Abstract
BACKGROUND AND PURPOSE:
Frontostriatal (including the putamen) circuit–mediated cognitive dysfunction has been implicated in frontotemporal lobar degeneration (FTLD), but not in Alzheimer disease (AD) or healthy aging. We sought to assess putaminal volume as a measure of the structural basis of relative frontostriatal dysfunction in these groups.
MATERIALS AND METHODS:
We measured putaminal volume in FTLD subtypes: frontotemporal dementia (FTD, n = 12), semantic dementia (SD, n = 13), and progressive nonfluent aphasia (PNFA, n = 9) in comparison with healthy controls (n = 25) and patients with AD (n = 18). Diagnoses were based on accepted clinical criteria. We conducted manual volume measurement of the putamen blinded to the diagnosis on T1 brain MR imaging by using a standardized protocol.
RESULTS:
Paired t tests (P < .05) showed that the left putaminal volume was significantly larger than the right in all groups combined. Multivariate analysis of covariance with a Bonferroni correction was used to assess statistical significance among the subject groups (AD, FTD, SD, PNFA, and controls) as independent variables and right/left putaminal volumes as dependent variables (covariates, age and intracranial volume; P < .05). The right putamen in FTD was significantly smaller than in AD and controls; whereas in SD, it was smaller compared with controls with a trend toward being smaller than in AD. There was also a trend toward the putamen in the PNFA being smaller than that in controls and in patients with AD. Across the groups, there was a positive partial correlation between putaminal volume and Mini-Mental State Examination (MMSE).
CONCLUSIONS:
Right putaminal volume was significantly smaller in FTD, the FTLD subtype with the greatest expected frontostriatal dysfunction; whereas in SD and PNFA, it showed a trend towards being smaller, consistent with expectation, compared to controls and AD; and in SD, compared with AD and controls. Putaminal volume weakly correlated with MMSE.
Frontotemporal lobar degeneration (FTLD) consists of 3 clinical subtypes: frontotemporal dementia (FTD), semantic dementia (SD), and progressive nonfluent aphasia (PNFA), conceptualized as a result of specific neuropathologic processes that may be visualized, in vivo, via MR imaging.1,2
The neuropathophysiology of FTLD involves frontostriatal neural circuits, which comprise the following: the origin from a prefrontal region, via the caudate, putamen, or nucleus accumbens, via the globus pallidus, via the thalamus, and thence, with feedback to the prefrontal cortex.1,3 Frontostriatal circuits serve several domains of cognitive function affected in FTLD.4,5 Differential cognitive dysfunction mediated via frontostriatal circuits in FTLD may be reflected in structural changes in components of that circuitry.
The striatum (nucleus accumbens, caudate nucleus, and putamen) serves as an entry point for afferent information from the periphery, as well as for afferents and efferents for functionally segregated regions of the cortex.2 Due to loss of afferent or efferent inputs from cortical atrophy, there may be neuroplastic reduction in the volume of the striatum. Severe atrophy of the caudate was noted long ago in FTLD.6–9 We previously demonstrated that volumetric differences in the caudate were consistent with the putative frontostriatal dysfunction in the respective subtype of FTLD.1
The putamen is functionally and structurally affected in FTLD. With positron-emission tomography, the putamen has been found to be hypometabolic in patients with FTLD with urinary incontinence, implying a role in the frontally mediated motor control of micturition in FTLD.10 Relative to healthy controls, left putaminal hypometabolism has been found in FTD.11 Hypometabolism may reflect reduced afferent/efferent activity—impaired activity in neural circuits traversing the putamen, such as the frontostriatal circuits—and, thus, result in atrophy. In neuropathologically confirmed FTLD, ante-mortem MR imaging–measured putaminal volumetric loss, in comparison with that in healthy controls, has been found in those with tau-predominant and ubiquitin-predominant intracellular inclusions.12 Such putaminal atrophy was associated with parkinsonism.
We used manual tracing of the putamen, with a standardized protocol based on our previous studies of the caudate, to measure putaminal volume.13,14 We sought to compare FTLD with a neurodegenerative dementia in which frontostriatal dysfunction is not a major feature, Alzheimer disease (AD), and with healthy controls, in whom there should be no neurodegeneration or cognitive dysfunction. On the basis of the clinical features, the different subtypes of FTLD may display differing degrees of frontostriatal circuit–mediated cognitive dysfunction. FTD, the behavioral variant with predominant frontal-executive cognitive dysfunction, would be expected to show the most involvement of frontostriatal circuit structures, such as the putamen. PNFA and SD show lesser levels of such frontal-executive cognitive dysfunction and may be expected to show less involvement of the putamen. We hypothesized the following:
1) There would be hemispheric asymmetry of putaminal volume.
2) The group average volume of the putamen would differ among groups of AD, FTLD (and subgroups), and healthy controls on the basis of the theoretic degree of frontostriatal circuit dysfunction (controls > AD > SD > PNFA > FTD).
3) Putaminal volume would correlate with a global measure of cognition, as an exploratory investigation to assess the feasibility of correlating putaminal volume with more detailed neuropsychological testing in each group.
Materials and Methods
Participants
Participants were recruited retrospectively from the Memory Clinic at the Karolinska University Hospital, Huddinge, Stockholm, Sweden, and have been described in our previous article on the caudate in FTLD.1 Routine dementia assessment was conducted in all participants. The study was approved by the local ethics committee.
Eighty subjects participated in the previous study: 34 patients with FTLD (12 FTD, 13 SD, 9 PNFA), 19 with AD, and 27 in a control group (Table 1).1 Three subjects, 2 controls and 1 patient with AD, had strokes in the putamen and were excluded from this and subsequent studies, yielding 77 subjects for this study (thus we had 18 with AD and 25 controls, with the other groups as mentioned above).
Table 1:
Demographic features of patients and controls*
| Control | FTD | SD | PNFA | AD | |
|---|---|---|---|---|---|
| No. | 27 | 12 | 13 | 9 | 19 |
| Sex (M/F) | 7:20 | 3:10 | 5:9 | 3:6 | 7:12 |
| Age (yr) | 61.1 (53–78) | 59.45 (42–72) | 63.77 (52–77) | 64.9 (57–78) | 61.8 (56–75) |
| MMSE | 28.7 (25–30) | 20.83 (10–30)†‡ | 22.92 (5–29)†‡ | 22.5 (15–28)†§ | 23.1 (7–29)†‡ |
| Disease duration (yr) | – | 1.65 (0.25–3.4) | 3.90 (1.3–7.7)†¶ | 3.56 (0.06–8.13) | 2.87 (0–4.97) |
Note:— – indicates not applicable; FTD, frontotemporal dementia; SD, semantic dementia; PNFA, progressive nonfluent aphasia; AD, Alzheimer disease; MMSE, Mini-Mental State Examination.
Numbers in parentheses indicate the range of values for age, MMSE, and disease duration, respectively.
Kruskal-Wallis test.
P < .01 compared with controls.
P < .05 compared with controls.
P < .01 compared with FTD.
AD diagnoses were based on clinical criteria including the Diagnostic and Statistical Manual of Mental Disorders, 4th text revision (DSM-IV-TR) and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10).15,16 Patients with FTLD were diagnosed according to consensus diagnostic criteria for FTLD syndromes presented by Neary et al.17 Diagnoses on all subjects included in this study were reviewed by an experienced neurologist.
All subjects in the studies underwent the standard investigation procedure for patients in the memory clinic. The clinical diagnosis was determined at a multidisciplinary consensus conference with physicians, neuropsychologists, speech-language pathologists, and nurses.
The medical examination included a history from a close informant, as well as assessment of physical, neurologic, and psychiatric status. Laboratory investigation of blood, CSF, and urine (including vitamin B12 and folic acid levels and thyroid function) was performed. Neuroradiologic examination consisted of MR imaging of the brain and single-photon emission CT imaging of cerebral blood flow. Detailed electroencephalographic, neuropsychological, and speech-language examinations were performed (described in our previous article1), including the global cognition assessment, Mini-Mental State Examination (MMSE).18
FTLD
Clinical criteria for the subtypes of FTLD were based on international consensus criteria.17 The subtypes included were FTD, SD, and PNFA. Only patients with a primary degenerative cerebral process were selected, excluding patients with signs of cerebrovascular or systemic disorders. Patients with FTLD at different stages of the disease were included.
AD
The diagnosis of AD was based on DSM-IV and ICD-10 criteria.15,16 Participants with AD displayed the development of multiple cognitive deficits including memory impairment and ≥1 of aphasia, apraxia, or agnosia, plus disturbance in executive functioning. These presented as an illness of gradual onset, with continuing decline from previous levels of functioning. These symptoms were not due to another dementing process or psychiatric disorder.
Controls
Controls comprised individuals who were found, after careful assessment, not to fulfill the criteria for FTLD, AD, or any other cognitive disorder, but to sometimes feel forgetful in everyday life. Objective impairment was ruled out through comprehensive neuropsychological assessment; impairment was defined as performance ≥1.5 SDs below the mean on any cognitive test. Accordingly, controls had no objective cognitive impairment by definition. To further minimize the risk of including participants with neurodegenerative disease in very early stages, we included only those participants whose condition did not deteriorate over a minimum of 2-years’ follow-up.
Imaging
Image Acquisition.
T1-weighted MR images were acquired on a 1.5T Magnetom Vision Plus scanner (Siemens Medical Systems, Erlangen, Germany). A 3D magnetization-prepared rapid acquisition of gradient echo pulse sequence (TR, 11.4 ms; TE, 4.4 ms; TI, 300 ms; FA, 10 °; NEX, 1) was used to obtain 72 contiguous coronal 2.5-mm sections with a 512 × 144 matrix and 230-mm FOV.
Image Analysis.
Volumetric analysis was performed by using HERMES (Nuclear Diagnostics, Stockholm, Sweden). Preprocessing of imaging data was performed by interpolation of the images to render them orthogonal and isometric in orientation (cubic voxels = 1 × 1 × 1 mm), followed by alignment via automated rigid-body registration19 to a customized local reference brain (in the anterior/posterior commissure orientation) for ease of tracing in the same orientation and format. Images were adjusted by histogram signal-intensity-analysis assist in outlining brain structures.
All preprocessing was performed via the Brain Map (BMAP) Morphy-Display Scaled software and associated modules designed by L.S. Using this custom-designed software, 1 experienced tracer (J.C.L.L.) analyzed all brain MR images blinded to clinical information (including diagnosis). On the basis of reference images, a standardized manual tracing protocol was used to trace and quantify the volume of the putamen in the axial plane by using reconstructed images (each voxel = 1 mm3) as follows:
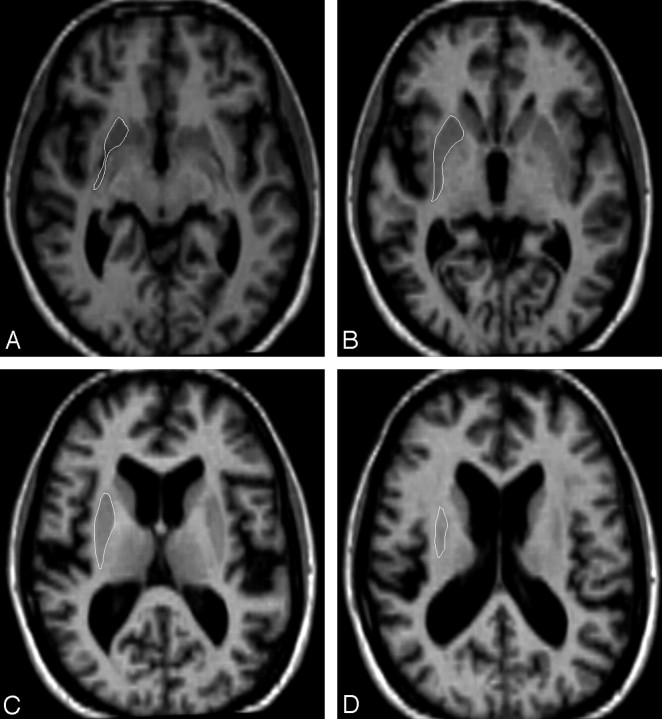
1) For the inferior boundary, we selected the first section in which the putamen is distinct from the head of the caudate, separated by the white matter of the internal capsule and separated from the globus pallidus by a thin lamina of white matter (Fig 1).
2) Reference images for representative sections were used, including the point of differentiation from the nucleus accumbens and caudate (Fig 1).
3) The anterior boundary was defined by separation from the caudate head by the anterior limb of the internal capsule.
4) The lateral border of the putamen was traced by following the margin along the white matter of the external capsule.
5) Small blood vessels representing cribriform change were included in the region of interest.
6) The medial border of the putamen was traced along the anterior limb of the internal capsule and then following the margin along the lamina of white matter separating it from the globus pallidus through to the genu of the internal capsule. In tracing this medial border, we used the most distinct boundary between the body of the putamen and the lamina of white matter separating it from the globus pallidus.
7) The superior boundary was the last section in which the putamen was visible (Fig 1).
8) We used symmetry of the right and left hemispheres, noting that the left putamen was somewhat larger than the right, to judge where the outline should be traced. Judgments as to the borders of the putamen were made by consulting the reference images.
9) We preferred tracing distinct boundaries to achieve reliability. We also consulted other detailed protocols, modifying as required.12,20,21
Fig 1.
Gray-scale axial reference images for tracing the putamen. A, The first section shows the most inferior point of the putamen, bounded by the anterior limb of the internal capsule anteriorly, the external capsule laterally, the internal capsule medially, and the posterior limb of the internal capsule posteriorly. B, The inferior section shows delineation from the claustrum, which appears as a thin gray matter band lateral to the external capsule. C, The midlevel section shows the clear demarcation from the external and internal capsules. D, The last section shows the last identifiable gray matter body interposed between the large white matter tracts.
Volumes obtained were assessed for covariance or normalized in reference to total intracranial volume (ICV) (see below). Total ICV was measured as follows: ICV was traced on coronal sections by a stereologic point-counting technique manually tracing the intracranial volume. Every fourth section was traced. The starting point was randomly chosen from one of the first to fourth sections at the anterior end of the brain. The landmarks for delineation and protocol were based on those used by Eritaia et al.14
Reliability of image analysis was assessed by using intraclass correlations performed via the Statistical Package for the Social Sciences (SPSS, Chicago, Ill). The intrarater class correlation was evaluated by repeating right and left putaminal measurements on 13 scans (26 comparisons) and was 0.93.22
Statistical Analysis
Volumetric Analysis.
Statistical analysis was performed by using SPSS 15.0:
1) Paired t tests were used to assess hemispheric differences in putaminal volume within subject groups with a significance level set at <.05. (Three subjects, 2 controls and 1 patient with AD, had strokes in the putamen and were excluded from this and subsequent studies.)
2) We used partial correlation to explore the relationship between putaminal volume and MMSE scores across all groups, while controlling for age and ICV. Four subjects had missing MMSE values and were excluded from the MMSE partial-correlation analysis. Preliminary analyses were performed to ensure no violation of the assumptions of normality, linearity, and homoscedasticity.
3) Multivariate analysis of covariance (MANCOVA) was used to test statistical significance among the subject groups (AD, FTD, SD, PNFA, and controls) as the independent variable and between raw right and left putaminal volumes as the independent variable at the within-subject level. With SPSS, checks of assumption of normality, linearity, homogeneity of variances and regression slopes, and reliable measurements of covariates for the data were satisfied as a prerequisite for MANCOVA. Covariates used in the MANCOVA were age and intracranial volume. Pairwise comparison of marginal means was conducted, with a Bonferroni correction for multiple comparisons. MMSE was not used as a covariate in the MANCOVA because the number of missing values would have significantly reduced the sample of PNFA (from 9 to 6) and SD (from 13 to 12) groups for the MANCOVA, and those without MMSE were retained in the MANCOVA. The significance level was set at <.05.
Results
Demographic Data
Although not specifically age-matched, the groups did not differ significantly in mean age. Similarly, MMSE scores were significantly different from those of controls but not across the dementia diagnoses. Illness duration was significantly different for the SD group versus the FTD group (Table 1).
Group Comparisons of Right and Left Putaminal Volume for Hemispheric Asymmetry
All Groups Combined.
The results for these comparisons are summarized in Table 2. Across all groups combined (AD, FTD, SD, PNFA, and controls), there was hemispheric asymmetry of putaminal volume, with the left putaminal volume significantly larger than the right at P = .002, eta-squared = 0.134.
Table 2:
Within-group comparisons of hemispheric putaminal volume*
| R Put Vol | R Put Vol (SEM) | L Put Vol | L Put Vol (SEM) | tValue | df | Significance (2-tailed) | η2 | |
|---|---|---|---|---|---|---|---|---|
| Overall (n = 77) | 3.505 | 0.069 | 3.676 | 0.069 | −3.428 | 76 | .002† | 0.134 |
| C (n = 25) | 3.771 | 0.092 | 3.753 | 0.111 | 0.242 | 24 | .811 | |
| AD (n = 18) | 3.746 | 0.110 | 3.915 | 0.123 | −1.647 | 17 | .118 | |
| FTD (n = 12) | 3.151 | 0.161 | 3.646 | 0.167 | −3.052 | 11 | .011† | 0.456 |
| SD (n = 13) | 3.277 | 0.192 | 3.480 | 0.210 | −1.458 | 12 | .170 | |
| PNFA (n = 9) | 3.084 | 0.219 | 3.310 | 0.197 | −1.870 | 8 | .098 |
Note:—C indicates control; R Put, right putamen; L Put, left putamen; Overall, all groups included; Vol, volume.
All volumes in cubic centimeters.
Significant at P < .05.
By Disease or Control Group.
Within the FTD group, the left putaminal volume was significantly larger than the right at P = .011, eta-squared = 0.458. Within the other groups of controls, AD, PNFA, and SD, no hemispheric asymmetry was found. Thus, most of the combined group effect of hemispheric asymmetry was contributed by the FTD group asymmetry.
Partial Correlations of MMSE with Putaminal Volume across Groups to Investigate Associations with Cognition
Combining all diagnostic groups (AD, FTD, SD, PNFA, and controls), we found a weak (r < 0.4) positive partial correlation (P < .05) between bilateral putaminal volumes and MMSE. Higher putaminal volumes were associated with higher MMSE scores. Inspection of the zero-order correlations for left (r = 0.258) and for right (r = 0.356) showed that controlling for age and ICV had little effect on the strength of the relationship between the variables.
The coefficient of determination was 0.061 for the left putamen, with MMSE explaining 6% of the variance of left putaminal volume. The coefficient of determination was 0.127 on the right, explaining 13% of the variance.
The partial correlations by groups are summarized in Table 3.
Table 3:
Partial correlations between putaminal volume and MMSE by subject group*
| Volume | Control(n = 25) | AD(n = 18) | FTD(n = 12) | SD(n = 12) | PNFA(n = 6) | All(n = 73) |
|---|---|---|---|---|---|---|
| R Put | ||||||
| R | −0.326 | 0.434 | −0.119 | 0.380 | 0.979 | 0.351 |
| Sig | 0.129 | 0.093 | 0.743 | 0.278 | 0.021† | 0.003† |
| L Put | ||||||
| R | −0.224 | 0.370 | 0.332 | 0.567 | 0.663 | 0.248 |
| Sig | 0.305 | 0.159 | 0.348 | 0.087 | 0.337 | 0.037† |
Note:—R, partial correlation value; Sig, 2-tailed significance for partial correlation value; All, across all groups: control, AD, FTD, SD, PNFA.
Partial correlation controlled for age and ICV.
Partial correlations significant at P < 05.
Combining All Group (AD, FTLD, SD, PNFA) and Control MANCOVAs to Assess Relative Volumes of Disease Groups (AD, FTLD, SD, PNFA) in Comparison with Controls
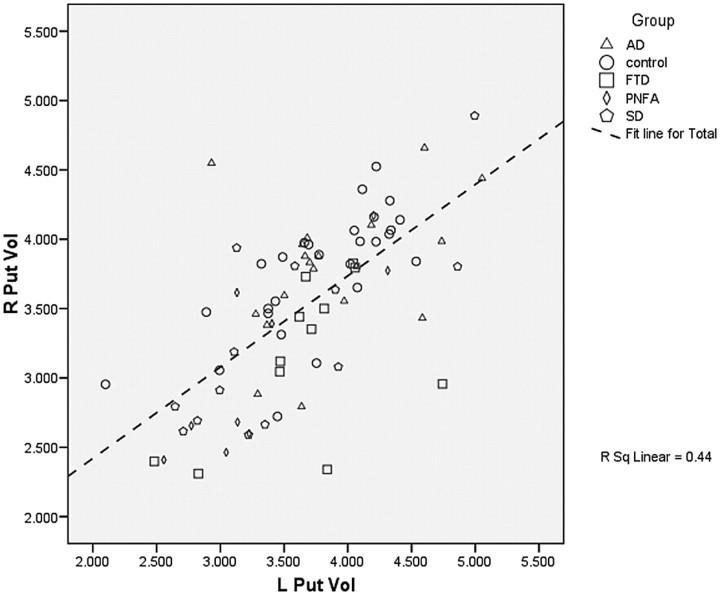
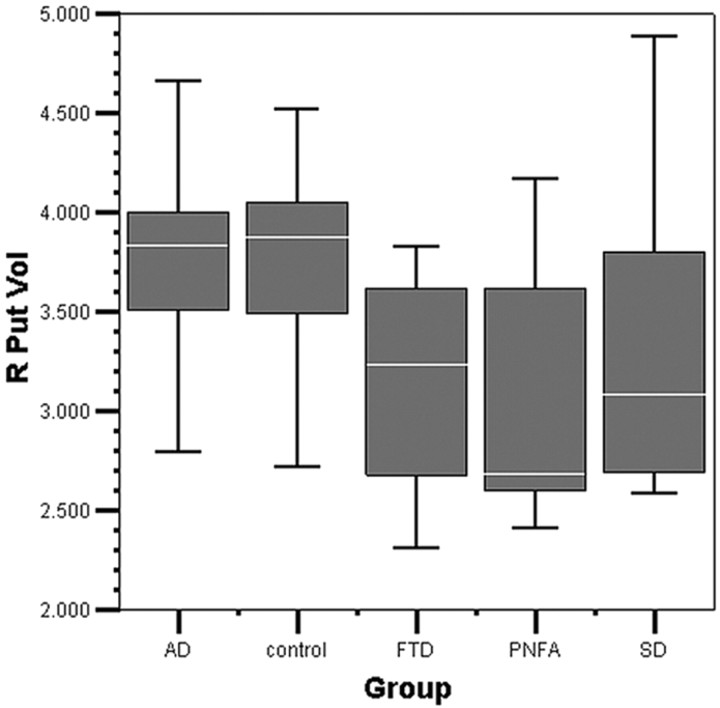
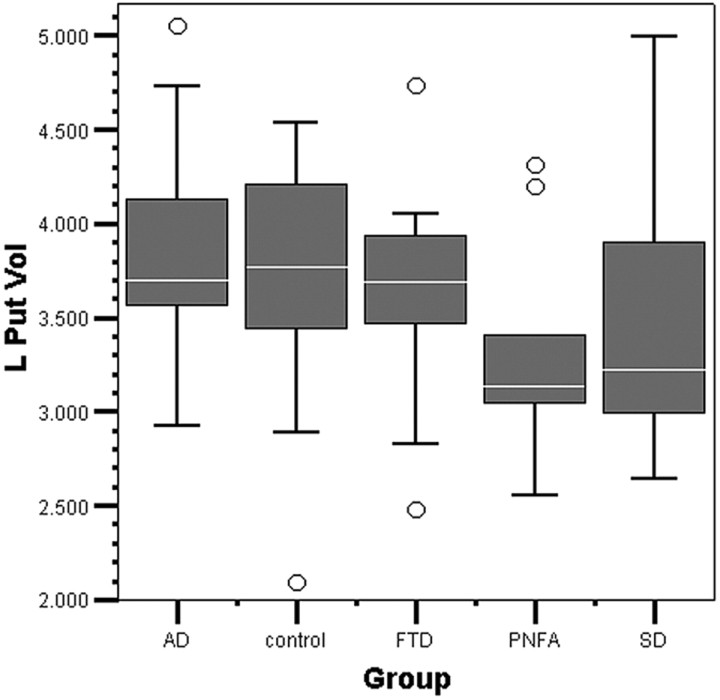
The results of the MANCOVA, combining all groups in the model (AD, FTLD, SD, PNFA, and controls), are displayed in Table 4. A scatterplot of right-versus-left putaminal volume across all groups is displayed in Fig 2, and boxplots of putaminal volume by group, in Figs 3 and 4.
Table 4:
Group MANCOVA: tests of between-subjects’ effects*
| Dependent Variable, Corrected Model Group (No.) | df | F-Statistic | Significance | Partial η2 | Observed Power | Covariates |
|---|---|---|---|---|---|---|
| R Put | ||||||
| AD=18 | 6 | 7.276 | .000* | 0.374 | 1.000 | Age: 61.982 yr |
| C=25 | ICV: 1411.77 | |||||
| FTD=12 | ||||||
| PNFA=9 | ||||||
| SD=13 | ||||||
| L Put | 6 | 3.987 | .002* | 0.247 | 0.960 |
Note:—Estimated marginal means from MANCOVA; Group MANCOVA: model, full factorial, sum of squares type III; dependent factor, R & L Put Vol; independent factors, Group (all groups): AD, control, FTD, PNFA, SD; compare main effects via pair-wise comparison for estimated marginal means.
Multivariate analysis of covariance significant at P < .05.
Fig 2.
Scatterplot of right versus left putaminal volume. R Put Vol indicates right putaminal volume (in cubic centimeters); L Put Vol, left putaminal volume (in cubic centimeters); R Sq Linear, linear fit line regression value.
Fig 3.
Right putaminal volume by group boxplot. The median line in the box is white. R Put Vol indicates right putaminal volume (in cubic centimeters).
Fig 4.
Left putaminal volume by group boxplot. The median line in the box is white; circles show outliers. L Put Vol indicates left putaminal volume (in cubic centimeters).
In the combined group model (AD, FTLD, SD, PNFA, and controls), bilateral putaminal volumes were significantly different. There was a significant difference in right putaminal volume among subjects by diagnosis (AD, control, FTD, PNFA, SD): F(6, 76)=7.276, P=.000 (P < .05), eta=0.374. Left putaminal volume was also significantly different among diagnostic groups F(6, 76)=3.987, P=.002, (P < .05), eta=0.247.
Pairwise Comparisons of Putaminal Volume to Assess Differences between Separate Diagnostic Groups (AD, FTLD, SD, PNFA) and Controls
Univariate pair-wise comparison of marginal means of the subject groups derived from the MANCOVA was conducted (AD, FTD, SD, PNFA, and controls) (n=77) with a Bonferroni correction for multiple comparisons. The results of the pair-wise comparison of the estimated marginal means from MANCOVA are summarized in Tables 5 and 6. Overall, there was a significant difference in right putaminal volume in the pair-wise comparisons of all diagnostic groups, F(4,73) = 6.679, P = .000, eta = 0.277. There was no significant difference in left putaminal volume via pair-wise comparison of all diagnostic groups.
Table 5:
Univariate tests
| Dependent Variable | Sum of Squares | df | Mean Square | F-Test* | Significance | Partial η2 | Power (α)† |
|---|---|---|---|---|---|---|---|
| R Put Vol | |||||||
| Contrast | 6.977 | 4 | 1.744 | 6.979 | .000 | .277 | .992 |
| Error | 18.244 | 73 | .250 | ||||
| L Put Vol | |||||||
| Contrast | 2.647 | 4 | .662 | 2.165 | .081 | .106 | .612 |
| Error | 22.314 | 73 | .306 |
The F-test tests the effect of group. This test is based on the linearly independent pair-wise comparisons among the estimated marginal means.
α computed using α = .05.
Table 6:
Pair-wise comparisons*
| Groups and Dependent Variables |
Mean Difference (I-J) | Standard Error | Significance (a)† | 95% Confidence Interval for Difference (α) |
||
|---|---|---|---|---|---|---|
| (I) Group | (J) Group | Upper Bound | Lower Bound | |||
| R Put Vol (cm3) | ||||||
| AD | Controls | .009 | .150 | 1.000 | −.425 | .444 |
| FTD | .698† | .186 | .003 | .161 | 1.236 | |
| PNFA | .576 | .206 | .065 | −.019 | 1.171 | |
| SD | .512 | .181 | .061 | −.012 | 1.037 | |
| Control | AD | −.009 | .150 | 1.000 | −.444 | .425 |
| FTD | .689‡ | .175 | .002 | .183 | 1.195 | |
| PNFA | .567 | .196 | .051 | −.001 | 1.134 | |
| SD | .503‡ | .171 | .045 | .006 | .999 | |
| FTD | AD | −.698‡ | .186 | .003 | −1.236 | −.161 |
| Control | −.689‡ | .175 | .002 | −1.195 | −.183 | |
| PNFA | −.123 | .228 | 1.000 | −.783 | .538 | |
| SD | −.186 | .204 | 1.000 | −.778 | .405 | |
| PNFA | AD | −.576 | .206 | .065 | −1.171 | .019 |
| Control | −.567 | .196 | .051 | −1.134 | .001 | |
| FTD | .123 | .228 | 1.000 | −.538 | .783 | |
| SD | −.064 | .219 | 1.000 | −.699 | .571 | |
| SD | AD | −.512 | .181 | .061 | −1.037 | .012 |
| Control | −.503‡ | .171 | .045 | −.999 | −.006 | |
| FTD | .186 | .204 | 1.000 | −.405 | .778 | |
| PNFA | .064 | .219 | 1.000 | −.571 | .699 | |
| L Put Vol (cm3) | ||||||
| AD | Control | .058 | .166 | 1.000 | −.422 | .539 |
| FTD | .231 | .205 | 1.000 | −.363 | .826 | |
| PNFA | .482 | .227 | .374 | −.176 | 1.140 | |
| SD | .438 | .200 | .322 | −.143 | 1.018 | |
| Control | AD | −.058 | .166 | 1.000 | −.539 | .422 |
| FTD | .173 | .193 | 1.000 | −.386 | .733 | |
| PNFA | .424 | .217 | .546 | −.204 | 1.052 | |
| SD | .379 | .190 | .492 | −.170 | .928 | |
| FTD | AD | −.231 | .205 | 1.000 | −.826 | .363 |
| control | −.173 | .193 | 1.000 | −.733 | .386 | |
| PNFA | .251 | .252 | 1.000 | −.480 | .981 | |
| SD | .206 | .226 | 1.000 | −.448 | .860 | |
| PNFA | AD | −.482 | .227 | .374 | −1.140 | .176 |
| control | −.424 | .217 | .546 | −1.052 | .204 | |
| FTD | −.251 | .252 | 1.000 | −.981 | .480 | |
| SD | −.045 | .243 | 1.000 | −.747 | .658 | |
| SD | AD | −.438 | .200 | .322 | −1.018 | .143 |
| control | −.379 | .190 | .492 | −.928 | .170 | |
| FTD | −.206 | .226 | 1.000 | −.860 | .448 | |
| PNFA | .045 | .243 | 1.000 | −.658 | .747 | |
Based on estimated marginal means.
Adjustment for multiple comparisons: Bonferroni.
The mean difference is significant at the .05 level.
Pair-Wise AD Comparisons with FTLD Subgroups
Right putaminal volume was significantly greater in AD compared with FTD: mean difference (MD) = 0.698 cm3, standard error of mean (SEM) = 0.186, P = .003. For the right putamen, there were weak trends toward AD putaminal volume being larger compared with SD (MD = 0.512 cm3, P = .061) and PNFA (MD = 0.576 cm3, P = .065).
There were no significant differences in right putaminal volume between AD and controls. There were no significant differences in left putaminal volume among AD, controls, and FTLD subgroups
Pair-Wise Comparisons among FTLD Subgroups and with Controls
Right putaminal volume was significantly larger in controls than in FTD: MD = 0.689 cm3, SEM = 0.175, P = .002. Right putaminal volume was not significantly different among subjects with FTD compared with those with PNFA and SD. Right putaminal volume was weakly significantly smaller in SD compared with controls: MD = −0.503 cm3, SEM = 0.171, P = .045. There was a trend toward a significant difference in right putaminal volume in subjects with PNFA compared with controls: MD = −0.567 cm3, SEM = 0.196, P = .051. Left putaminal volume was not significantly different in FTD compared with AD, controls, PNFA, and SD. Bilaterally, there was no significant difference in putaminal volume among subtypes of FTLD.
Mean Putaminal Volumes for Each Disease Group (AD, FTD, SD, PNFA) as a Percentage of Control Volume
The combined group model MANCOVA estimated marginal mean putaminal volumes across the all groups; the grand mean is displayed in Table 7. The estimated marginal means in each diagnostic group are displayed as a percentage of control putaminal volumes in Table 8 and graphically in Figs 3 and 4. The AD group was the largest in mean bilateral volume of the diagnostic groups (101% of control volume), followed by the FTD group (89%), SD group (89%), and PNFA group (87%).
Table 7:
Estimated marginal means: grand mean*
| Dependent Variable | Mean | Standard Error | 95% Confidence Interval |
|
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| R Put Vol ( cm3) | 3.418 | .060 | 3.299 | 3.538 |
| L Put Vol (cm3) | 3.612 | .067 | 3.479 | 3.744 |
Covariates appearing in the model are evaluated at the following values: age = 61.88 yr, ICV = 1408.0000.
Table 8:
Marginal mean estimates*
| Groups and Dependent Variables |
Standard Error | 95% Confidence Interval |
% Control Volume | ||
|---|---|---|---|---|---|
| Group | Mean | Lower Bound | Upper Bound | ||
| R Put Vol (cm3) | |||||
| AD | 3.778 | .115 | 3.549 | 4.006 | 100 |
| Control | 3.768 | .097 | 3.576 | 3.961 | 100 |
| FTD | 3.079 | .146 | 2.787 | 3.371 | 82 |
| PNFA | 3.202 | .170 | 2.863 | 3.541 | 85 |
| SD | 3.266 | .140 | 2.986 | 3.545 | 87 |
| L Put Vol (cm3) | |||||
| AD | 3.854 | .127 | 3.601 | 4.106 | 102 |
| Control | 3.795 | .107 | 3.582 | 4.008 | 100 |
| FTD | 3.622 | .162 | 3.300 | 3.945 | 95 |
| PNFA | 3.371 | .188 | 2.996 | 3.746 | 89 |
| SD | 3.416 | .155 | 3.107 | 3.725 | 90 |
Note:—% Control vol indicates putaminal volume as a percentage of control volume (in cubic centimeters).
Covariates appearing in the model are evaluated at the following values: age = 61.88 yr, ICV = 1408.0000.
Discussion
We found that the left putamen is generally greater in volume than the right in all disease groups (AD and FTLD) but is symmetric in healthy controls. Previous studies on healthy persons have found that left caudate and putaminal volumes were greater than those on the right.23–26 In the AD and FTLD groups, hemispheric asymmetry may be enhanced by disease processes, whereas less pronounced asymmetry in controls may require larger groups to show a significant asymmetry.
For the group MANCOVA, there was evidence of a significant difference in putaminal volume bilaterally across all AD, FTLD subgroups, and controls combined.
The estimated marginal mean volumes from the MANCOVA for the right putamen showed AD = controls > SD > PNFA > FTD. However, in the right putamen, the pair-wise comparisons of the FTLD subtypes showed no significant difference, only trends, among subtypes. The right putamen in FTD was significantly smaller in volume than that in AD and controls. The right putamen in SD was significantly smaller than that in controls.
The estimated marginal mean volumes for the left putamen showed AD = controls > FTD > SD > PNFA, with no significant pair-wise differences among any diagnostic groups. For the left putamen, there were no significant differences among FTLD subgroups.
In general, the differences in putaminal volume are right-sided with weak-to-moderate effect size. The lateralized nature of differences in putaminal volume to the right hemisphere is puzzling and cannot easily be explained, other than to conclude that the left putamen is relatively preserved, being larger than the right in AD and FTLD, because in the control group putaminal volumes are symmetric. This conclusion is supported in part by the finding in the combined group MANCOVA that left putaminal volume is significantly different across all groups with a weak-to-moderate eta-squared. The use of small subtype groups and a manual tracing method may have been insufficient to detect smaller left-sided volumetric losses. Small subtype groups of FTLD are common in most imaging studies due to the difficulties in characterizing and recruiting subjects; whereas our manual tracing method shows reasonable intrarater reliability.
Hypertrophy of brain structures associated with emotion may reflect growth of the structure due to hyperactivity.27 As a corollary, neurodegenerative processes, such as in FTLD, may result in atrophy and underactivity of the putamen. FTLD is characterized by emotional and behavioral disturbances considered to arise from neuropathology involving frontal cognitive dysfunction, structurally implicating frontostriatal circuits in each subtype of FTLD.3,4,28–32 Differences in putaminal volume in subtypes of FTLD may reflect the relative frontostriatal dysfunction of the subtype. Dysfunction in such circuits may be due to, or the result of, disconnections or structural change.29,31 Differences in frontostriatal putaminal dysfunction in FTLD may, therefore, manifest structurally as differential putaminal volumes among FTLD subtypes.1
Frontostriatal dysfunction is not prominent in AD and is not present in controls. Accordingly, we would expect no significant differences in volume of the putamen between AD and controls; and overall, these volumes should be the largest due to lack of involvement of the putamen in the theoretic neuropathophysiology. This expectation is consistent with our findings for the right putamen alone.
Examining the FTLD subtypes, we found the degree of atrophy was, in the right putamen alone, partially consistent with the expected theoretic frontostriatal dysfunction in each subtype.
The right putamen in AD was 100% of the volume of controls, whereas in SD (87%), PNFA (85%), and FTD (82%), it was respectively smaller. The FTD group would be expected to have the greatest dysfunction due to greater involvement of frontostriatal pathology33; and on the right, this pattern was consistent with the expected frontostriatal dysfunction. In contrast, left putaminal volume in FTD was 95% of that of controls.
SD is believed to involve less frontostriatal dysfunction, and bilateral putaminal volume (90% of that in controls) showed a trend toward being smaller than that in AD and was (weakly) significantly different from control putaminal volume on the right.
Patients with PNFA also displayed frontostriatal dysfunction, and those with this subtype had the smallest bilateral putaminal volume (89% of controls), showing a trend toward a difference on the right from AD and controls. Language dysfunction in PNFA may include a motor or cognitive processing component secondary to putaminal atrophy. A previous study of subcortical cerebrovascular lesions showed that anterior striatal lesions did not affect speech but that posterior striatal or extensive putaminal infarction did.34
We also found that putaminal volume was weakly, but significantly, correlated with a basic global measure of cognition measured via MMSE, with lower volume correlating with poorer cognition. Further studies will be required to assess whether cognition related to frontostriatal circuit–mediated cognition may be correlated with relative putaminal volume differences.
In postmortem examination–confirmed cases of FTLD, those with tau-positive neuropathology had greater reduction in gray matter volume in the region of the bilateral putamen compared with those with ubiquitin-positive neuropathology (FTLD-U).12 There was a significant reduction of frontal white matter in the FTLD-U group, representing possible disconnection of neural pathways to the putamen. Differences in tau- or ubiquitin-based neuropathology among clinical subtypes may have contributed to the putaminal volume loss that we have noted, with a greater preponderance of tau-positive pathology in those with the smallest volumes. The consequences of putaminal atrophy on motor function are intriguing and could independently contribute to neuropsychological dysfunctions in FTLD subtypes. We hope to perform similar clinical neuropathologic correlation studies in the future.
One limitation of this study is the use of a subjective memory–complaint cohort as the control. However, this group was comprehensively assessed for objective cognitive dysfunction, and those with objective changes were excluded. Given the exigencies of recruiting people with subtypes of FTLD, AD, and controls, age and sex matching was not possible. However, apart from female preponderance, duration of illness, and MMSE scores, there were no other significant differences among groups. Adjustments were made via the MANCOVA for the covariates age and total intracranial volume, but not for MMSE, due to significant missing values for this variable and concerns that language dysfunction in FTLD subtypes may artificially reduce MMSE scores in SD and PNFA. Although we acknowledge that automated tracing protocols may have greater reliability, greater validity is achieved with expert observer tracing.13 Automated algorithmic methods for segmentation and quantification of the putamen are not necessarily superior. Automated algorithms are validated by comparison with manual tracing by experienced tracers. Thus manual tracing remains the gold standard for in vivo measurement of putaminal volume.
Clinically, our findings contribute to the understanding of the neuropathophysiology of FTLD and the functional significance of the putamen. The right putaminal volume is significantly different among FTD, AD, and controls and between SD and controls. There are trends toward putaminal-atrophy differences between SD and AD and among PNFA, AD, and controls. However, there are no significant differences in the left putaminal volume in any diagnostic subgroup (FTLD or other). Putaminal atrophy on MR imaging may be potentially useful in clinical subtyping of FTLD, in combination with patterns of atrophy in other cortical and subcortical regions.1,35
These results contrast with our previous study of bilateral caudate volume in FTLD, in which a clear gradient of atrophy, with volumes of controls = AD > SD > PNFA > FTD, was consistent with the expected frontostriatal dysfunction in each subtype of FTLD and for AD and controls. Thus, the putamen seems less clearly implicated as a substrate in frontostriatal circuit dysfunction in FTLD. Decreased putaminal volume is weakly associated with poorer cognition across all groups, suggesting that further exploration of the correlation of neuropsychological function and putaminal volume may be worthwhile. Associations of putaminal volume with motor function and parkinsonism should also be explored. Further exploration of the structural and functional integrity of frontostriatal circuits as a substrate for cognitive and behavioral change is warranted. Larger samples are likely to clarify whether such anatomic differences hold true.
Conclusions
In relation to hypothesis 1, we have shown that there is an overall group hemispheric asymmetry of the putamen, but this may be due to selective atrophy on the right in the disease groups. For hypothesis 2, there was no clear gradient of putaminal atrophy among subtypes of FTLD, consistent with the expected frontostriatal dysfunction, but there remained interesting results. AD subjects, with theoretically minimal frontostriatal circuit dysfunction, had larger right putaminal volumes—equivalent healthy controls—than those in all FTLD subtypes. The volume of the right putamen was significantly smaller in comparison with that in AD and controls in the FTD subtype of FTLD, the subtype considered to have the greatest degree of frontostriatal cognitive dysfunction. The right putaminal volume in SD was significantly smaller compared with that in controls, with a trend to a difference from AD. There was a trend toward difference for the right putamen in PNFA being smaller compared with that in AD and controls. Thus, all FTLD subtypes showed evidence of right putaminal atrophy compared with controls and AD, but no evidence of pair-wise differences among the subtypes themselves.
There are no corresponding significant differences in the left putamen. Perhaps the putaminal atrophic process in FTLD is lateralized to the right; thus, the putamen is less clearly implicated as a substrate in frontostriatal circuit dysfunction in FTLD than the caudate.1 That the changes are confined to the right putamen is puzzling, recalling by analogy, Socrates’ comment on Heraclitus’ philosophy: “The part I understand is excellent, and so too is, I dare say, the part I do not understand.”36 Finally, for hypothesis 3, putaminal volume is weakly correlated with global cognition as assessed by MMSE across AD, FTLD, and controls, indicating that further investigation of correlations with cognition may be warranted.
We plan to explore the striatal volume in larger samples via studying interrelationships with cortical regions in subtypes of FTLD, morphologic changes via further shape analysis, and correlations with neuropsychological and behavioral features.
Acknowledgments
This research made use of the Stockholm Medical Imaging Laboratory at Karolinska University Hospital, Stockholm, Sweden. Christian Andersen, MD, performed neurologic assessments for diagnosis of FTLD subtypes.
References
- 1. Looi JCL, Lindberg O, Zandbelt BB, et al. Caudate nucleus volumes in frontotemporal lobe dementia: differential atrophy in subtypes. AJNR Am J Neuroradiol 2008; 29: 1537– 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schroeter ML, Razcka K, Neumann J, et al. Towards a nosology for frontotemporal lobar degenerations: a meta-analysis involving 267 subjects. Neuroimage 2007; 36: 497– 510 [DOI] [PubMed] [Google Scholar]
- 3. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9: 357– 81 [DOI] [PubMed] [Google Scholar]
- 4. Hodges JR, Patterson K. The neuropsychology of frontotemporal dementia. In Hodges JR. Frontotemporal Dementia Syndromes. Cambridge, UK: Cambridge University Press; 2007: 102– 33 [Google Scholar]
- 5. Leh SE, Ptito A, Chakravaty MM, et al. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci Lett 2007; 419: 113– 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex 2007; 16: 1508– 21 [DOI] [PubMed] [Google Scholar]
- 7. von Braunmühl A, Picksche K. In: Bumke O. Handbuch der Geisteskrankheiten. vol. 11, part VII Berlin, Germany: Springer-Verlag; 1930: 673– 715 [Google Scholar]
- 8. von Bagh K. Klinische und pathologisch-anatomische Studien an 30 Fällen systematischer umschriebener Atrophie der Grosshirnrinde (Pickscher Krankheit): Annales Academiae Scientiarum Fennicae—Series A.V. Medica-Anthropologica. Helsinki, Finland: Suomalaisen Tiedeakatemia; 1946 [Google Scholar]
- 9. Lüers T, Spatz H. Picksche Krankheit: Progressive umschriebene Großhirnatrophie. In: Lubarsch O, Rössle F, Henke F. Handbuch der speziellen pathologischen Anatomie und Histologie. vol. 13, part 1A Berlin, Germany: Springer-Verlag; 1957: 614– 715 [Google Scholar]
- 10. Perneczky R, Diehl-Schmid J, Förstl H, et al. Urinary incontinence and its functional anatomy in frontotemporal lobar degenerations. Eur J Nucl Med Mol Imaging 2008; 35: 605– 10 [DOI] [PubMed] [Google Scholar]
- 11. Jeong Y, Cho SS, Park JM, et al. 18FDG-PET findings in frontotemporal dementia: an SPM analysis of 29 patients. J Nuc Med 2005; 46: 233– 39 [PubMed] [Google Scholar]
- 12. Kim EJ, Rabinovici GD, Seeley WW, et al. Patterns of MRI atrophy in tau positive and ubiquitin positive frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry 2007; 78: 1375– 78. Epub 2007 Jul 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Looi JC, Lindberg O, Liberg B, et al. Volumetrics of the caudate nucleus: reliability and validity of a new manual tracing protocol. Psychiatry Res Neuroimag 2008; 163: 279– 88. Epub 2008 Jul 25 [DOI] [PubMed] [Google Scholar]
- 14. Eritaia J, Wood SJ, Stewart GW, et al. An optimized method for estimating intracranial volume from magnetic resonance images. Magn Reson Med 2000; 44: 973– 77 [DOI] [PubMed] [Google Scholar]
- 15. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- 16. World Health Organization. International Statistical Classification of Diseases and Related Health Problems: 10th Revision Version for 2007. Available at: http://www.who.int/classifications/apps/icd/icd10online. Accessed January 11, 2007
- 17. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51: 1546– 54 [DOI] [PubMed] [Google Scholar]
- 18. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res 1975; 12: 189– 98 [DOI] [PubMed] [Google Scholar]
- 19. Woods RP, Grafton ST, Holmes CJ, et al. Automated image registration. I. General methods and intrasubject, intramodality validation. J Comput Assisted Tomogr 1998; 22: 139– 52 [DOI] [PubMed] [Google Scholar]
- 20. Langen M, Durston S, Staal WG, et al. Caudate nucleus is enlarged in high-functioning medication-naïve subjects with autism. Biol Psychiatry 2007; 62: 262– 66 [DOI] [PubMed] [Google Scholar]
- 21. Raz N, Rodrigue KM, Kennedy KM, et al. Differential aging of the human striatum: longitudinal evidence. AJNR Am J Neuroradiol 2003; 24: 1849– 56 [PMC free article] [PubMed] [Google Scholar]
- 22. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979; 86: 420– 28 [DOI] [PubMed] [Google Scholar]
- 23. Szabo CA, Lancaster JL, Xiong J, et al. MR imaging volumetry of subcortical structures and cerebellar hemispheres in normal persons. AJNR Am J Neuroradiol 2003; 24: 644– 47 [PMC free article] [PubMed] [Google Scholar]
- 24. Gur RE, Maany V, Mozley PD, et al. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry 1998; 155: 1711– 17 [DOI] [PubMed] [Google Scholar]
- 25. DeCarli C, Hatta J, Fazilat S, et al. Extratemporal atrophy in patients with complex partial seizures of left temporal origin. Ann Neurol 1998; 43: 41– 45 [DOI] [PubMed] [Google Scholar]
- 26. Murphy DG, DeCarli C, Schapiro MB, et al. Age-related differences in volumes of subcortical nuclei, brain matter, and cerebrospinal fluid in healthy men as measured with magnetic resonance imaging. Arch Neurol 1992; 49: 839– 45 [DOI] [PubMed] [Google Scholar]
- 27. Krishnamoorthy E. A differential role for the hippocampus and amygdala in neuropsychiatric disorders. J Neurol Neurosurg Psychiatry 2007; 78: 1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res 2002; 53: 647– 54 [DOI] [PubMed] [Google Scholar]
- 29. Cummings JL. Frontal subcortical circuits and human behavior. Arch Neurol 1993; 5: 873– 80 [DOI] [PubMed] [Google Scholar]
- 30. Andrewes DG. Neuropsychology: From Theory to Practice. East Sussex, UK: Psychology Press; 2001 [Google Scholar]
- 31. Barker-Collo S, McCarthy D. Neuropsychological assessment. In: Feigin VL, Bennett DA. Handbook of Clinical Neuroepidemiology. New York: Nova Science; 2007: 621– 48 [Google Scholar]
- 32. Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev 1995; 20: 91– 127 [DOI] [PubMed] [Google Scholar]
- 33. Neary D, Snowden J, Mann D. Frontotemporal dementia. Lancet Neurol 2005; 4: 771– 80 [DOI] [PubMed] [Google Scholar]
- 34. Alexander MP, Naeser MA, Palumbo CL. Correlations of subcortical CT lesion sites and aphasia profiles. Brain 1987; 110: 961– 91 [DOI] [PubMed] [Google Scholar]
- 35. Lindberg O, Östberg P, Zandbelt BB, et al. Cortical morphometric subclassification of frontotemporal lobar degeneration. AJNR Am J Neuroradiol 2009; 30: 1233– 39 Epub 2009 Apr 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gottlieb A. The Dream of Reason. London, UK: Penguin; 2000: 41 [Google Scholar]