Abstract
BACKGROUND AND PURPOSE:
The embolization of aneurysms with hydrogel filaments allow postprocedural CT and MR imaging studies without artifacts. We compared the performance of 3 hydrogel filament formulations in rabbit experimental aneurysms by using angiography and histologic samples.
MATERIALS AND METHODS:
Embolization of 35 rabbit elastase or bifurcation aneurysms was performed with 3 different formulations of detachable hydrogel filaments, including 1) polyethylene glycol opacified with aromatic iodine (PEG-I; n = 12), 2) polyethylene glycol opacified with barium sulfate (PEG-B; n = 12), or 3) polypropylene glycol opacified with barium sulfate (PPG-B; n = 11). Follow-up angiography was performed before the rabbits were killed at 2 (n = 7), 6 (n = 9), 10 (n = 8), or 26 (n = 11) weeks. Angiographic occlusion was scored according to the Raymond scale, and interval changes were assessed. The harvested aneurysms were evaluated on histologic examination. From the sections, we determined the percentage of the sac excluded from the vasculature and occupied by embolic devices by using image analysis. We compared results using the analysis of variance/t test or χ2 test.
RESULTS:
The mean number of devices used to treat aneurysms in the PPG-B group was significantly greater than that used for the other 2 groups, though aneurysm volumes were similar among groups. Compared with immediate posttreatment occlusion scores, mean angiographic occlusion at follow-up was increased for all 3 hydrogel filament groups. On histologic examination, thrombus organization, neointima formation, and inflammation were similar to that observed in rabbit experimental aneurysms with other embolic devices containing platinum coils.
CONCLUSIONS:
The embolization of experimental aneurysms with hydrogel filaments resulted in durable angiographic and histologic occlusion from 2 to 26 weeks. With improvements, hydrogel filaments free from metallic coils show promise for endovascular use.
Embolization of intracranial aneurysms with detachable platinum coils is an accepted alternative to neurosurgical clipping and has become the method of choice for many lesions. However, coiling of aneurysms may not result in a definitive treatment, especially for wide-neck and large aneurysms,1 and surveillance imaging to detect recanalization or regrowth is required. Indeed, initial results of the International Subarachnoid Aneurysm Trial showed that coiling aneurysms was superior to clipping,2 but recent data analysis suggests that the advantages of coiling cannot be assumed for patients younger than 40 years because of concerns of late rebleeding.3
The question of the long-term durability of platinum coil treatment has led to the development of numerous modified embolic devices. These modified devices usually comprise a platinum filament backbone for radiopacity and deliverability, along with some type of polymeric material in an attempt to alter the physical or biologic characteristics of the implant. Biodegradable polymers, usually a copolymer of lactic acid and glycolic acid, have been used to amplify the rate of thrombus organization. Initial clinical reports generally show that the addition of degradable polymers to platinum coils does not decrease rates of recurrence.4,5 In an alternative sense, expansile hydrogels have been used to increase the volumetric occlusion rate. Increased volumetric occlusion with platinum coils is associated with better angiographic stability and lower retreatment rates.6,7 The use of devices with expansile hydrogels increases the volumetric filling above that of platinum coils8,9 and may enhance the durability of the treatment.10
Notwithstanding their early promise, devices constructed with expansile polymers (HydroCoil; MicroVention Terumo, Aliso Viejo, Calif) have some limitations. The devices are stiffer than comparable platinum coils, in part because of the inflexible nature of the dried hydrogel. Although the expansion of the HydroCoil device overcomes the limitations of stiffness regarding volumetric occlusion,8 even higher rates of volumetric occlusion might be obtained with an expansile device that is relatively soft and thus is easily packed into aneurysms. Another limitation common to detachable coils is the presence of transition metals, the elements in groups 3 to 12 on the periodic table. Radiographs are unable to penetrate some of these metals, such as platinum, tungsten, and gold, and such radiopacity leads to their use in embolic devices. The use of transition metals to impart radiopacity also causes beam-hardening artifacts on CT imaging, greatly limiting this technique. With the widespread availability of CT angiography, this imaging technique may be beneficial for the noninvasive follow-up of aneurysm embolizations.
One approach to address the limitations of current embolic devices is with hydrogel filaments.11 We have evaluated detachable embolic devices prepared from cylindric pieces of radiopaque hydrogel possessing a secondary helical shape and a defined length that are under development by MicroVention Terumo (Aliso Viejo, Calif). This study presents our initial preclinical evaluations of the use of 3 prototype formulations in rabbit bifurcation and elastase experimental aneurysms.
Materials and Methods
The rabbits with bifurcation aneurysms were cared for in accordance with the Austrian regulations governing animal experiments, and the experimentation was approved by the committee for animal experiments from Land Salzburg, Austria. The rabbits with elastase aneurysms were cared for in accordance to the policies and principles established by the Animal Welfare Act and the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Experimental Design
For these initial evaluations, we were interested in deployment characteristics, durability of angiographic and histologic occlusion, thrombus organization, and durability of the hydrogel filaments. As each experimental aneurysm model has advantages and disadvantages, we used rabbit models for this work because thrombus organization is delayed in rabbits compared with dogs.12 Experimental bifurcation and elastase aneurysms were created in 7 and 28 New Zealand white rabbits, respectively. We used both bifurcation and elastase aneurysms to determine the deployment characteristics of the hydrogel filaments in differing anatomies. As shown in Table 1, 2 or 3 bifurcation aneurysms and 9 or 10 elastase aneurysms were embolized in each of the hydrogel filament groups. Fewer bifurcation aneurysms were embolized because of their larger size compared with elastase aneurysms, which permitted the tracking and deployment characteristics of a greater number of devices to be evaluated per aneurysm.
Table 1:
Treatment groups
| Duration | Number of Subjects |
|||||
|---|---|---|---|---|---|---|
| PEG-I |
PEG-B |
PPG-B |
||||
| Bifurcation | Elastase | Bifurcation | Elastase | Bifurcation | Elastase | |
| 2 Weeks | 0 | 1 | 0 | 3 | 0 | 3 |
| 6 Weeks | 0 | 2 | 0 | 4 | 0 | 3 |
| 10 Weeks | 0 | 2 | 0 | 3 | 0 | 3 |
| 26 Weeks | 3 | 4 | 2 | 0 | 2 | 0 |
| Total | 3 | 9 | 2 | 10 | 2 | 9 |
Note:—PEG-I indicates polyethylene glycol opacified with aromatic iodine; PEG-B, polyethylene glycol opacified with barium sulfate; PPG-B, polypropylene glycol opacified with barium sulfate.
Table 2:
Procedural results
| PEG-I (n = 12) | PEG-B (n = 12) | PPG-B (n = 11) | |
|---|---|---|---|
| Dome (mm) | 3.8 ± 1.7 | 4.7 ± 1.5 | 4.0 ± 0.9 |
| Length (mm) | 8.3 ± 2.7 | 10.9 ± 1.3 | 9.9 ± 2.0 |
| Neck (mm) | 3.5 ± 1.4 | 4.1 ± 0.8 | 3.7 ± 1.8 |
| Volume (cm3) | 0.14 ± 0.20 | 0.15 ± 0.07 | 0.18 ± 0.12 |
| Number of devices | 2.6 ± 2.3 | 2.8 ± 1.2 | 5.1 ± 2.1 |
| Device length (cm) | 25 ± 23 | 34 ± 13 | 36 ± 14 |
| Volumetric occlusion | NA | 86 ± 24 | 69 ± 23 |
Note:—The aneurysm length in the PEG-B group was significantly larger than that of the PEG-I group. The number of devices utilized in the PPG-B group was significantly larger than in both the PEG-I and PEG-B groups. No other statistically significant differences were observed between the groups.
Table 3:
Angiographic results
| Group | Posttreatment |
Follow-Up |
||||||
|---|---|---|---|---|---|---|---|---|
| Complete | Dog Ear | Residual Neck | Residual Aneurysm | Complete | Dog Ear | Residual Neck | Residual Aneurysm | |
| PEG-I | 5 (42%) | 3 (25%) | 1 (8%) | 3 (25%) | 6 (50%) | 2 (17%) | 3 (25%) | 1 (8%) |
| PEG-B | 1 (8%) | 4 (33%) | 5 (42%) | 2 (17%) | 8 (67%) | 1 (8%) | 3 (25%) | 0 (0%) |
| PPG-B | 3 (25%) | 2 (17%) | 4 (33%) | 2 (17%) | 6 (50%) | 3 (25%) | 0 (0%) | 2 (17%) |
The experimentation in this study was performed sequentially. The polyethylene glycol opacified with aromatic iodine (PEG-I) embolizations were performed first, followed by the polyethylene glycol opacified with barium sulfate (PEG-B) embolizations, and then finally the polypropylene glycol opacified with barium sulfate (PPG-B) embolizations. Limitations of the embolic devices were identified and resolved, either partially or completely, in the next iteration of device design. In the beginning of the experimentation, it was unknown if the use of hydrogel filaments was a viable approach to endovascular embolization of aneurysms. The long-term durability and the foreign-body response of the hydrogel filaments were unknown. Also, although administration of iodinated contrast has minimal complications, rare, serious anaphylactic reactions have been reported.13 In an effort to understand the long-term biocompatibility of iodine-containing aromatic compounds implanted in experimental aneurysms, the follow-up of the aneurysms treated with PEG-I devices was focused on longer durations. The results of the PEG-I embolized aneurysms showed that the approach was viable and the replacement of iodine with barium sulfate eliminated the anaphylaxis concerns. As a result, subsequent follow-ups of PEG-B and PPG-B were evenly dispersed to determine the time course of thrombus organization and foreign-body response inside the aneurysm sac. Table 1 summarizes the follow-up duration of the aneurysms in this study.
Creation of Aneurysms
Rabbit Bifurcation.
The microsurgical construction of the carotid bifurcation aneurysms in 7 rabbits was performed according to previously described methods.14 For all operative procedures, the animals were anesthetized with an intramuscular injection of 20 to 30 mg/kg of ketamine hydrochloride and 0.2 mL of 2% xylazine hydrochloride, followed by maintenance anesthesia by intravenous injection of a saline solution of ketamine and xylazine (5:1:5; 0.5–1 mL/h/kg).
Both common carotid arteries (CCA) were exposed, and a permanent ligature was placed proximal to the left CCA bifurcation and transected. Using single knot sutures with 10-0 Prolene (Ethicon, Cincinnati, Ohio), we performed an end-to-side anastomosis of the left to the right CCA and we constructed the aneurysm sac with a venous graft pouch from the left external jugular vein.
Rabbit Elastase.
We created elastase-induced aneurysms in 28 rabbits by using previously published methods.15 The right CCA was exposed surgically and ligated distally. A 3F Fogarty balloon (Baxter Healthcare, Irvine, Calif) was inserted through a 5F sheath, placed at the origin of the right CCA under fluoroscopic guidance, and inflated with contrast. An approximately 200-U/mL solution of porcine elastase (Worthington Biochemical, Lakewood, NJ) was incubated above the balloon for 20 minutes.
Embolic Devices
The embolic devices used in this study were developed by MicroVention Terumo. Detailed descriptions of the preparation, characterization, and physical evaluation of the hydrogel filaments are given elsewhere.11 In brief, the PEG-I filament consisted of 60% w/w triiodophenyl penta-4-enoate, 20% w/w acrylic acid, and 19% w/w polyethylene glycol. The iodine content was approximately 700 mg/cm3. Two types of detachable devices with PEG-I filaments were evaluated. The first type was delivered through a 3F microcatheter (n = 21 devices). A variety of lengths (7–10 cm) were used to embolize the aneurysm sac. The second type was delivered through a 5F catheter (n = 10 devices). A variety of lengths (7–10 cm) were used to embolize the aneurysm sac. The PEG-I filaments underwent a 2.3-fold increase in volume after hydration. Sufficient iodine could not be loaded into the PEG-I hydrogel filaments that were deliverable through a 3F microcatheter to impart sufficient radiopacity for visualization during fluoroscopy. To overcome this limitation, we added a stretched platinum coil to some of the 3F devices, and we prepared and delivered larger devices through a 5F catheter. The larger diameter of these devices resulted in sufficient radiopacity.
The PEG-B filament consisted of 52% w/w barium sulfate, 27% N,N′-methylenebisacrylamide, 12% w/w polyethylene glycol, and 9% w/w acrylic acid. A total of 33 detachable devices with a variety of heat set diameters (4–8 mm) and lengths (5–20 cm) were delivered through a 3F microcatheter. The PEG-B filaments underwent a 3.5-fold increase in volume after hydration.
The PPG-B filament consisted of 66% w/w barium sulfate, 17% w/w polypropylene glycol, 15% w/w acrylic acid, and 2% w/w N,N′-methylenebisacrylamide. A total of 56 detachable devices with a variety of heat set diameters (4–8 mm) and lengths (1–10 cm) were delivered through a 3F microcatheter. The PPG-B filaments underwent a 2.8-fold increase in volume after hydration.
Aneurysm Embolization and Follow-Up
With the animals under anesthesia and sterile conditions, the right femoral artery was surgically exposed and a 5F vascular sheath was placed. After intravenous administration of heparin (100 U/kg), a 5F guiding catheter was advanced into the right CCA. We measured the aneurysm dimensions using digital subtraction angiography (DSA) with a radiopaque ball bearing (7.9 mm) as a sizing reference. A microcatheter was advanced into the aneurysm sac.
Embolization was performed by use of either PEG-I, PEG-B, or PPG-B devices under fluoroscopic guidance. All aneurysms were embolized as completely as possible while minimizing protrusion of embolic devices into the parent artery. After performing DSA to document contrast filling into the aneurysm sac, we removed the sheath and all catheters. The proximal aspect of the femoral artery was ligated with 6-0 silk suture. At the time of follow-up, the rabbits were anesthetized and a 5F sheath was placed in the left femoral artery. We performed follow-up angiography using a 5F guiding catheter positioned in the brachiocephalic artery or right CCA. The rabbits were killed by a lethal injection of sodium pentobarbital or an overdose of the anesthetic agents and 2 mL of T61 euthanasia solution (Veterinaria, Zurich, Switzerland).
Histologic Processing
The aneurysm-parent arteries complex was first rinsed in situ with saline solution and then perfusion fixed with 10% neutral buffered formalin. After surgical excision, the specimen was placed in fresh fixative.
Because the metallic content of the aneurysms in this study was very low or none at all, the use of a number of types of media to embed the specimens was possible. Three embedding media—paraffin, optimal cutting temperature (OCT), and methyl methacrylate—were evaluated in this study for suitability. To preserve the embolic device-tissue interface, we embedded half of the aneurysms in methyl methacrylate. To permit immunohistochemical analyses, we embedded the other half of the aneurysms in paraffin or OCT.
A total of 17 aneurysms were embedded in methyl methacrylate and sawed longitudinally through the neck of the aneurysm. We prepared 6 μ sections using a rotary microtome. Twelve aneurysms were embedded in paraffin and bisected longitudinally through the neck of the aneurysm. We prepared 5 μ sections using a rotary microtome. Six aneurysms were embedded in OCT, bisected longitudinally through the neck of the aneurysm, and solidified in liquid nitrogen-cooled isopentane. Although feasible, the use of OCT was determined to be cumbersome and did not add additional value over paraffin embedding. For all embedding media, the sections were stained with hematoxylin-eosin and Movat pentachrome stains.
Evaluation Criteria
Procedural.
Using the preembolization angiogram with the external sizing device, we recorded the dome, length, and neck of the aneurysm. The volume of the aneurysm was calculated from the dome and length assuming cylindrical geometry. In addition, the number of devices and the total device length were recorded. We determined the volume of the embolic devices using the total length and the volumetric expansion of the filaments. We then determined the volumetric occlusion by dividing the volume of the embolic devices by the volume of the aneurysm.
Angiographic.
To assess postembolization and follow-up results, 2 of the authors (M.K. and D.F.K.) scored the angiograms in accordance with the Raymond scale.16 Complete occlusion, dog ear, residual neck, and residual aneurysms were scored 1, 2, 3, and 4, respectively. Given the large interobserver variability in the scoring of aneurysm occlusion,17 the average of the 2 scores, without adjudication, was used to reflect aneurysm occlusion posttreatment and at follow-up. In the event that the averaged score was not an integer, the score was rounded up to permit Raymond classification. To assess for treatment durability, follow-up angiograms were compared with the posttreatment angiogram by use of the following categories: stable, progressive occlusion (any decrease in contrast opacification within the aneurysm cavity), or recurrence (any increase in contrast opacification within the aneurysm cavity).
Histologic.
In addition to general observation of the nature of thrombus organization, neck coverage, and inflammation, at least 2 histologic sections from all the aneurysms in this study underwent computerized morphometry quantification.12 We photographed the sections using an AxioCam MRc5 digital camera mounted on an AxioImager A1 microscope with a 1.25X objective and analyzed them using AxioVision 4.6 software (Carl Zeiss Microimaging, Jena, Germany). The aneurysm area, the occluded area, and the area occupied by hydrogel filaments were quantified.
Statistical Analysis
We performed statistical analyses using JMP 7.0 (SAS Institute, Cary, NC). We assessed differences in continuous data using analysis of variance. If a difference was identified in the overall test, individual differences were assessed with a t test. Differences in discrete data were assessed with a χ2 test. Statistical significance was accepted at P ≤ .05.
Results
Procedural.
All 35 aneurysms were successfully embolized. All rabbits were in good health without any observable neurologic deficit during the course of the experiment. The procedural results are summarized in Table 2. The aneurysm length of the PEG-B group was significantly larger than that of the PEG-I group (P = .005). The number of devices used to embolize the PPG-B group was significantly larger than that of the other 2 groups (P = .005). No other statistically significant differences between the groups were observed.
The embolic devices used in this study had fluoroscopic visibility and delivery problems. In the opinion of the operators (M.K. and D.F.K.), the PEG-I devices were only slightly visible during fluoroscopy, whereas the PEG-B and PPG-B devices had visibility comparable with platinum coils. A comparison of the radiopacity of the evaluated devices to platinum coils is shown in Fig 1. Although tracking the devices through the microcatheter was reproducible, deployment of the device out of the microcatheter was difficult, particularly so for the PPG-B devices. The devices did not have sufficient column strength to be pushed through the aorta/brachiocephalic artery tortuosity in the rabbit elastase model. Deployment was easier, but not clinically acceptable, in the rabbit bifurcation model. To overcome the deployment issue, we used devices with short lengths.
Fig 1.
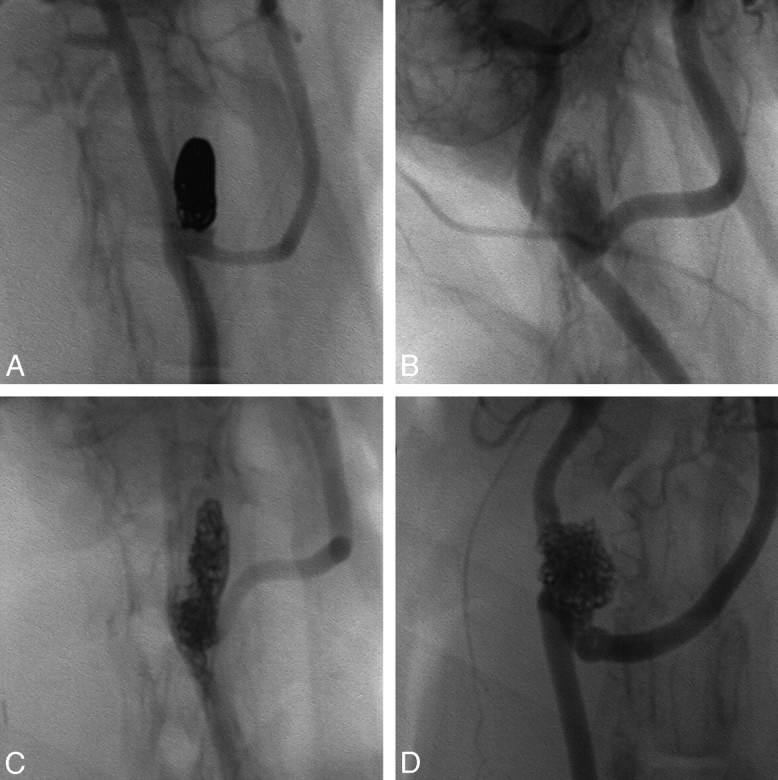
Radiopacity comparison. Nonsubtracted angiography of rabbit bifurcation aneurysms embolized with platinum coils (A), PEG-I devices (B), PEG-B devices (C), and PPG-B devices (D).
With the difficulties visualizing the devices, large parent artery incursions were present in 10 (83%) of 12 aneurysms in the PEG-I group. During the course of the experiment, the animals did not exhibit symptoms of thrombus embolization distal to the parent artery incursions. At follow-up, all 12 vessels were patent with normal flow. However, the large amount of the devices outside the aneurysm cavity inflated the volumetric occlusion calculation to meaninglessness.
Angiographic.
The scoring of the posttreatment and follow-up angiograms according to the Raymond scale is shown in Table 3. Statistically significant differences were not present in posttreatment or follow-up Raymond scores. The choice of embolic device did not affect the posttreatment occlusion score. At follow-up, increased occlusion was observed for all 3 groups.
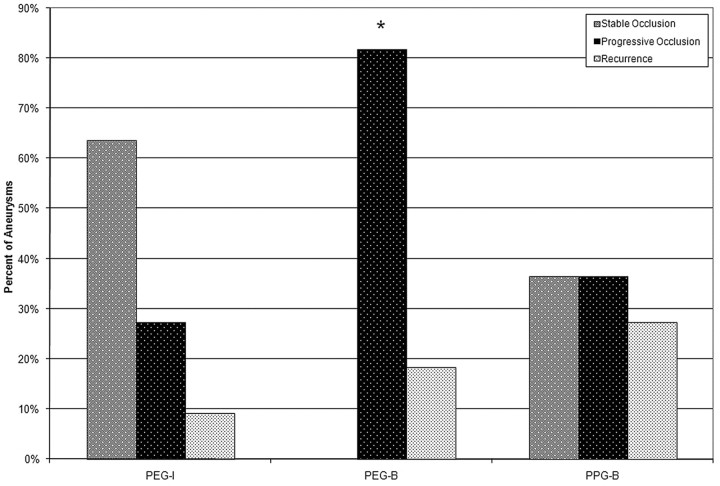
Statistically significant differences were present in the durability results, as shown in Fig 2. Progressive occlusion was significantly greater in the PEG-B group compared with the other 2 groups (P = .004). Approximately 90%, 80%, and 70% of the aneurysms in the PEG-I, PEG-B, and PPG-B groups, respectively, had stable or progressive occlusion. Sample angiographic results for the PEG-I, PEG-B, and PPG-B groups are shown in the upper panels of Figs 3–5, respectively.
Fig 2.
Durability of angiographic stable occlusion or progressive occlusion was observed in 29 (83%) of 35 of the aneurysms treated with hydrogel filaments. The progressive occlusion observed in the PEG-B was significantly higher than that of the PEG-I and PPG-B groups.
Fig 3.
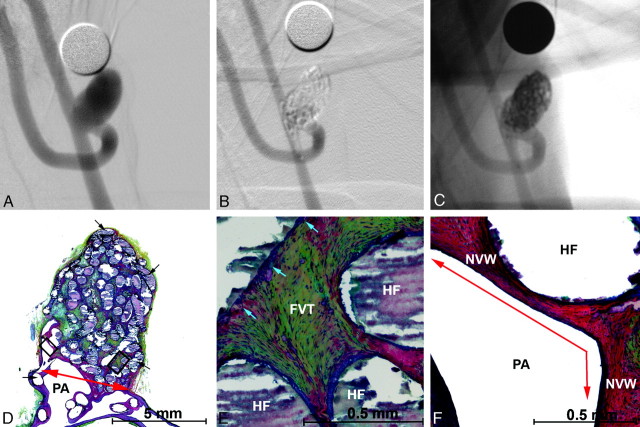
Angiographic and histologic images of aneurysm treated with PEG-I devices. A, Preembolization DSA, 4.5 × 10.5 mm (dome x length). B, Posttreatment DSA showing complete occlusion with parent artery compromise. C, A 6-month follow-up DSA showing complete occlusion with parent artery compromise. D–F, Methyl methacrylate embedded, hematoxylin- eosin–stained microtome sections of the aneurysm. D, Aneurysm overview showing hydrogel filaments (black arrows), neointima covered neck (red arrow), the parent artery (PA), and the protrusion into the parent artery (P). E, Enlarged upper square in D. Fibrovascular tissue (FVT) between hydrogel filaments (HF) and mild inflammation. Platinum coil winds added to the hydrogel filament to improve radiopacity are visible (brown arrows). Mild inflammation is present at the surface of the hydrogel filaments. F, Enlarged lower square from D. Hydrogel filaments (HF) supporting a thick, endothelialized new vessel wall (NVW) that traverses the neck of the aneurysm (red arrow) and adheres to the parent artery wall (PAW).
Fig 4.
Angiographic and histologic images of aneurysm treated with PEG-B devices with a 3F microcatheter. A, Preembolization DSA, 6.0 × 9.3 mm (dome x length). B, Posttreatment DSA showing dog ear filling with parent artery compromise. C, Posttreatment, nonsubtracted angiographic image showing the radiopacity of the hydrogel filaments. D–F, Methyl methacrylate embedded, Movat pentachrome–stained microtome sections of the aneurysm. D, Aneurysm overview showing hydrogel filaments (black arrows), neointima covered neck (red arrow), and the parent artery (PA). E, Enlarged right-hand square from D. Fibrovascular tissue (FVT) between hydrogel filaments (HF) and mild inflammation (blue arrows). F, Enlarged left-hand square from D. Hydrogel filaments (HF) supporting a thick new vessel wall that traverses the aneurysm neck (red arrow).
Fig 5.
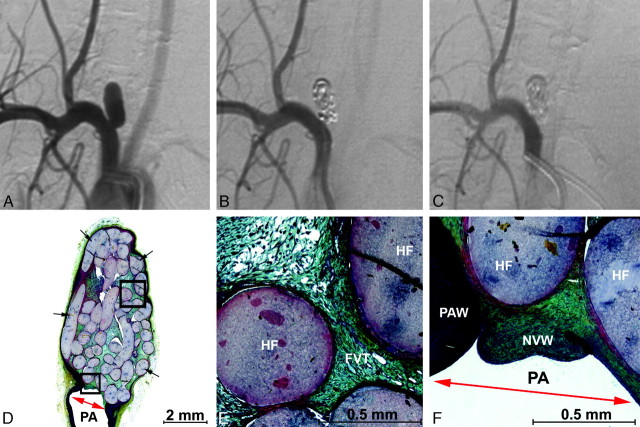
Angiographic and histologic images of aneurysm treated with PPG-B devices with a 3F microcatheter. A, Preembolization DSA, 3.0 × 8.0 mm (dome × length). B, Posttreatment DSA showing complete occlusion without parent artery compromise. C, A 6-week follow-up DSA showing complete occlusion without parent artery compromise. D–F, Methyl methacrylate embedded, Movat pentachrome–stained microtome sections of the aneurysm. D, Aneurysm overview showing densely packed hydrogel filaments (black arrows) and a new vessel wall covering the neck (red arrow). E, Enlarged upper square from D. Fibrovascular tissue (FVT) between hydrogel filaments (HF) and mild inflammation. F, Enlarged lower square from D. Hydrogel filaments (HF) supporting a thick new vessel wall that traverses the aneurysm neck (red arrow) and adheres to the parent artery wall (PAW).
Histologic.
Sections were successfully prepared from paraffin, methyl methacrylate, and OCT embedding media. The choice of the embedding media did not affect the analysis of the sections. In the PEG-I group, fibrous connective tissue between the hydrogel embolic devices and neointima traversing the neck were observed in 10 of 12 aneurysms. The aneurysm evaluated at 2 weeks was filled with unorganized thrombus, and one of the aneurysms evaluated at 26 weeks was filled with mostly organized thrombus. In a likewise fashion, these 2 aneurysms had thrombus without endothelialization covering their necks. Inflammation was minimal to mild in 11 aneurysms and marked in 1, consisting of macrophages, lymphocytes, and giant cells. In 3 cases, recanalization was evident by a concave neck shape.
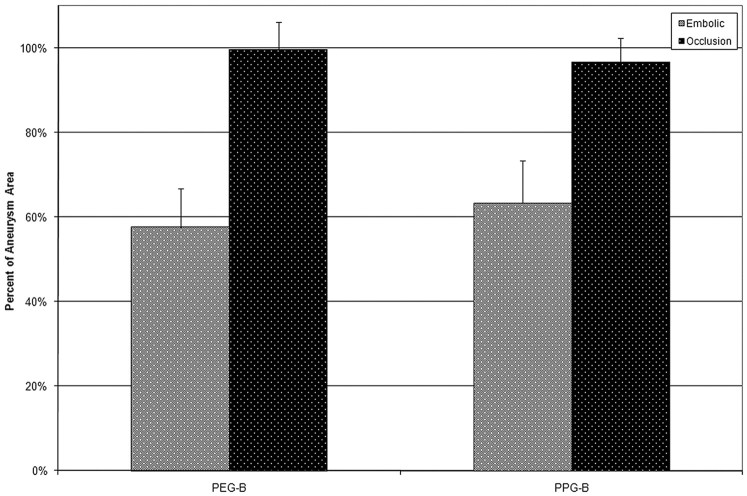
At 2 weeks in the PEG-B and PPG-B groups, the aneurysm sacs were filled with unorganized thrombus and early neointima formation was noted across the neck. At 6 weeks, thrombus organization was underway inside the sac and neointima formation was continuing across the neck. At 10 and 26 weeks, the sacs were filled with fibrous connective tissue and neointima formation with endothelialization was complete. Inflammation was minimal to mild, consisting of macrophages and giant cells. Sample histologic results for the PEG-I, PEG-B, and PPG-B groups are shown in the lower panels of Figs 3 to 5, respectively. The quantification results of the histologic sections are shown in Fig 6.
Fig 6.
Quantification of embolic and occluded areas from histologic sections. Because of the large amount of protrusion into the parent artery, the results from the PEG-I group are omitted from this figure. For both the PEG-B and PPG-B filaments, the average percentage of the aneurysm cavity occupied by embolic devices on the evaluated slides was approximately 60%. The average percentage of the aneurysm cavity that was occluded was approximately 100%.
Discussion
In this study, we evaluated initial prototype devices prepared from 3 types of radiopaque, expansile hydrogel filaments in experimental aneurysms. Although problems common in the early development stage, such as radiopacity and deliverability, were encountered, embolization with the hydrogel filaments resulted in high levels of volumetric occlusion and durable angiographic occlusion from 2 to 26 weeks. These results suggest that, with improvement, polymeric, transition metal-free embolics could be used to improve aneurysm occlusion.
The hydrogel filament devices are under development in an effort to improve the durability of aneurysm occlusion through increased volumetric occlusion. Several studies with platinum coils have correlated better angiographic durability with increased volumetric occlusion in experimental18 and clinical aneurysms.6,7 Although the use of HydroCoil devices can increase the volumetric occlusion above what is typically achieved with platinum coils,8,9 the increased stiffness of the devices hampers their deployment inside an aneurysm cavity. With the hydrogel filaments, the goal is a device that increases volumetric occlusion through expansion without sacrificing attenuated packing.
Although the embolization posttreatment was frequently incomplete, the volumetric occlusion of the PEG-B and PPG-B groups was comparable with the volumetric occlusion of canine bifurcation, canine sidewall, and rabbit elastase experimental aneurysms by use of HydroCoil devices and was greater than that of platinum coils.12 Another way to determine the volumetric occlusion is to measure the area occupied by embolic materials on a histologic section. With image analysis software, the areas of the embolic devices and the aneurysm can be accurately determined. Multiple sections from the aneurysm can be used to determine the packing attenuation across the depth of the aneurysm. With this technique, the area percentage occupied by the hydrogel filaments compared favorably with the average embolic area percentage of canine bifurcation, canine sidewall, and rabbit elastase experimental aneurysms by use of HydroCoil devices and was greater than that of platinum coils.12 With use of 2 measurement techniques, increased volumetric occlusion with the hydrogel filament devices was demonstrated.
As seen in Table 1 and Fig 2, the high volumetric occlusion led to angiographic durability. Posttreatment for all 3 groups, the mean angiographic occlusion score was dog ear filling and all 3 groups had lower Raymond scores at follow-up. In this study, 6 (17%) of 35 aneurysms had decreased angiographic occlusion at follow-up compared with posttreatment. This result compares favorably to the 33% of rabbit elastase aneurysms treated with Matrix (Boston Scientific, Fremont, Calif) devices that exhibited decreased occlusion at follow-up and is similar to the results with HydroCoil devices and platinum coils.19 The histologic results in this study confirmed the angiographic occlusion results. As seen in Fig 6, quantification of the aneurysm occlusion from the histologic sections showed that the hydrogel filaments maintained a stable occlusion.
Parent artery protrusions were common in this study. Significant protrusions were common in the PEG-I group as a result of difficulties visualizing and deploying the devices. Most protrusions in the PEG-B and PPG-B groups were minor, consisting of several loops of the filaments in the parent artery. Despite the protrusions, flow was maintained through the parent artery during the course of the experiment. On histologic examination, the protrusions were covered with endothelialized neointima, as shown in Figs 3 and 4.
This study, although encouraging for the development of a new type of embolic device, had limitations. Our study did not have a control group of platinum coils; however, the performance of platinum coils in the rabbit elastase and bifurcation models is well studied.19–22 Furthermore, the concordance between experimental and human aneurysms was unknown and was probably low. The hemodynamics of aneurysms located near the heart were probably not reflective of intracranial aneurysms. Furthermore, thrombus organization was much more rapid in experimental aneurysms compared with human aneurysms.
As we move ahead, hydrogel filaments with acceptable radiopacity and reproducible delivery into the aneurysm cavity are under development. These improvements will increase the clinical applicability of the devices. In the future, we also envision the incorporation of gadolinium or iron oxide into the hydrogel filament to permit visibility of the device under fluoroscopic, CT, and MR imaging. An MR-embolic device could allow for MR-guided embolizations in the future.
Conclusions
The embolization of experimental aneurysms with hydrogel filaments without transition metals resulted in durable angiographic and histologic occlusion from 2 to 26 weeks. Thrombus organization, neointima formation, and inflammation were similar to experimental aneurysms embolized with other types of embolic devices. With improvements in the delivery of the hydrogel filaments, they show promise for use in the endovascular treatment of aneurysms.
Acknowledgments
We thank MicroVention Terumo for funding our study and Drs. R. Agic, M. Kral, L. Ritter, D. Lewis, D. Dai, and R. Kadirvel for their contributions in conducting the preclinical studies.
Footnotes
This study was funded by MicroVention Terumo, Aliso Viejo, Calif.
References
- 1. Gruber A, Killer M, Bavinzski G, et al. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery 1999; 45: 793– 803 [DOI] [PubMed] [Google Scholar]
- 2. Molyneux AJ, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366: 809– 17; discussion 803–04 [DOI] [PubMed] [Google Scholar]
- 3. Mitchell P, Kerr R, Mendelow AD, et al. Could late rebleeding overturn the superiority of cranial aneurysm coil embolization over clip ligation seen in the International Subarachnoid Aneurysm Trial? J Neurosurg 2008; 108: 437– 42 [DOI] [PubMed] [Google Scholar]
- 4. Butteriss D, Gholkar A, Mitra D, et al. Single-center experience of Cerecyte coils in the treatment of intracranial aneurysms: initial experience and early follow-up results. AJNR Am J Neuroradiol 2008; 29: 53– 56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pierot L, Leclerc X, Bonafe A, et al. Endovascular treatment of intracranial aneurysms with Matrix detachable coils: midterm anatomic follow-up from a prospective multicenter registry. AJNR Am J Neuroradiol 2008; 29: 57– 61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kai Y, Hamada J, Morioka M, et al. Evaluation of the stability of small ruptured aneurysms with a small neck after embolization with Guglielmi detachable coils: correlation between coil packing ratio and coil compaction. Neurosurgery 2005; 56: 785– 92; discussion 785–92 [DOI] [PubMed] [Google Scholar]
- 7. Slob MJ, Sluzewski M, van Rooij WJ. The relation between packing and reopening in coiled intracranial aneurysms: a prospective study. Neuroradiology 2005; 47: 942– 45 [DOI] [PubMed] [Google Scholar]
- 8. Cloft HJ, Kallmes DF. Aneurysm packing with HydroCoil embolic system versus platinum coils: initial clinical experience. AJNR Am J Neuroradiol 2004; 25: 60– 62 [PMC free article] [PubMed] [Google Scholar]
- 9. Gaba RC, Ansari SA, Roy SS, et al. Embolization of intracranial aneurysms with hydrogel-coated coils versus inert platinum coils: effects on packing density, coil length and quantity, procedure performance, cost, length of hospital stay, and durability of therapy. Stroke 2006; 37: 1443– 50 [DOI] [PubMed] [Google Scholar]
- 10. Deshaies EM, Adamo MA, Boulos AS. A prospective single-center analysis of the safety and efficacy of the HydroCoil embolization system for the treatment of intracranial aneurysms. J Neurosurg 2007; 106: 226– 33 [DOI] [PubMed] [Google Scholar]
- 11. Constant MJ, Keeley EM, Cruise GM. Preparation, characterization, and evaluation of radiopaque hydrogel filaments for endovascular embolization. J Biomed Mater Res B Appl Biomater 2009; 89: 306– 13 [DOI] [PubMed] [Google Scholar]
- 12. Cruise GM, Shum JC, Plenk H., Jr. Hydrogel-coated and platinum coils for intracranial aneurysm embolization compared in three experimental models using computerized angiographic and histologic morphometry. J Mater Chem 2007; 17: 3965– 73 [Google Scholar]
- 13. Tramer MR, von Elm E, Loubeyre P, et al. Pharmacological prevention of serious anaphylactic reactions due to iodinated contrast media: systematic review. BMJ 2006; 333: 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forrest MD, O'Reilly GV. Production of experimental aneurysms at a surgically created arterial bifurcation. AJNR Am J Neuroradiol 1989; 10: 400– 02 [PMC free article] [PubMed] [Google Scholar]
- 15. Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices American Roentgen Ray Society. AJR Am J Roentgenol 2000; 174: 349– 54 [DOI] [PubMed] [Google Scholar]
- 16. Raymond J, Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery 1997; 41: 1235– 45; discussion 1245–46 [DOI] [PubMed] [Google Scholar]
- 17. Cloft HJ, Kaufmann T, Kallmes DF. Observer agreement in the assessment of endovascular aneurysm therapy and aneurysm recurrence. AJNR Am J Neuroradiol 2007; 28: 497– 500 [PMC free article] [PubMed] [Google Scholar]
- 18. Reul J, Spetzger U, Weis J, et al. Endovascular occlusion of experimental aneurysms with detachable coils: influence of packing density and perioperative anticoagulation. Neurosurgery 1997; 41: 1160– 65; discussion 1165–68 [DOI] [PubMed] [Google Scholar]
- 19. Ding YH, Dai D, Lewis DA, et al. Angiographic and histologic analysis of experimental aneurysms embolized with platinum coils, Matrix, and HydroCoil. AJNR Am J Neuroradiol 2005; 26: 1757– 63 [PMC free article] [PubMed] [Google Scholar]
- 20. Fujiwara NH, Kallmes DF. Healing response in elastase-induced rabbit aneurysms after embolization with a new platinum coil system. AJNR Am J Neuroradiol 2002; 23: 1137– 44 [PMC free article] [PubMed] [Google Scholar]
- 21. Dai D, Ding YH, Kadirvel R, et al. A longitudinal immunohistochemical study of the healing of experimental aneurysms after embolization with platinum coils. AJNR Am J Neuroradiol 2006; 27: 736– 41 [PMC free article] [PubMed] [Google Scholar]
- 22. Bavinzski G, Richling B, Binder BR, et al. Histopathological findings in experimental aneurysms embolized with conventional and thrombogenic/antithrombolytic Guglielmi coils. Minim Invasive Neurosurg 1999; 42: 167– 74 [DOI] [PubMed] [Google Scholar]