SUMMARY:
Transarterial embolization in the external carotid artery (ECA) territory has a major role in the endovascular management of epistaxis, skull base tumors, and dural arteriovenous fistulas. Knowledge of the potential anastomotic routes, identification of the cranial nerve supply from the ECA, and the proper choice of embolic material are crucial to help the interventionalist avoid neurologic complications during the procedure. Three regions along the skull base constitute potential anastomotic routes between the extracranial and intracranial arteries: the orbital, the petrocavernous, and the upper cervical regions. Branches of the internal maxillary artery have anastomoses with the ophthalmic artery and petrocavernous internal carotid artery (ICA), whereas the branches of the ascending pharyngeal artery are connected to the petrocavernous ICA. Branches of both the ascending pharyngeal artery and the occipital artery have anastomoses with the vertebral artery. To avoid cranial nerve palsy, one must have knowledge of the supply to the lower cranial nerves: The petrous branch of the middle meningeal artery and the stylomastoid branch of the posterior auricular artery form the facial arcade as the major supply to the facial nerve, and the neuromeningeal trunk of the ascending pharyngeal artery supplies the lower cranial nerves (CN IX–XII).
In the last 20 years, the role of embolization of the external carotid artery (ECA) territory has become increasingly more important, mainly for transarterial endovascular treatment of dural arteriovenous fistulas,1,2 treatment of epistaxis, and preoperative embolization of head and neck tumors to decrease surgical blood loss.3–6 However, because embryologic and phylogenetic development closely links the ECAs to the intracranial arteries, there are certain common anastomotic routes that have to be kept in mind while doing the embolization procedures to avoid possible major complications such as embolic stroke or cranial nerve palsies.7 Most of these anastomotic channels follow the cranial nerves along the neural foramen. Although they may not be visualized on routine (ie, global) catheter angiographies, they are always present and will therefore necessarily open under the following circumstances: 1) with increased intra-arterial pressure (eg, during embolization procedures or superselective injections8), 2) in the presence of high-flow shunts as a consequence of the “sump effect,” or 3) as collateral routes when occlusions of the major intracranial arteries occur.9–12
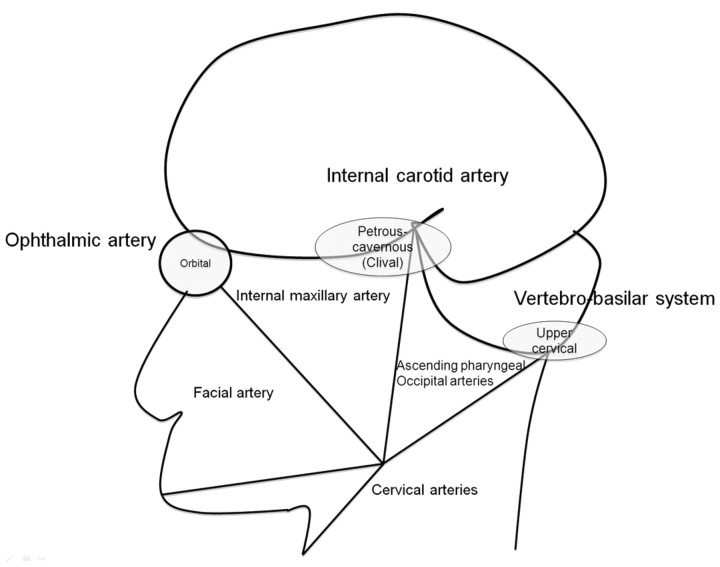
The functional vascular anatomy concept, as introduced by Lasjaunias et al7 and Berenstein et al in 1983,13 analyzes the arterial anatomy of the head and neck by their territories, which include the internal maxillary, linguofacial, pharyngo-occipital, thyroidal, cervical, internal carotid, and vertebral territories. The adjacent territories have inter-relationships and will function as potential vascular collaterals in case of vascular occlusion. There are 3 regions within these territories (Fig 1) that serve as the major extracranial–intracranial anastomotic pathways:
1) The orbital region via the ophthalmic artery that is the interface between the internal maxillary and internal carotid territories.
2) The petrous-cavernous region via the inferolateral trunk (ILT), the petrous branches of the internal carotid artery (ICA), and the meningohypophyseal trunk to the carotid artery.
3) The upper cervical region via the ascending pharyngeal, the occipital, and the ascending and deep cervical arteries to the vertebral artery.
Fig 1.
Diagram of the functional vascular anatomy of the head and neck with the 3 major extracranial–intracranial anastomotic pathway regions: the orbital, petrous-cavernous-clival, and upper cervical regions.
The major extracranial and intracranial anastomoses are summarized in Table 1.
Table 1:
Summary of the major extra- and intracranial anastomoses
| Extracranial |
Intracranial |
|||
|---|---|---|---|---|
| Major artery | Location | Branch | Branch | Artery |
| Internal maxillary artery | Proximal | MMA | Orbital branches, anterior branch (anterior falcine artery) | Ophthalmic artery |
| Cavernous branches | ILT | |||
| Petrous branch | CN VII supply | |||
| Proximal | AMA | Artery of foramen ovale | ILT | |
| Distal | Vidian artery | Petrous ICA | ||
| Distal | Artery of foramen rotundum | ILT | ||
| Distal | Anterior deep temporal artery | Ophthalmic artery | ||
| Superficial temporal artery | Frontal branch | Supraorbital branch | Ophthalmic artery | |
| Ascending pharyngeal artery | Pharyngeal trunk | Superior pharyngeal artery | Carotid branch (foramen lacerum) | Lateral clival artery |
| Neuromeningeal trunk | Odontoid arch | Vertebral artery (C1) | ||
| Hypoglossal and jugular branch | Meningohypophyseal trunk of ICA | |||
| Posterior auricular-occiptal artery | Stylomastoid branch | CN VII supply | ||
| Occipital artery | Muscular branches | Vertebral artery (C1–C2) | ||
| Ascending and deep cervical arteries | Vertebral artery (C3–C7) | |||
Note:—MMA indicates middle meningeal artery; AMA, accessory meningeal artery; ILT, inferolateral trunk; ICA, internal carotid artery; CN, cranial nerve.
Orbital Region (anastomoses to the ophthalmic artery)
The most important risk when embolizing within this region is occlusion of the central retinal artery, which results in blindness of the patient. Infrequently, embolic stroke can also occur through retrograde filling of the ICA. Because the central retinal artery typically originates with or close to the posterior ciliary arteries (either the medial or lateral branches) from the ophthalmic artery,7 the choroidal blush, best seen on the lateral angiographic view in the capillary and early venous phases, is often used as a landmark to identify its origin. The anastomoses between the ECA and ophthalmic artery are summarized in Table 2.
Table 2:
Summary of the branches of the ophthalmic artery and their anastomoses to the ECA
| Ophthalmic Artery Branch | Origin of the Ophthalmic Branch | ECA Branch |
|---|---|---|
| Proximal lacrimal artery (main branch) | Second portion of OA | MMA (through superior orbital fissure) |
| Distal lacrimal artery (inferior branch) | Second portion of OA | Anterior deep temporal artery and infraorbital artery (IMA) |
| Anterior ethmoidal arteries | Third portion of OA | Septal arteries: sphenopalatine artery (IMA), MMA |
| Posterior ethmoidal arteries | Second or third portion of OA | Sphenopalatine artery, greater palatine artery (IMA), MMA |
| Supraorbital artery | Third portion of OA | STA |
| Dorsal nasal artery | Terminal branch of OA | Angular termination of FA, infraorbital artery |
Note:—OA indicates ophthalmic artery; ECA, external carotid artery; IMA, internal maxillary artery; STA, superficial temporal artery; FA, facial artery.
Proximal Internal Maxillary Collaterals
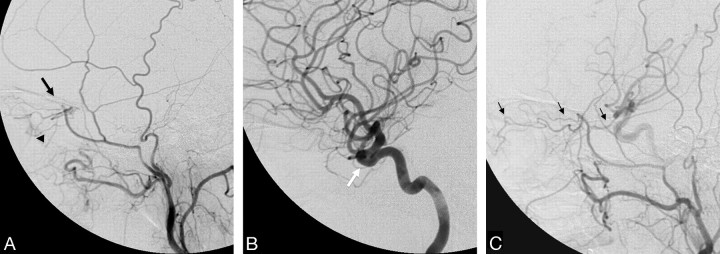
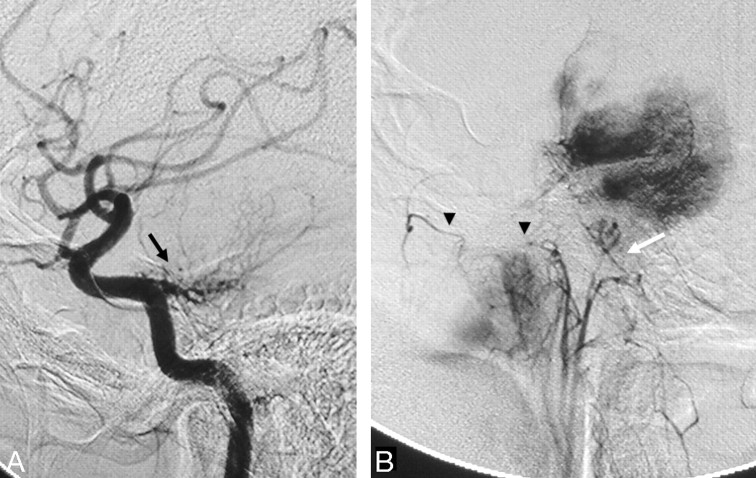
There are several potential orbital collateral routes from the ECA to the ophthalmic artery. The most frequently encountered is the meningo-ophthalmic artery (Fig 2).14 This artery is the extreme variation in which the remnant of the embryologic stapedial artery (ie, the middle meningeal artery [MMA] and the distal internal maxillary artery [IMA]) takes over the entire orbital supply from the primitive ophthalmic artery.7 This results in supply to the distal ophthalmic artery, including the central retinal artery and ciliary arteries only from the MMA as the remnant of the stapedial system. The important clues that suggest this variation are nonvisualization of the ophthalmic artery from the ICA and a choroidal blush on the ECA injection (Fig 2A, -B). In this setting, even proximal occlusion of the MMA carries a high risk because the MMA is the sole supply to the distal ophthalmic artery and must be avoided.
Fig 2.
Left ECA (A) and left ICA (B) angiograms (lateral view) demonstrate a meningo-ophthalmic artery arising from the left MMA, just before it crosses the sphenoid ridge on the lateral view (black arrow), contributing supply to the entire orbit with the absence of the ophthalmic artery from the ICA (white arrow). Note the choroidal blush (arrowhead). C, Right ECA angiogram of the same patient shows anastomosis between the MMA and the ophthalmic artery through the lacrimal system with retrograde filling of the ICA (thin black arrows).
The stapedial artery originally arises from the ICA and is later annexed by the ECA and, therefore, will constitute a potential anastomotic route to the orbit. These other MMA collaterals to the ophthalmic artery are through the superficial recurrent meningeal branch of the lacrimal artery, which anastomose with the orbital branches through the superior orbital fissure15 and the anterior falcine artery, a meningeal branch arising from the distal ophthalmic artery supplying the anterior falx, anastomosing indirectly with the anterior branch of the MMA through the meninges.7 The orbital branches can be best seen on the lateral projection, typically just inferior to the sphenoid ridge (Fig 2C), similar to the location of the meningo-ophthalmic artery origin. These above-mentioned anastomoses are always present (except for cases with a meningo-ophthalmic artery). However, they are usually not visualized on global injections and only open with increased pressure during embolization procedures of the MMA.
Distal IMA Collaterals
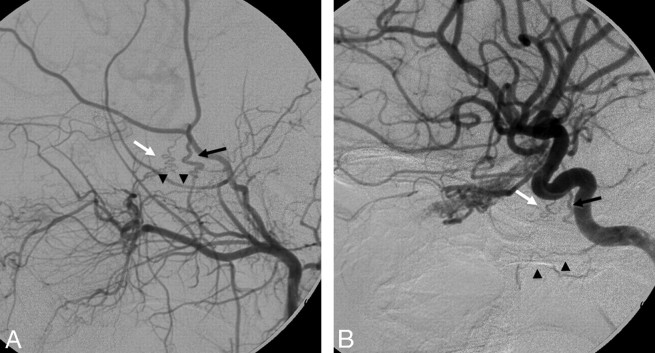
The distal IMA has anastomoses with the inferior branch of the lacrimal artery of the ophthalmic artery through the anterior deep temporal artery (Fig 3) and through the infraorbital artery.7,14,15 The sphenopalatine artery, also from the distal IMA, is connected with the anterior and posterior ethmoidal arteries of the ophthalmic artery through the septal arteries, which are classically seen in large juvenile angiofibromas.16 The sphenopalatine artery is one of the targets in the embolization of epistaxis; therefore, these anastomoses have to be kept in mind. Because their diameter is <80 μm, embolization of the branches with particles greater than this size is considered safe.
Fig 3.
Left ECA (A) and left ICA (B) angiograms (lateral view) pre- and postballoon embolization of a traumatic carotid cavernous fistula reveal anastomosis between the anterior deep temporal artery from the distal IMA through the lacrimal artery to the ophthalmic artery (black arrow). Note the retrograde filling of the proximal ophthalmic artery; the same curve is also seen from the ICA injection (white arrows).
Cutaneous Collaterals
The frontal branch of the superficial temporal artery (STA) connects with the ophthalmic artery through the supraorbital artery. The distal end of the facial artery (FA) anastomoses with the dorsal nasal artery at the angular termination.7,17 Both the FA and the STA branches thereby classically connect to the distal (third) portion of the ophthalmic artery.
Cavernous-Petrous Region (anastomoses to the ICA)
Within this region, there are 3 major anastomotic areas to the ICA: the petrous, the clivus, and the cavernous territories. The corresponding branches of the ICA are the mandibular artery (remnant of the dorsal part of the first aortic arch) and the caroticotympanic artery (remnant of the embryologic hyoid artery) for the petrous territory, the meningohypophyseal trunk (remnant of the embryologic primitive maxillary artery) and the lateral clival artery for the clival territory, and the ILT (remnant of the embryologic dorsal ophthalmic artery) for the cavernous region.7,18,19 The major anastomotic routes to the ILT are summarized in Table 3.
Table 3:
Summary of the ILT branches of the cavernous ICA and their anastomoses
| ILT Branches | Course | ECA Anastomosis | ECA Branch Origins |
|---|---|---|---|
| Superior or tentorial branch | Roof of the cavernous sinus | Cavernous branches of MMA | Proximal IMA |
| Anteromedial branch | Superior orbital fissure | Orbital branches of MMA, ophthalmic artery | Proximal IMA |
| Anterolateral branch | Foramen rotundum | Artery of foramen rotundum | Distal IMA |
| Posteromedial branch | Foramen ovale | Superior division of AMA | Proximal IMA |
Ascending Pharyngeal Collaterals
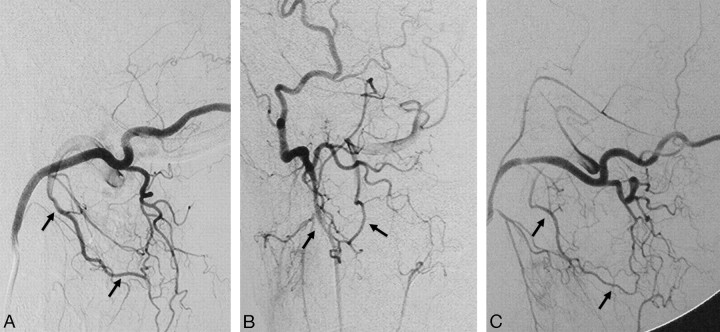
The ascending pharyngeal artery is the remnant of the embryologic artery of the third brachial arch and divides into 2 major trunks: the pharyngeal and neuromeningeal trunks, which connect to the petrous and cavernous ICA through several channels.20,21 The anteriorly located pharyngeal trunk gives off 3 branches: the inferior, middle, and superior pharyngeal arteries. The latter has the most important anastomotic routes, including the eustachian tube anastomotic circle that connects with the mandibular artery from the petrous ICA and an anastomosis with the accessory meningeal artery (AMA) and pterygovaginal artery from the distal IMA (Fig 4). Finally, this artery has a carotid canal branch that enters the cranium through the foramen lacerum to the cavernous sinus to join with the recurrent artery of the foramen lacerum, (branch of the lateral clival artery, which arises from the C5 portion of the carotid siphon) and the ILT (which connects to the cavernous ICA). The distal neuromeningeal trunk branches into 2 major arteries, the jugular and hypoglossal branches, and enters the cranial cavity through the corresponding foramen.7,20,21 Both branches give off medial and lateral clival branches immediately after exiting the hypoglossal and jugular foramen, respectively. These anastomose with the clival branches from the lateral clival artery (Fig 5) and the meningohypophyseal trunk, which can often be seen as participating arteries in the supply to meningiomas that develop in this region (Fig 6). The inferior tympanic artery, which may arise from the main trunk of the ascending pharyngeal artery or one of its major branches, enters the tympanic cavity through the inferior tympanic foramen with Jacobson's nerve and retains the embryologic connection of the artery of the third brachial arch (ascending pharyngeal artery) and the hyoid artery (or caroticotympanic artery) from the petrous ICA. Within the middle ear, the inferior tympanic artery also anastomoses with the superior tympanic artery, which arises from the petrous branch of the MMA, the anterior tympanic artery from the proximal IMA, the stylomastoid artery from the posterior auricular-occipital artery, and the mandibular branch of the ICA.7,19
Fig 4.
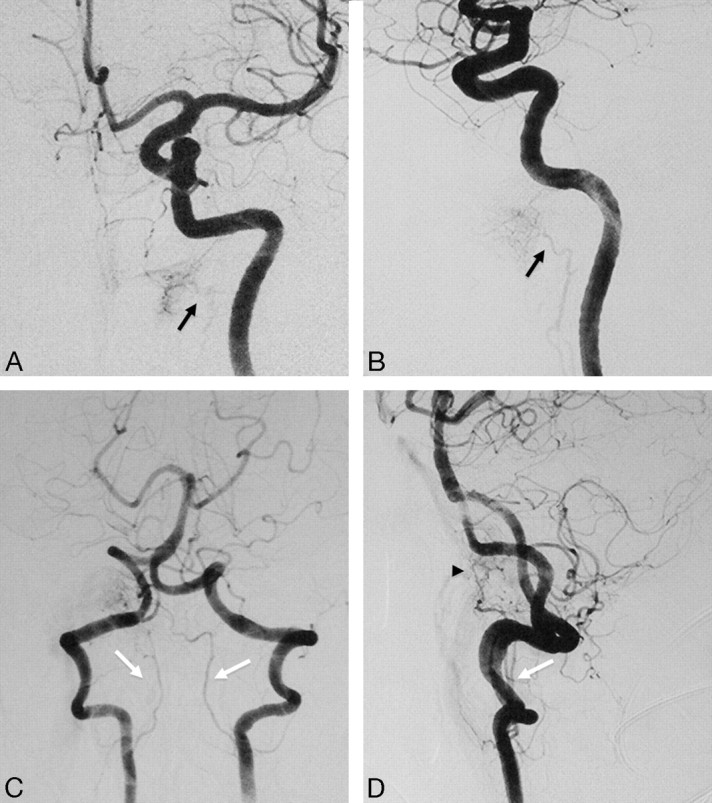
Left ICA angiogram in anteroposterior (A) and lateral (B) views demonstrates the anastomosis between the mandibular artery arising from the petrous ICA and the superior pharyngeal branch of the ascending pharyngeal artery (black arrows) in a patient with a right hypoglossal canal (anterior condyloid) dural arteriovenous fistula. Left vertebral artery angiogram in anteroposterior (C) and lateral (D) views shows arterial feeders from the vertebral arteries through the odontoid arch, arising from the C3 level (white arrows), anastomosing with the hypoglossal branch of the ascending pharyngeal artery (arrowhead).
Fig 5.
Right ascending pharyngeal-occipital angiogram in anteroposterior (A) and lateral (B) views reveals the anastomosis between the neuromeningeal trunk (black arrows) of the ascending pharyngeal artery and the lateral clival artery (white arrows) from the cavernous ICA.
Fig 6.
Right ICA (A) and right ascending pharyngeal (B) angiograms in a lateral view reveal the typical “sunburst” appearance of a clival meningoma supplied by the meningohypophyseal trunk from the ICA (black arrow) and clival branches from the neuromeningeal trunk of the ascending pharyngeal artery (white arrow). Retrograde filling of the pterygovaginal artery of the distal IMA from the superior pharyngeal artery through the eustachian tube anastomotic circle is also seen (arrowheads).
Typically these anastomoses are not visualized on the cerebral angiograms but may open up during embolization. In some rare cases, agenesis of the cervical ICA and persistence of the embryologic hyostapedial artery will result in the so-called “aberrant ICA,” in which the ICA enters the skull base through the inferior tympanic canal, and the cervical part of the ICA is actually the ascending pharyngeal artery.7,22,23
Proximal IMA Collaterals
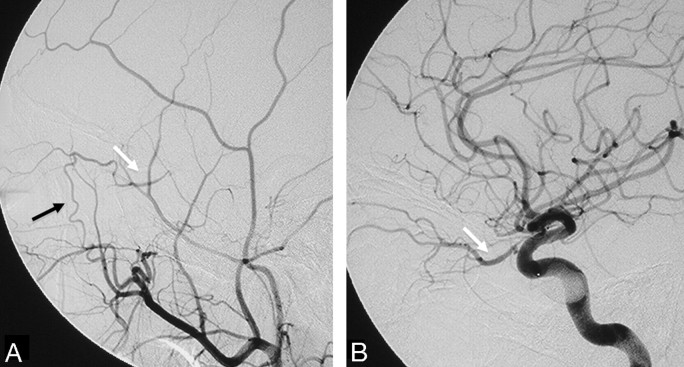
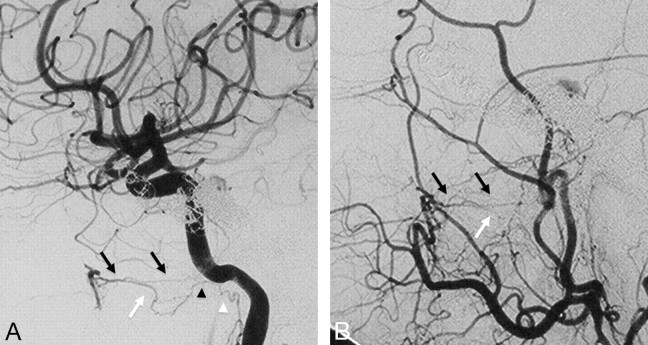
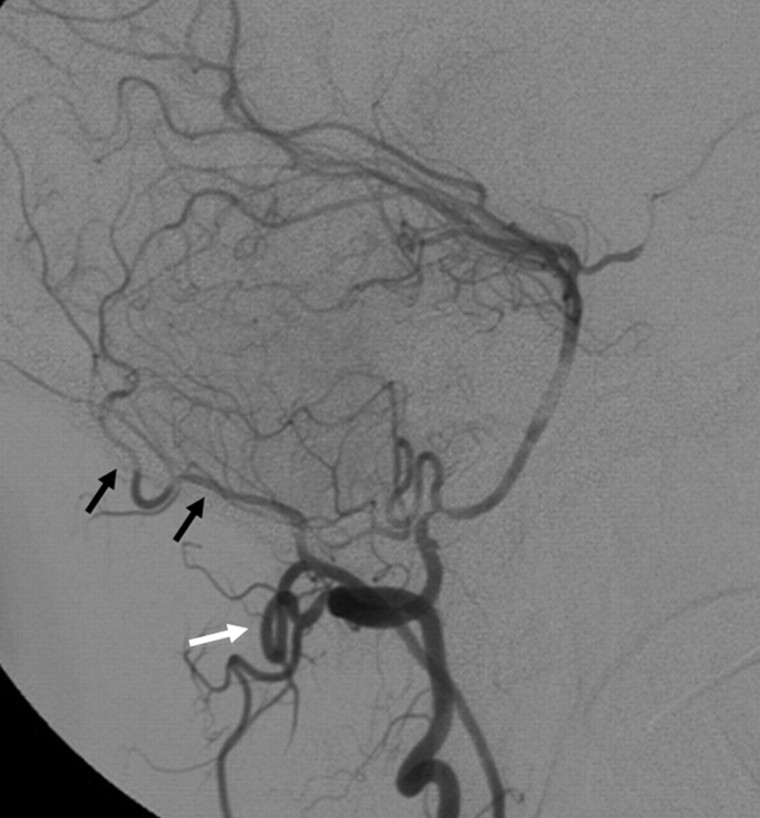
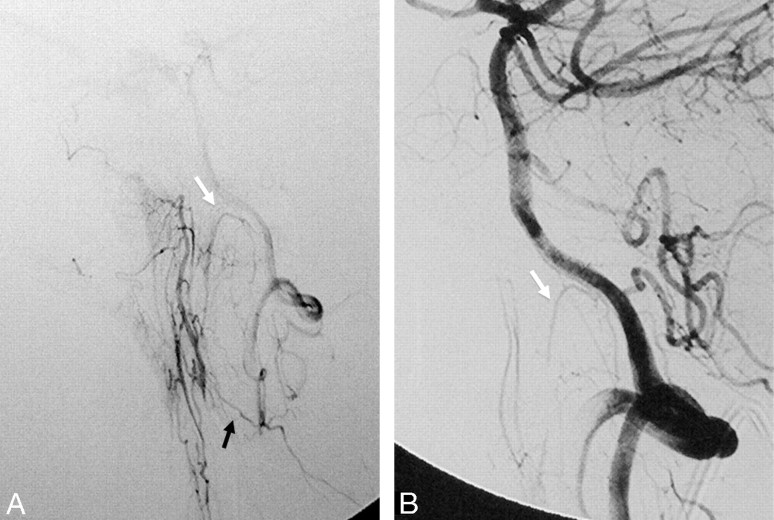
The major anastomotic route for the MMA and AMA is through the ILT to the cavernous ICA.18,19,24 After exiting the foramen spinosum, the MMA gives off cavernous branches that anastomose with the superior or tentorial branch of the ILT. The orbital branches of the MMA, besides their connection to the ophthalmic artery, also anastomose with the anteromedial branch of the ILT within the superior orbital fissure. The superior division of the AMA enters the cavernous sinus through the foramen ovale to anastomose with the posteromedial branch of the ILT (Figs 7 and 8), which courses under the trigeminal ganglion and accompanies CN V2.25 In 20% of cases, the AMA may take over the entire supply of the ILT territory.7 These cavernous collaterals are often enlarged and serve as the arterial feeders in cavernous dural arteriovenous fistulas (Fig 7).
Fig 7.
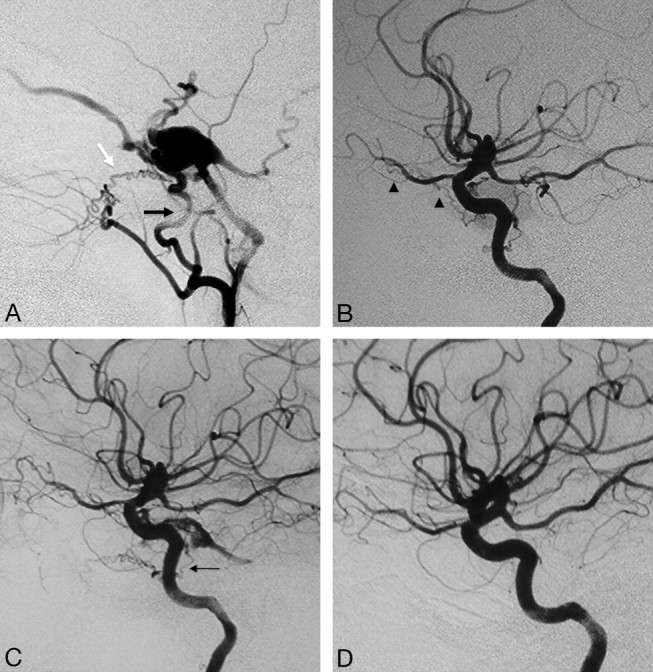
Right ECA (A) and right ICA (B) angiograms in the lateral view reveal supply to a cavernous dural arteriovenous fistula from the accessory meningeal artery through the foramen ovale (black arrow), artery of the foramen rotundum (white arrow), and recurrent meningeal branch of the ophthalmic artery (arrowheads). C, Right ICA angiogram immediately after the embolization demonstrates better visualization of the anastomosis between the artery of the foramen rotundum and the lateral clival artery (thin black arrow). D, On 3-month follow-up, note remodeling with a decreased size of the collaterals, which are no longer seen on global injections.
Fig 8.
Left ECA (A) and left ICA (B) angiograms in the lateral view of a patient with cerebrofacial arteriovenous metameric syndrome (type II) and an arteriovenous malformation of the optic nerve reveal an anastomosis between the AMA through the foramen ovale (black arrows) and the artery of the foramen rotundum (white arrows) to the ILT and the vidian artery (arrowheads), arising from the distal IMA to the vidian branch of the mandibular artery.
The petrosquamous or the posterior branch of the MMA also anastomoses with the marginal artery of the tentorium, which can arise from either the ILT, the ophthalmic artery, or the meningohypophyseal trunk of the ICA.7 Apart from the cavernous anastomosis, the AMA also has smaller anastomotic channels surrounding the eustachian tube, as mentioned previously, with the superior pharyngeal branch of the ascending pharyngeal artery, the mandibular branch of the petrous ICA, and the pterygovaginal artery from the distal IMA.
Distal IMA Collaterals
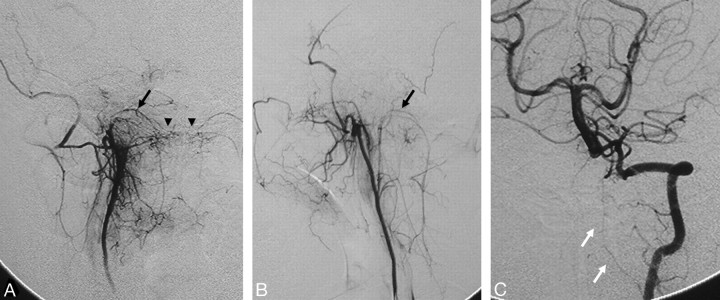
There are several branches of the distal IMA that have intracranial anastomoses with the ICA. The first is the artery of the foramen rotundum, which, as implied by its name, runs through the foramen rotundum and is best seen in the lateral view, where it can be identified with its corkscrew appearance, connecting with the anterolateral branch of the ILT (Fig 8)18,19 or, in rare cases, with the lateral clival artery (Fig 7C). The vidian artery has a distinctly horizontal course, which can be easily identified on the lateral view from the distal IMA through the vidian canal to the foramen lacerum, where it anastomoses with the corresponding vidian branch of the mandibular artery arising from the petrous ICA (Figs 8 and 9).12,26–29 The pterygovaginal artery, which often originates adjacent to the vidian artery, has a more inferior course through the pterygovaginal canal along the roof of the nasopharynx, ending at the anastomotic circle around the eustachian tube.7
Fig 9.
Right ICA (A) and right ECA (B) angiograms in lateral view, posttransvenous coiling of a cavernous dural arteriovenous malformation, demonstrate the anastomosis between the vidian artery from the distal IMA to the vidian branch of the mandibular artery. The vidian artery has a characteristic horizontal course on the lateral view (black arrows), thus differentiating it from the more inferior course of the pterygovaginal artery (white arrows), which also anastomoses with a branch of the mandibular artery (black arrowhead) and the superior pharyngeal artery (white arrowhead) around the eustachian tube.
Upper Cervical Regions (anastomoses to the vertebral artery)
Occipital Collaterals
The occipital artery is a remnant of the embryologic type I and type II proatlantal arteries, which correspond to the C1 and C2 segmental arteries.7,30 In the fetal period, both embryologic arteries serve as connection routes between the carotid and vertebrobasilar systems. Full persistence of these segmental arteries is extremely rare31,32; however, the occipital artery, as their remnant, still retains its connection from the external carotid system to the vertebral artery through the posterior anastomotic radicular branches, which arise from the horizontal portion of the occipital arteries at both the C1 and C2 levels (Figs 10 and 11). These are the major collaterals from the vertebral artery to the carotid system, which are often seen in case of common carotid artery ligation or occlusion.10,33–35 The stylomastoid artery, which may arise from either the posterior auricular or the occipital artery, supplies the meninges of the posterior fossa and forms anastomoses with other meningeal branches from the MMA, the ascending pharyngeal artery, the ILT, the meningohypophyseal trunk, and the posterior meningeal artery from the vertebral artery.7
Fig 10.
Right vertebral artery angiogram in the lateral view, after coiling of a right vertebral artery aneurysm, reveals anastomosis between the right vertebral artery and the occipital artery (black arrows) through the posterior radicular branches at the C1 level (white arrow).
Fig 11.
Right occipital artery angiograms in lateral (A) and anteroposterior (B) views demonstrate the anastomosis between the occipital artery and the right vertebral artery at the C2 level through the posterior radicular branches (arrows). C, Similar findings are also observed on the contralateral left occipital artery angiogram.
Ascending Pharyngeal Collaterals
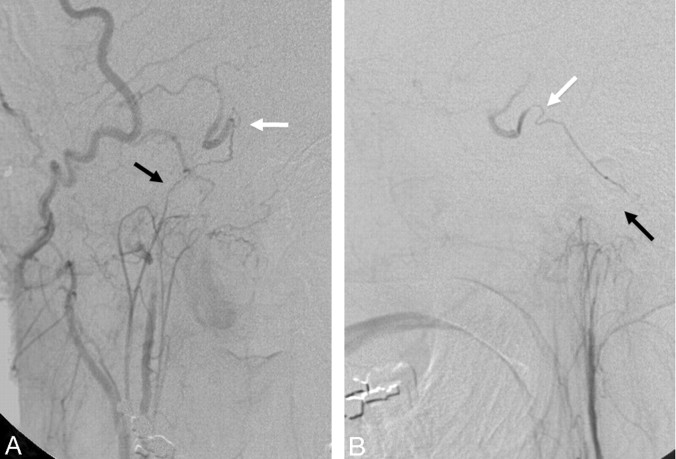
As a remnant of the embryologic hypoglossal artery, the ascending pharyngeal artery keeps its connection with the vertebral artery through 2 proximal branches.7,36,37 The lowest, usually the most proximal branch of the ascending pharyngeal artery, is the musculospinal branch, which anastomoses laterally with the C3 radicular anastomotic artery of the vertebral artery (Fig 12). The second is the prevertebral branch, located on the ventral surface of the C1–C2 vertebrae and anastomoses with the odontoid arch.38,39 This branch typically arises from the neuromeningeal trunk but may also arise directly from the main ascending pharyngeal artery. It has a characteristic U-shaped curve, seen on the lateral view before anastomosing medially on the surface of the dens with the C3 collaterals from the vertebral artery (Figs 12 and 13).
Fig 12.
Left ascending pharyngeal artery (A) and left vertebral artery (B) angiograms in the lateral view demonstrate the anastomosis of the musculospinal branch of the ascending pharyngeal artery (black arrow) to the left vertebral artery at the C3 level and the contribution to the odontoid arch from the neuromeningeal trunk and C3 left vertebral artery branch (white arrows).
Fig 13.
Right ascending pharyngeal artery (A) and left vertebral artery (C) angiograms in the anteroposterior view and left ascending pharyngeal artery angiogram in the lateral view (B) show the anastomosis around the odontoid arch (arrowheads) with branches from the neuromeningeal trunks (black arrows) and the C3 segment of the left vertebral artery (white arrows).
Cervical Collaterals
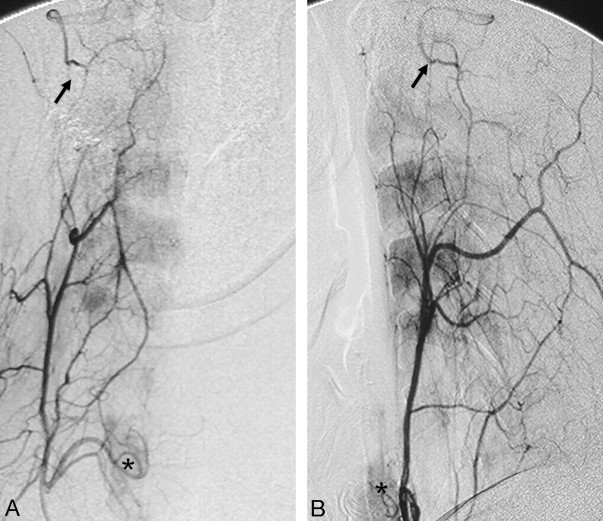
The ascending cervical artery and the deep cervical artery are the major anastomotic channels of the vertebral artery at the C2–C4 levels (Fig 14).7,40,41 Both arise from the subclavian arteries. More specifically, the ascending cervical artery typically originates from the thyrocervical trunk, whereas the deep cervical artery arises from the costocervical trunk. Several case reports have described anastomoses between the vertebral artery and these cervical arteries, including the aberrant origin of the vertebral artery from the thyrocervical trunk,40 and the use of these anastomotic channels for thrombolysis of basilar artery thrombosis in a case with proximal vertebral artery occlusion.41
Fig 14.
Right ascending cervical angiogram in anteroposterior (A) and lateral (B) views demonstrates anastomosis with the vertebral artery at the C2 level (black arrows). Note the inferior thyroidal artery with capillary staining of the thyroid gland (asterisks), which also arises from the thyrocervical trunk.
Cranial Nerve Supply
The arterial supply of all cranial nerves is summarized in Table 4.7,42–45 The most important ECA supplies to the cranial nerves are the supplies to CN VII (facial nerve) and the lower cranial nerves (IX–;XII). Supply of the geniculate ganglion of the facial nerve is through the facial arcade, which, in most of the cases, consists of the petrous branch of the MMA and the stylomastoid branch of the posterior auricular artery (Fig 15). The stylomastoid branch can also arise from a common trunk of the posterior auricular and occipital arteries, a variation seen in 50% of patients. Although the cisternal portions of the lower cranial nerves are supplied from the ipsilateral vertebral arteries, the supply to their foraminal parts is mainly through the neuromeningeal trunk of the ascending pharyngeal artery, which divides into 2 branches before entering the cranial cavity. The jugular branch contributes supply to CN IX and X as it traverses the jugular foramen, and the hypoglossal branch supplies CN XII within the hypoglossal canal. The supply of CN XI (accessory spinal nerve), however, is from the musculospinal branch, which arises from the neuromeningeal trunk before entering the foramen magnum and is proximal to the odontoid arch. Proximal embolization of these branches with particles can result in cranial nerve palsies, which are temporary and can recover following steroid therapy and with development of collaterals.
Fig 15.
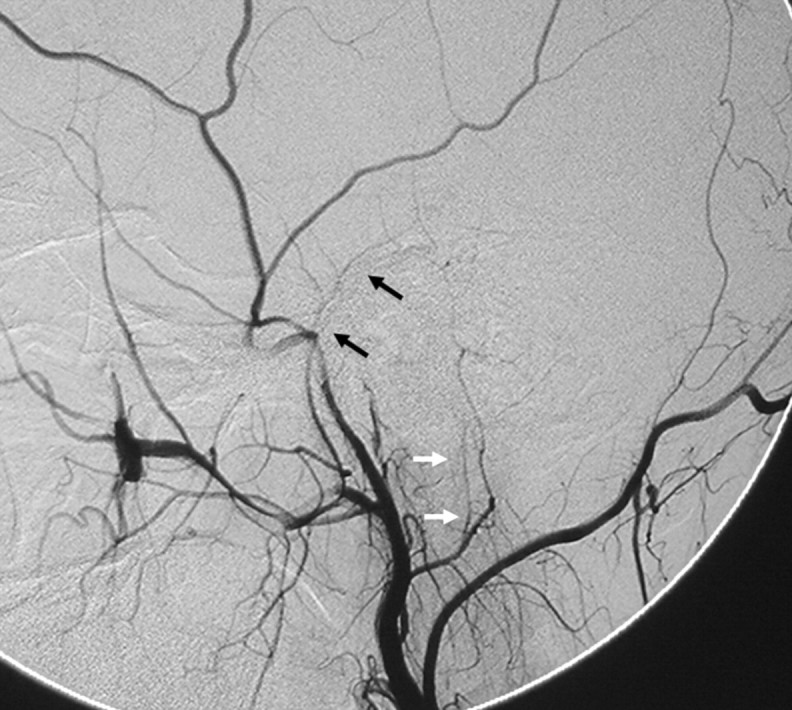
Right ECA angiogram in the lateral view shows the petrous branch of the MMA (black arrows) and the stylomastoid branch (white arrows) arising from the posterior auricular artery, contributing to the facial arcade, the main supply for the facial nerve.
Table 4:
Summary of the cranial nerve supply
| Cranial Nerve | Location | Arterial supply | |
|---|---|---|---|
| III, IV | Cisternal | Mesencephalic perforators (common trunk for CN III) | Vertebrobasilar system |
| Cavernous sinus | CN III: ILT only; CN IV: marginal artery of the tentorium cerebelli (meningohypophyseal trunk) + ILT | ||
| Superior orbital fissure | Anteromedial branch of ILT | ||
| VI | Cisternal | Vertebrobasilar system | |
| Dorsum sella | Jugular branch of AscPhA, medial branch of lateral clival artery, meningohypophyseal trunk | ||
| Cavernous sinus | ILT | ||
| Superior orbital fissure | Anteromedial branch of ILT | ||
| V | Cisternal | Basilar vestige of trigeminal artery (between SCA and AICA) | |
| Meckel cave | Lateral artery of trigeminal ganglion, cavernous branch of MMA, carotid branch of AscPhA, ILT | ||
| V2 | Foramen rotundum | Artery of foramen rotundum | ILT, distal IMA |
| V3 | Foramen ovale | Posteromedial branch | ILT, AMA |
| VII, VIII | Cisternal + IAC | Internal auditory artery | AICA |
| Geniculate ganglion | Petrosal branch of MMA, stylomastoid branch of posterior auricular/occipital artery | ||
| IX, X | Cisternal, jugular foramen | Jugular branch of neuromeningeal trunk | VA, AscPhA |
| XI | Spinal root | C3 segmental branch | Cervical arteries, musculospinal branch of AscPhA |
| Cranial root | Jugular branch of neuromeningeal trunk | AscPhA | |
| XII | Cisternal hypoglossal canal | Hypoglossal branch of neuromeningeal trunk | VA, AscPhA |
Note:—AscPhA indicates ascending pharyngeal artery; SCA, superior cerebellar artery; IAC, internal auditory canal; AICA, anterior inferior cerebellar artery; VA, vertebral artery.
On the other hand, distal embolization with either particles that are smaller than 80 μm or liquid materials will lead to permanent cranial nerve palsy or can even open connections with the intracranial contributions, (ie, the vertebral artery in case of the lower cranial nerves via the above-mentioned ascending pharyngeal artery anastomoses) and then result in embolic stroke of the posterior fossa.
Embolic Materials
Particles and liquid embolic materials are the 2 most commonly used embolization materials in the ECA system. Although the penetration capacity of particles (the most commonly used are Gelfoam sponge and Gelfoam powder [Gelfoam; Upjohn, Kalamazoo, Mich], and polyvinyl alcohol particles) is dependent on the size, liquid embolic materials such as glue (n-butyl 2-cyanoacrylate) and Onyx (ev3, Irvine, Calif) can open and enter small anastomotic channels that are not visualized on the initial angiograms and, therefore, should be used with increased caution.46,47 In general, the size of the nonvisualized anastomotic arteries ranges from 50 to 80 μm; therefore, particles that are >150 μm will not penetrate these anastomoses and will avoid potential embolic complications. The visualization of an anastomotic channel is not a contraindication for embolization of the particular artery. There are several techniques that can be used to prevent embolic material from entering the collaterals, including simple proximal mechanical blockage of the collateral branch with large particles or coils before the embolization, or flow-reversal methods using a proximal balloon occlusion in the target ECA vessel, which leads to flow redirection from the ICA to the ECA territory.47
Conclusions
Transarterial embolization of the ECA and cervical arteries has become an established technique in the treatment of tumors and arteriovenous shunts of the head and neck region. Due to its embryologic relationship with the intracranial circulation, various anastomotic channels exist between the 2 systems, though they may not be visualized on angiography. Knowledge of these potential anastomotic routes and the proper choice of embolic material are crucial to help the interventionalist avoid embolic complications to the brain and cranial nerves during the procedure.
References
- 1. Agid R, Terbrugge K, Rodesch G, et al. Management strategies for anterior cranial fossa (ethmoidal) dural arteriovenous fistulas with an emphasis on endovascular treatment. J Neurosurg 2009; 110: 79– 84 [DOI] [PubMed] [Google Scholar]
- 2. Shi ZS, Ziegler J, Gonzalez NR, et al. Transarterial embolization of clival dural arteriovenous fistulae using liquid embolic agents. Neurosurgery 2008; 62: 408– 15 [DOI] [PubMed] [Google Scholar]
- 3. Gupta AK, Purkayastha S, Bodhey NK, et al. Preoperative embolization of hypervascular head and neck tumours. Australas Radiol 2007; 51: 446– 52 [DOI] [PubMed] [Google Scholar]
- 4. Kasper GC, Welling RE, Wladis AR, et al. A multidisciplinary approach to carotid paragangliomas. Vasc Endovascular Surg 2006; 40: 467– 74 [DOI] [PubMed] [Google Scholar]
- 5. Persky MS, Setton A, Niimi Y, et al. Combined endovascular and surgical treatment of head and neck paragangliomas: a team approach. Head Neck 2002; 24: 423– 31 [DOI] [PubMed] [Google Scholar]
- 6. Roche PH, Paris J, Regis J, et al. Management of invasive juvenile nasopharyngeal angiofibromas: the role of a multimodality approach. Neurosurgery 2007; 61: 768– 77 [DOI] [PubMed] [Google Scholar]
- 7. Lasjaunias P, Berenstein A, ter Brugge K. Surgical Neuroangiography: 1 Clinical Vascular Anatomy and Variations. Berlin, Germany: Springer-Verlag; 2001 [Google Scholar]
- 8. Ohata K, Nishio A, Takami T, et al. Sudden appearance of transdural anastomosis from middle meningeal artery to superior cerebellar artery during preoperative embolization of meningioma. Neurol India 2006; 54: 328. [DOI] [PubMed] [Google Scholar]
- 9. Countee RW, Vijayanathan T. External carotid artery in internal carotid artery occlusion: angiographic, therapeutic, and prognostic considerations. Stroke 1979; 10: 450– 60 [DOI] [PubMed] [Google Scholar]
- 10. Holodny AI. Supply of the unilateral circulation of the brain by an occipital artery anastomosis: a case report. Angiology 2005; 56: 93– 95 [DOI] [PubMed] [Google Scholar]
- 11. Liebeskind DS. Collateral circulation. Stroke 2003; 34: 2279– 84 [DOI] [PubMed] [Google Scholar]
- 12. Takeuchi M, Kuwayama N, Kubo M, et al. Vidian artery as a collateral channel between the external and occluded internal carotid arteries: case report. Neurol Med Chir (Tokyo) 2005; 45: 470– 71 [DOI] [PubMed] [Google Scholar]
- 13. Berenstein A, Lasjaunias P, Kricheff II. Functional anatomy of the facial vasculature in pathologic conditions and its therapeutic application. AJNR Am J Neuroradiol 1983; 4: 149– 53 [PMC free article] [PubMed] [Google Scholar]
- 14. Hayreh SS. Orbital vascular anatomy. Eye 2006; 20: 1130– 44 [DOI] [PubMed] [Google Scholar]
- 15. Perrini P, Cardia A, Fraser K, et al. A microsurgical study of the anatomy and course of the ophthalmic artery and its possibly dangerous anastomoses. J Neurosurg 2007; 106: 142– 50 [DOI] [PubMed] [Google Scholar]
- 16. Casasco A, Houdart E, Biondi A, et al. Major complications of percutaneous embolization of skull-base tumors. AJNR Am J Neuroradiol 1999; 20: 179– 81 [PubMed] [Google Scholar]
- 17. Lopez R, Lauwers F, Paoli JR, et al. The vascular system of the upper eyelid: anatomical study and clinical interest. Surg Radiol Anat 2008; 30: 265– 69 [DOI] [PubMed] [Google Scholar]
- 18. Lasjaunias P, Moret J, Mink J. The anatomy of the inferolateral trunk (ILT) of the internal carotid artery. Neuroradiology 1977; 13: 215– 20 [DOI] [PubMed] [Google Scholar]
- 19. Tubbs RS, Hansasuta A, Loukas M, et al. Branches of the petrous and cavernous segments of the internal carotid artery. Clin Anat 2007; 20: 596– 601 [DOI] [PubMed] [Google Scholar]
- 20. Hacein-Bey L, Daniels DL, Ulmer JL, et al. The ascending pharyngeal artery: branches, anastomoses, and clinical significance. AJNR Am J Neuroradiol 2002; 23: 1246– 56 [PMC free article] [PubMed] [Google Scholar]
- 21. Lasjaunias P, Moret J. The ascending pharyngeal artery: normal and pathological radioanatomy. Neuroradiology 1976; 11: 77– 82 [DOI] [PubMed] [Google Scholar]
- 22. Roll JD, Urban MA, Larson TC, 3rd, et al. Bilateral aberrant internal carotid arteries with bilateral persistent stapedial arteries and bilateral duplicated internal carotid arteries. AJNR Am J Neuroradiol 2003; 24: 762– 65 [PMC free article] [PubMed] [Google Scholar]
- 23. Silbergleit R, Quint DJ, Mehta BA, et al. The persistent stapedial artery. AJNR Am J Neuroradiol 2000; 21: 572– 77 [PMC free article] [PubMed] [Google Scholar]
- 24. Capo H, Kupersmith MJ, Berenstein A, et al. The clinical importance of the inferolateral trunk of the internal carotid artery. Neurosurgery 1991; 28: 733– 37, discussion 737–38 [DOI] [PubMed] [Google Scholar]
- 25. Lasjaunias P, Theron J. Radiographic anatomy of the accessory meningeal artery. Radiology 1976; 121: 99– 104 [DOI] [PubMed] [Google Scholar]
- 26. Osawa S, Rhoton AL, Jr, Tanriover N, et al. Microsurgical anatomy and surgical exposure of the petrous segment of the internal carotid artery. Neurosurgery 2008; 63: 210– 38, discussion 239 [DOI] [PubMed] [Google Scholar]
- 27. Osborn AG. The vidian artery: normal and pathologic anatomy. Radiology 1980; 136: 373– 78 [DOI] [PubMed] [Google Scholar]
- 28. Quisling RG. Intrapetrous carotid artery branches: pathological application. Radiology 1980; 134: 109– 13 [DOI] [PubMed] [Google Scholar]
- 29. Quisling RG, Rhoton AL., Jr Intrapetrous carotid artery branches: radioanatomic analysis. Radiology 1979; 131: 133– 36 [DOI] [PubMed] [Google Scholar]
- 30. Lasjaunias P, Theron J, Moret J. The occipital artery: anatomy—normal arteriographic aspects–embryological significance. Neuroradiology 1978; 15: 31– 37 [DOI] [PubMed] [Google Scholar]
- 31. Gumus T, Onal B, Ilgit ET. Bilateral persistence of type 1 proatlantal arteries: report of a case and review of the literature. AJNR Am J Neuroradiol 2004; 25: 1622– 24 [PMC free article] [PubMed] [Google Scholar]
- 32. Kurose K, Kishi H, Nishijima Y. Type 2 proatlantal artery associated with a ruptured aneurysm: case report. Neurol Med Chir (Tokyo) 1990; 30: 191– 93 [DOI] [PubMed] [Google Scholar]
- 33. Miyachi S, Negoro M, Sugita K. The occipital-vertebral anastomosis as a collateral pathway: hemodynamic patterns—case report. Surg Neurol 1989; 32: 350– 55 [DOI] [PubMed] [Google Scholar]
- 34. Spetzler RF, Modic M, Bonstelle C. Spontaneous opening of large occipital-vertebral artery anastomosis during embolization: case report. J Neurosurg 1980; 53: 849– 50 [DOI] [PubMed] [Google Scholar]
- 35. Papon X, Pasco A, Fournier HD, et al. Anastomosis between the internal carotid and vertebral artery in the neck. Surg Radiol Anat 1995; 17: 335– 37 [DOI] [PubMed] [Google Scholar]
- 36. Meguro T, Terada K, Hirotsune N, et al. Unusual variant of persistent primitive hypoglossal artery. Br J Radiol 2007; 80: e314– 16 [DOI] [PubMed] [Google Scholar]
- 37. Nakamura M, Kobayashi S, Yoshida T, et al. Persistent external carotid-vertebrobasilar anastomosis via the hypoglossal canal. Neuroradiology 2000; 42: 821– 23 [DOI] [PubMed] [Google Scholar]
- 38. Lasjaunias P, Moret J, Theron J. The so-called anterior meningeal artery of the cervical vertebral artery: normal radioanatomy and anastomoses. Neuroradiology 1978; 17: 51– 55 [DOI] [PubMed] [Google Scholar]
- 39. Haffajee MR. A contribution by the ascending pharyngeal artery to the arterial supply of the odontoid process of the axis vertebra. Clin Anat 1997; 10: 14– 18 [DOI] [PubMed] [Google Scholar]
- 40. Strub WM, Leach JL, Tomsick TA. Left vertebral artery origin from the thyrocervical trunk: a unique vascular variant. AJNR Am J Neuroradiol 2006; 27: 1155– 56 [PMC free article] [PubMed] [Google Scholar]
- 41. Wang H, Fraser K, Wang D, et al. Successful intra-arterial basilar artery thrombolysis in a patient with bilateral vertebral artery occlusion: technical case report. Neurosurgery 2005; 57: E398. [DOI] [PubMed] [Google Scholar]
- 42. Lapresle J, Lasjaunias P. Cranial nerve ischaemic arterial syndromes: a review. Brain 1986; 109 ( Pt 1): 207– 16 [DOI] [PubMed] [Google Scholar]
- 43. Marinkovic S, Gibo H. The neurovascular relationships and the blood supply of the oculomotor nerve: the microsurgical anatomy of its cisternal segment. Surg Neurol 1994; 42: 505– 16 [DOI] [PubMed] [Google Scholar]
- 44. Krisht A, Barnett DW, Barrow DL, et al. The blood supply of the intracavernous cranial nerves: an anatomic study. Neurosurgery 1994; 34: 275– 79 [DOI] [PubMed] [Google Scholar]
- 45. Ozanne A, Pereira V, Krings T, et al. Arterial vascularization of the cranial nerves. Neuroimaging Clin N Am 2008; 18: 431– 39, xii [DOI] [PubMed] [Google Scholar]
- 46. Kagetsu NJ, Berenstein A, Choi IS. Interventional radiology of the extracranial head and neck. Cardiovasc Intervent Radiol 1991; 14: 325– 33 [DOI] [PubMed] [Google Scholar]
- 47. Lasjaunias P, Berenstein A, ter Brugge K. Surgical Neuroangiography: 2 Clinical and Endovascular Treatment Aspects in Adults. Berlin, Germany: Springer-Verlag; 2004 [Google Scholar]