontal cortex (wide arrow).
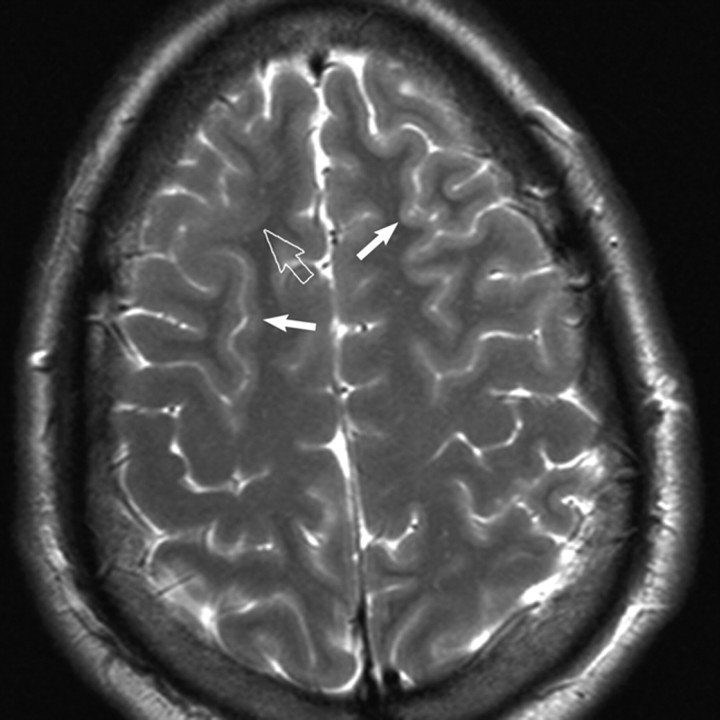
Fig 2.
Axial T2-weighted MR image reveals subtle cortical thickening and blurring of the gray-white matter junction (open arrow) in the right frontal lobe. Subtle gyral expansion is questioned. Note areas of normal thin-appearing cortex in the ipsilateral and contralateral hemisphere for comparison (closed arrows).
Histology revealed oligodendroglial hyperplasia, which is characterized by an increased number of oligodendrocytes in the juxtacortical white matter. Nuclei were uniform with no nucleoli. Immunoperoxidase stain for Ki-67 showed no staining, yielding a labeling index of essentially zero. Dr Peter Burger at Johns Hopkins Reference Laboratories, Baltimore, Md, also provided a consultative review, which concurred with the original diagnosis. The results of fluorescent in situ hybridization showed no evidence for 1p and 19q chromosomal deletions. No evidence for neoplasia was identified.
The patient experienced a decrease in seizure frequency immediately following surgery, though his seizures were not completely eliminated. Postoperative imaging suggested a small area of residual abnormality that may have accounted for incomplete resolution of seizures, though at the time of this review, no repeat resection had been performed.
Discussion
Oligodendroglial hyperplasia is a benign disorder, which is found with increased frequency in patients with epilepsy. It has previously been considered an occult lesion on imaging.1 Although benign, it may be the underlying cause for seizures in some patients. The ability to identify its presence with imaging allows the possibility of surgical cure.
Surgical resection offers the best hope for durable seizure control in patients with partial complex-type epilepsy, with the potential to decrease or even eliminate antiepileptic medication use. Surgical resection is the preferred treatment technique in patients refractory to medication.2 Many patients also experience negative drug-related side effects, and long-term medical therapy is expensive, so surgery is an attractive option.3 However, surgery relies on accurate lesion localization, so good-quality imaging is important for preoperative planning to allow complete resection.4–6
Histologically, oligodendroglial hyperplasia is characterized by an increased number of normal-appearing oligodendroglial cells in the cortex and juxtacortical white matter. It is considered at the mildest end of the spectrum of neuronal migrational disorders or microdysgenesis. Cortical migrational abnormalities, including forms of microdysgenesis, may be responsible for epilepsy. As with other disorders of cortical migration, oligodendroglial hyperplasia can also be found in patients without a history of seizures, so correlation with the patient's clinical presentation is important.1
High-resolution high-field MR imaging best evaluates epilepsy. Our practice requires good-quality imaging with whole-brain coverage, because we usually do not know the physiologic localization before scanning. We include the following unenhanced sequences on our 3T scanners by using an 8-channel parallel imaging−enabled head coil for epilepsy evaluation:
3D coronal T1-weighted turbo field-echo (TR, 8.5 ms/TE, 3.9 ms); acquisition matrix, 240 × 168 (reconstructed isovoxel size, 1 mm); FOV, 200–230 mm; section thickness, 1 mm (0 gap); section (frequency) reduction factor, 2; time, 6 minutes 16 seconds.
Axial turbo spin-echo T2-weighted sequence (TR, 3000 ms/TE, 80 ms; acquisition matrix, 416 × 271 (reconstructed voxel size, 0.43 in-plane); FOV, 200–230 mm; flip angle, 90°; section thickness, 4.0 mm (0.4-mm gap); phase (P) reduction factor, 1.7; time, 1 minute 57 seconds.
Coronal 2D short τ inversion recovery (TR, 8250 ms/TE, 20 ms; TI, 250 ms; acquisition matrix, 420 × 279 (reconstructed voxel size, 0.45 mm in-plane); FOV, 180–220 mm; section thickness, 2.0 mm (0.2-mm gap); P reduction factor, 3; phase oversampling, 2; time, 5 minutes 30 seconds.
Coronal fluid-attenuated inversion recovery (FLAIR) (TR, 11,000 ms/TE, 120 ms; TI, 2800 ms); acquisition matrix, 352 × 209 (reconstructed voxel size, 0.45 mm in-plane); FOV, 220–240 mm; section thickness, 4.0 mm (0.4-mm gap); P reduction factor, 1.7; time, 3 minutes 51 seconds.
Sagittal T1 FLAIR (TR, 2000 ms/TE, 10 ms; TI, 800 ms); FOV, 200–230 mm; section thickness, 4 mm (0.4-mm gap); acquisition matrix, 272 × 232 (reconstructed voxel size, 0.43 mm in-plane); P reduction factor, 1.7; time, 4 minutes 50 seconds.
In summary, the current case demonstrated blurring and thickening of cortex in the affected area at 3T imaging (though not visible on 1.5T MR imaging), which likely reflected the increased number of oligodendrocytes within juxtacortical white matter. Higher field MR imaging may be important for identifying forms of microdysgenesis such as oligodendroglial hyperplasia, which may be responsible for epilepsy.
References
- 1.Burger PC, Scheithauer BW, Vogel FS. The brain: surgery for seizures. In: Surgical Pathology of the Nervous System and Its Coverings. 4th ed. New York: Churchill Livingstone;2002
- 2.Phal PM, Usmanov A, Nesbit GM, et al. Qualitative comparison of 3-T and 1.5-T MRI in the evaluation of epilepsy. AJR Am J Roentgenol 2008;191:890–95 [DOI] [PubMed] [Google Scholar]
- 3.Cascino GD. Improving quality of life with epilepsy surgery: the seizure outcome is the key to success. Neurology 2007;68:1967–68 [DOI] [PubMed] [Google Scholar]
- 4.Langfitt JT, Holloway RG, McDermott MP, et al. Health care costs decline after successful epilepsy surgery. Neurology 2007;68:1290–98 [DOI] [PubMed] [Google Scholar]
- 5.Wyllie E, Lachhwani DK, Gupta A, et al. Successful surgery for epilepsy due to early brain lesions despite generalized EEG findings. Neurology 2007;69:389–97 [DOI] [PubMed] [Google Scholar]
- 6.Urbach H, Binder D, von Lehe M, et al. Correlation of MRI and histopathology in epileptogenic parietal and occipital lobe lesions. Seizure 2007;16:608–14. Epub 2007 Jun 11 [DOI] [PubMed] [Google Scholar]