Abstract
BACKGROUND AND PURPOSE:
As modifications are made to coils, monitoring the safety profile, ability to achieve high packing attenuation, and durability of occlusion as compared to the standard bare platinum coils is of paramount importance. We compared packing attenuation, initial occlusion, and recanalization rates between Cerecyte and bare platinum coils in the treatment of ruptured and unruptured cerebral aneurysms.
MATERIALS AND METHODS:
We compared 63 patients (67 aneurysms) treated with Cerecyte coils with 65 patients (70 aneurysms) treated by using bare platinum coils. Results were classified by the Raymond score. Clinical outcomes were assessed by using a modified Rankin Scale. Angiographic and clinical follow-ups were performed routinely at 6 and 12 months after the intervention.
RESULTS:
In the Cerecyte group, complete occlusion of the aneurysm (grade 1) was accomplished in 49% (33/67), a small residual neck (grade 2) was seen in 21% (14/67), and dome filling (grade 3) was seen in 30% (20/67). In the platinum group, 41% (29/70) were grade 1, 39% (27/70) were grade 2, and 20% (14/70) were grade 3 immediately postembolization. Mean packing attenuation was 43 ± 28% in the Cerecyte group and 40 ± 23% in the bare platinum group (P = .68). Twelve-month follow-up data were available for 54% (36/67) of the Cerecyte population and 43% (30/70) of the bare platinum population. There were 5 cases of neck recanalization (11%) in the Cerecyte group and 11 cases (23%) in the bare platinum group (P = .17). No rebleeds were noted in the follow-up period.
CONCLUSIONS:
Cerecyte coils have a satisfactory safety profile. We were able to achieve high packing attenuations and initial occlusion rates similar to those obtainable with platinum coils.
Endovascular embolization of intracranial aneurysms with detachable coils is associated with lower morbidity and mortality rates compared with traditional microsurgical clipping.1–3 However, recanalization of the aneurysm sac after coil embolization occurs in 20%–40% of patients.4–8 Platinum is a biologically inert material. As such, it produces a delayed aneurysmal thrombosis and neointimal proliferation.9,10 It has been hypothesized that accelerating aneurysm sac fibrosis may result in reduced recanalization rates. To reach this goal, investigators developed coils coated with bioabsorbable polymeric material (BPM) (such as polyglycolic acid [PGA]), expandable material, cytokines, ion implantation, protein coating, and fibroblast tissue allografts.11–18
Recently, a novel PGA-loaded coil (Cerecyte; Micrus Endovascular, San Jose, Calif) was developed to overcome coil friction and compartmentalization. Because the PGA is inside the primary wind of the coil, this new system is supposed to deliver aneurysm framing and filling comparable with that of bare platinum coils while delivering a bioactive copolymer. Bendszus et al19 reported a single-center experience with Cerecyte coils in 55 prospective aneurysms. The authors compared the results with a retrospective matched group (size and location) of 55 aneurysms treated with bare platinum coils. Angiographic outcome immediately after embolization and at 6 months showed a recanalization rate of 16% with bare platinum coils and 4% with Cerecyte. Retreatment rate at 6 months was 11% with bare platinum and 2% with Cerecyte.
Some authors have reported that high packing attenuation may result in decreased recanalization rates.20,21 Due to the conformability of Cerecyte coils, we investigated the following: 1) if by using Cerecyte coils, we could achieve a packing attenuation similar to that of bare platinum coils; and 2) if at a similar packing attenuation, the presence of the PGA could result in lower recanalization rates.
Materials and Methods
Patients and Techniques
This study was a retrospective analysis of a prospectively acquired data base of patients with ruptured or unruptured aneurysms treated with PGA-loaded coils (Cerecyte) between July 2005 and August 2008. We compared this group with patients with ruptured and unruptured intracranial aneurysms who were treated with bare platinum coils only during the same time period. Inclusion criteria were consecutive patients >18 years of age with either ruptured or unruptured intracranial aneurysms treated with Cerecyte coils or with platinum coils. Patients treated with other bioactive coils (ie, Matrix detachable coil, Boston Scientific, Natick, Mass; or HydroCoil, MicroVention, Aliso Viejo, Calif) were excluded. Informed consent was obtained before each procedure from either the patient or the closest family member.
Angiographic and Endovascular Procedures
All diagnostic angiograms were discussed with the vascular neurosurgeon to decide the best approach to the lesion (ie, endovascular embolization versus microsurgical clipping). Criteria that would favor an endovascular approach were the following: 1) >50 years of age; 2) size; 3) location (posterior circulation); 4) unruptured; 5) if ruptured, presence of vasospasm; 6) poor grade; and 7) operator preference and/or availability. Criteria that would favor the use of Cerecyte coils versus platinum coils were operator preference and coil availability.
All endovascular procedures were performed by 2 neurointerventionalists (A.K.W., I.L.) by using a biplane angiography unit with 3D capability (Allura Xper FD20/20; Philips Medical Systems, Best, the Netherlands) with patients under general anesthesia. Endovascular access was obtained by a standard transfemoral approach. Each aneurysm was then assessed angiographically by using standard projections. Aneurysms were coiled as densely as possible with either Cerecyte coils alone or in combination with bare platinum coils or exclusively with bare platinum coils at the judgment of the operator and coil material availability. Aneurysm occlusion was estimated by 2 independent reviewers (M.J.D., M.J.G.) by using a 3-point Raymond score (RS) (RS 1 = complete obliteration of aneurysm and neck, RS 2 = small neck remnant without contrast filling the aneurysm sac, and RS 3 = contrast filling the aneurysm sac).8 After the procedure, patients were transferred to the neurosurgical intensive care unit. Aneurysm volume calculations were performed by using aneurysm measurements in 3 standard angiographic projections (maximum aneurysm length, width, and height) as previously described.22 Coil volume for each aneurysm was calculated by assuming that the coil was a cylinder, using the primary coil diameter given in the manufacturers' specifications.22 Packing attenuation (percentage) was calculated as the ratio of coil volume to aneurysm volume × 100.
Clinical Evaluation
A complete neurologic examination was performed in all patients at baseline, immediately after the procedure, and at 6 and 12 months after coil embolization by either A.K.W., I.L., or a neurologist not involved in the treatment. A modified Rankin Scale (mRS) score was included in the 30-day and 6-month follow-up evaluations. Primary adverse events included death and stroke. Secondary adverse events recorded were transient ischemic attack, need for re-intervention, and presence of hematomas. Residual aneurysmal size was determined by angiography. Medical histories, procedural reports, and clinical outcomes were recorded in a prospective data base maintained for quality assurance purposes by the interventional neuroradiology service.
Angiographic Follow-Up
In the Cerecyte group, follow-up conventional angiography was performed in 67% (45/67) and 54% (36/67) of patients at 6 and 12 months, respectively. Sixty-seven percent (47/70) and 43% (30/70) of patients underwent 6- and 12-month follow-up, respectively, in the bare platinum population. Each follow-up study used the same projections as those acquired during the embolization procedure. Aneurysms were assessed by using the same RS as described above. Recanalizations of the aneurysm sac were defined as an increase in RS.
Statistical Analysis
Comparisons between the 2 groups (Cerecyte versus bare platinum) were performed by using either the Fisher exact or the Mann-Whitney U test of significance in GraphPad InStat (Version 3.06; http://www.brothersoft.com/graphpad-instat-5898.html). Significant differences were established for P ≤ .05.
Results
From July 2005 to August 2008, 224 patients with either ruptured or unruptured cerebral aneurysms were treated with coil embolization at the University of Massachusetts. Sixty-three patients with 67 aneurysms were treated with Cerecyte coils. Thirteen aneurysms were treated with Cerecyte coils alone, whereas 54 were treated with Cerecyte coils in combination with bare platinum coils. In aneurysms treated with a combination of Cerecyte and bare platinum coils, the mean ratio of the length of the Cerecyte coils to the length of all coils was 53 ± 23% (range, 11%–93%). For comparison, we analyzed 65 patients with 70 aneurysms treated exclusively with bare platinum coils during the same time period.
Cerecyte Group
Patient demographics and aneurysm characteristics are summarized in Table 1. In the Cerecyte cohort, patient age ranged from 26 to 87 years (43 women). Mean aneurysm size was 5.7 ± 2.4 mm, and mean neck size was 3.7 ± 1.8 mm. Twenty-six aneurysms were ruptured at presentation (39%). Three were fusiform/dissecting aneurysms, and 2 were recurrent aneurysms post-microsurgical clipping. There were 19 anterior communicating artery (AcomA) or A1/A2 (anterior cerebral artery [ACA]), 12 internal carotid artery (5 ICA trunk, 2 supraclinoid, 3 ophthalmic, 2 superior hypophyseal), 8 middle cerebral artery (MCA), 8 basilar tip, 9 posterior communicating artery (PcomA), 2 posterior inferior cerebellar artery (PICA) (a representative case is shown in Fig 1), 1 superior cerebellar artery (SCA), 5 pericallosal artery, and 1 each of anterior choroidal, posterior cerebral, and vertebral artery aneurysms. Balloon-assisted coil embolization was performed in 12 patients (18%), and stent-assisted coil embolization was carried out in 10 patients (15%).
Table 1:
Patient demographics and aneurysm characteristics
| Demographics | Bare Platinum (%) | Cerecyte (%) | P Value |
|---|---|---|---|
| No. patients | 65 | 63 | |
| No. aneurysms | 70 | 67 | |
| Age | 25–85 | 26–87 | .06 |
| Female | 44 (68) | 43 (68) | 1.0 |
| Aneurysm size | 5.7 ± 2.6 | 5.7 ± 2.4 | .99 |
| Neck size | 3.2 ± 1.4 | 3.7 ± 1.8 | .17 |
| Ruptured | 29 (41) | 26 (39) | .86 |
| 6-Month FU | 47 (67) | 45 (67) | |
| 12-Month FU | 30 (43) | 36 (54) | |
| Balloon-assisted | 12 (17) | 12 (18) | 1.0 |
| Stent-assisted | 11 (16) | 10 (15) | 1.0 |
Note:—FU indicates follow-up
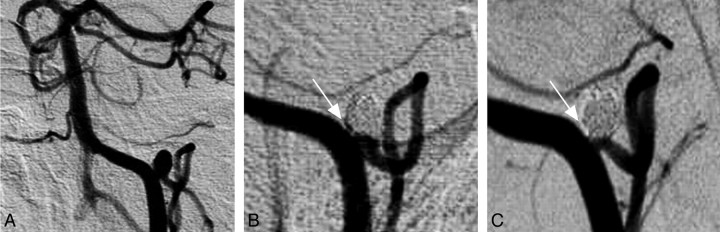
Fig 1.
Sample case. A, A 61-year-old woman developed subarachnoid hemorrhage (Hunt and Hess scale, grade 4) secondary to a ruptured left PICA aneurysm. Four Cerecyte coils (28.5-cm total length; packing attenuation, 50%) were placed in the aneurysm. B, On immediate postprocedural control angiography, the arrow indicates a small area of contrast filling at the base of the aneurysm. C, This area of contrast filling is no longer visualized at 6-month follow-up (arrow), and the PICA remains patent.
Bare Platinum Group
For the bare platinum series, patients ranged from 25 to 85 years of age (44 women). Mean aneurysm size was 5.7 ± 2.6 mm, and mean neck size was 3.2 ± 1.4 mm. Thirty-six aneurysms were ruptured at presentation (51%). Two were fusiform/dissecting aneurysms. In this group, there were 23 AcomA or A1/A2 (ACA), 17 ICA (4 ICA trunk, 1 supraclinoid, 6 ophthalmic, 6 superior hypophyseal), 9 MCA, 2 basilar tip, 12 PcomA, 3 SCA, 3 pericallosal, and 1 vertebral artery aneurysm. Balloon-assisted embolization was performed in 12 patients (17%), whereas stent-assistance was used in 11 patients (16%).
Postprocedure Occlusion
Results of coil embolization are summarized in Table 2. Initial complete occlusion of the aneurysm (RS = grade 1) in the Cerecyte group was accomplished in 33 patients (49%) and 29 patients (41%) in the bare platinum group (P = .39). Fourteen patients were RS grade 2 (21%) and 20 patients were grade 3 (30%) in the Cerecyte group. These scores compare with 39% RS grade 2 and 20% grade 3 in the bare platinum population. Follow-up angiography revealed 5 cases of aneurysm recanalization (11%) that required recoiling in the Cerecyte population. One patient was RS grade 2 following initial intervention, and 3 were grade 3. Twenty aneurysms demonstrated progressive occlusion in the follow-up period (ie, from initial RS 2/3 to RS 1). A representative case of progressive occlusion is shown in Fig 2.
Table 2:
Results of coil embolization
| Bare Platinum (%) | Cerecyte (%) | P Value | |
|---|---|---|---|
| RS 1 | 29 (41) | 33 (49) | .39 |
| RS 2 | 27 (39) | 14 (21) | |
| RS 3 | 14 (20) | 20 (30) | |
| Recanalizations | 11 (23) | 5 (11) | .17 |
| Complications | 2 Intraop ruptures, 1 arterial dissection | 2 Intraop ruptures | |
| Abciximab | 7 (10) | 10 (15) | .44 |
| Packing attenuation | 40 ± 23 | 43 ± 28 | .68 |
Note:—Intraop indicates intraoperative; RS, Raymond score.
Fig 2.
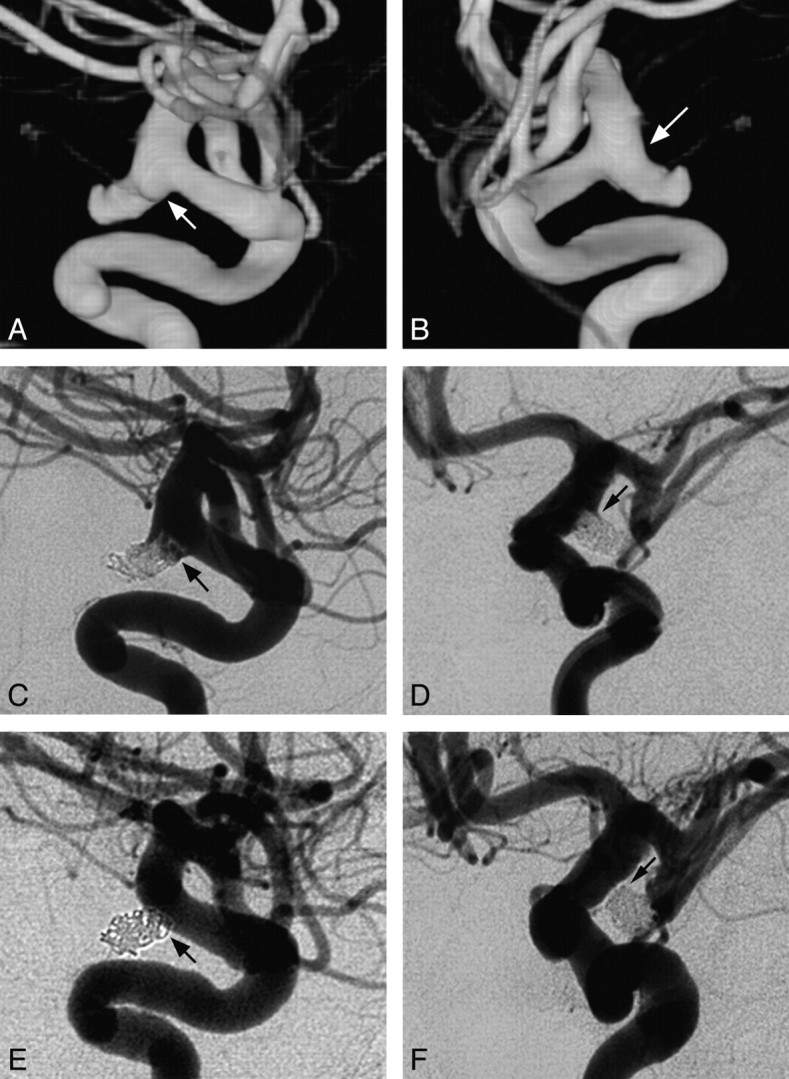
Sample case. A and B, 3D reconstructive rotational angiograms reveal a ruptured PcomA in a 60-year-old woman with subarachnoid hemorrhage (Hunt and Hess scale, grade 3). A bleb is seen at the inferior aspect of the aneurysm (arrow, A), and a shoulder is visualized at the superior portion (arrow, B ). Coil embolization is performed by using a combination of bare platinum and Cerecyte coils (50% Cerecyte; packing attenuation, 21%). C and D, Immediate postcoiling control angiograms demonstrate residual filling of the bleb (arrow, C ) and shoulder (arrow, D). E and F, Six-month follow-up angiograms demonstrate complete aneurysm occlusion with obliteration of both the bleb (arrow, E) and the shoulder (arrow, F).
There were 11 recanalizations (23%) in the follow-up period in the bare platinum population. One patient had 2 aneurysms that recanalized at 36-month follow-up. These aneurysms were then retreated by using Cerecyte coils. These aneurysms were RS grade 1 following re-intervention, and follow-up is pending.
Safety
Safety data are summarized in Table 2. In each population, 2 patients had intraprocedural aneurysm rupture, with no long-lasting clinical sequelae. In the Cerecyte population, 10 patients (15%) had intraprocedural clot-on-coil formations that were successfully treated with intra-arterial (IA) abciximab infusion. One patient of these 10 developed a small infarction on postoperative day 1. This compares with 7 cases (10%) of clot on coil successfully treated with IA abciximab in the bare platinum population (P = .44). In the follow-up period, there was 1 death from unrelated causes and no rebleeds.
Packing Attenuation
Mean packing attenuation in the Cerecyte group was 43 ± 28% and 40 ± 23% in the bare platinum group (P = .68). Mean packing attenuation was 21 ± 14% in the recanalized Cerecyte aneurysms and 35 ± 25% in the recanalized bare platinum aneurysms. Significance was not calculated for the packing attenuations of the recanalized aneurysms because of the small number of packing-attenuation calculations.
Discussion
Cerecyte coils had a good safety profile in both ruptured and unruptured cerebral aneurysms. We were able to achieve a mean packing attenuation of 43% with Cerecyte coils, similar to that accomplished with bare platinum coils (40%). There were no differences in the demographics between the 2 groups. There was no significant difference in recanalization rates between the Cerecyte group and the bare platinum group (11% versus 23%).
Despite less peri- and postprocedural morbidity and mortality compared with microsurgical clipping, aneurysmal recanalization is more common with the endovascular approach.2,3,9,10 Animal models have shown that coils coated with BPMs can lead to an early and complete neointimal formation at the neck, with the aim of decreasing the rate of aneurysmal recanalization.11 However, single-center case series with Matrix coils did not unequivocally show lower rates of aneurysmal recanalization when compared with bare platinum coils. In Matrix-treated aneurysms, the overall the recanalization rate was 37% (26% for small aneurysms with small necks) and retreatment rate was 23% (14% for small aneurysms with small necks). The recanalization rate for large aneurysms was 75%, with a 58% retreatment rate.23 In another single-center experience, 57% of aneurysms embolized either completely or partially with Matrix coils recanalized during 1 year (23% retreatment rate) and a similar recanalization rate for large aneurysms (82%) was reported.24 The authors suggested that the high amount of BPM coating on the coil seems to lead to coil friction and compartmentalization causing incomplete aneurysmal packing.
Initial complete aneurysmal occlusion and high packing attenuation appear to be significant factors in decreasing recanalization rates.21 In particular, Gonzalez et al6 reported that recanalization rates increased significantly from 6.3% to 18.7% when a neck remnant was present immediately after coil embolization. An in vitro study showed that platinum coils designed with multiple break points allowed the coil to form a complex tridimensional shape resulting in a packing attenuation higher than 30%.25 The relationship between high packing attenuation and reduced recanalization rates has been hypothesized and reported previously. Specifically, packing attenuations of >20% resulted in lower rates of coil compaction.20,21
Sluzewski et al20 retrospectively reviewed 160 patients with aneurysmal subarachnoid hemorrhage who had 18-month follow-up angiography after coil embolization. The authors reported that packing attenuation of ≥24% protected against recanalization in aneurysms with volumes <600 mm3. Packing attenuations between 20% and 23.9% resulted in no recanalization if the aneurysm volume was <200 mm3. Recently, Wakhloo et al21 reported data on 79 aneurysms treated with complex-shaped platinum coils to achieve high rates of initial occlusion and high packing attenuation. In their study, aneurysms had a volume of 273 ± 506 mm3 (diameter, 7.1 ± 3.4 mm) and a neck size of 4.2 ± 1.9 mm (range, 1.5–12 mm). Despite including in the analysis wide-neck and basilar apex aneurysms, complete or near-complete occlusion was achieved in all aneurysms. Packing attenuation was 37.9 ± 15.5%. At follow-up conventional angiography, a small recanalization at the neck was present in 5/33 aneurysms (15%), including 3 basilar tip aneurysms.
Cerecyte coils are loaded with PGA, rather than coated with BPM. In addition, the coil was designed with a complex shape to try to conform to the aneurysm sac better. The goal was to increase the rates of complete initial occlusion, achieve high packing attenuation, deliver a bioactive polymer, and ultimately decrease the rates of recanalization.
Previous studies have demonstrated that the Cerecyte coil system is safe and can achieve low recanalization rates.19,26–28 Bendszus and Solymosi28 first analyzed a cohort of 24 patients with 25 aneurysms treated with Cerecyte. Mean aneurysm dome size was 5.2 mm with a mean neck size of 2.8 mm. A mean packing attenuation of 23.6% was achieved in these aneurysms. There were no significant complications secondary to the coil system or the procedure itself. The authors reported complete angiographic occlusion of 88% at 6-month follow-up, higher than that in several previously published studies2,8,29; however, small aneurysms are less likely to recanalize. The authors concluded that the bioactive nature of Cerecyte coils may explain their low recurrence rates despite relatively low packing attenuation. Therefore, a study describing occlusion of larger wide-neck aneurysms that also analyzed the effect of higher packing attenuations was needed.
Recently, Butteriss et al26 reported their results in 68 aneurysms treated with Cerecyte coils. The mean aneurysm size was similar to that in previous studies at 5.8 mm. However, 40% of the aneurysms were classified as wide-neck (>4 mm; or neck-dome ratio, >2). The authors described initial occlusion rates similar to those in previous studies with a 20.6% recurrence rate at 6-month follow-up. This study had the benefit of analyzing a larger cohort of patients but did not include data on packing attenuation within these aneurysms.
To our knowledge, no study has yet investigated whether Cerecyte coils can achieve high packing attenuation. Furthermore, no study has analyzed differences in packing attenuation achievable between Cerecyte and bare platinum coils. Veznedaroglu et al27 recently reported data on 89 aneurysms treated with Cerecyte coils. The authors described an immediate postoperative angiographic occlusion of 45%, residual neck in 52%, and fundus-filling in 3 aneurysms. In our series, we were able to achieve initial complete occlusion of the Cerecyte-treated aneurysmal sac (RS score = grade 1) in 33 aneurysms (49%); 14 patients were grade 2 (21%), and 20 patients were grade 3 (30%). This result compares with 41% grade 1, 39% grade 2, and 20% grade 3 in the bare platinum group. We were able to achieve packing attenuations of >40% by using both Cerecyte coils and standard bare platinum coils. Our Cerecyte packing attenuation of 43% is higher than that previously published using Matrix29 or bare platinum coils.21 The high initial complete occlusion rate and high packing attenuation that we achieved may account for the observed 11% recanalization rate at the neck in the Cerecyte group. This compares with 23% recanalization mostly at the neck in the platinum group, though the difference is not statistically significant.
It has been hypothesized that bioactive coils may cause more incidence of clot on coil compared with platinum coils.29 In our experience, the rate of intraoperative clot-on-coil formation requiring intra-arterial infusion of abciximab at the site of occlusion was similar between the Cerecyte group (15%) and the platinum group (10%). These findings are similar to those described in previous studies.21 We conclude that the Cerecyte system has a good safety profile. In our series, the morbidity rate was in line with the previously published data, because 1 patient had a small stroke on postoperative day 1 (2%) with no long-term neurologic deficit. The 1 death that occurred in the follow-up period was due to causes unrelated to the coiling procedure.
Study Limitations
The present work is limited by the retrospective nature of the study design. Without a blinded study, it is difficult to draw conclusions on which coil system provides optimal occlusion. The nonrandomized nature of the 2 groups (Cerecyte and bare platinum) could lead to selection bias. However, there were no differences in the demographics of the 2 groups. Our findings add to a growing body of literature supporting the Cerecyte Coil Trial, a randomized-control trial designed to assess different coil types (platinum and Cerecyte) in aneurysm embolization. We also recognize that the total number of aneurysms and the number of recanalizations are both small (not all patients had angiographic follow-up); and as such, this study may not be powered to detect differences in recanalization rates between the 2 groups.
Conclusions
In both ruptured and unruptured cerebral aneurysms, Cerecyte coils may achieve high initial occlusion rates and packing attenuations similar to those in platinum coils, with a satisfactory safety profile.
References
- 1. Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002; 360: 1267– 74 [DOI] [PubMed] [Google Scholar]
- 2. Molyneux AJ, Kerr RS, Yu LM, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366: 809– 17 [DOI] [PubMed] [Google Scholar]
- 3. Johnston SC, Zhao S, Dudley RA, et al. Treatment of unruptured cerebral aneurysms in California. Stroke 2001; 32: 597– 605 [DOI] [PubMed] [Google Scholar]
- 4. Murayama Y, Nien YL, Duckwiler G, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg 2003; 98: 959– 66 [DOI] [PubMed] [Google Scholar]
- 5. Ng P, Khangure MS, Phatouros CC, et al. Endovascular treatment of intracranial aneurysms with Guglielmi detachable coils: analysis of midterm angiographic and clinical outcomes. Stroke 2002; 33: 210– 17 [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez N, Murayama Y, Nien YL, et al. Treatment of unruptured aneurysms with GDCs: clinical experience with 247 aneurysms. AJNR Am J Neuroradiol 2004; 25: 577– 83 [PMC free article] [PubMed] [Google Scholar]
- 7. Gallas S, Pasco A, Cottier JP, et al. A multicenter study of 705 ruptured intracranial aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol 2005; 26: 1723– 31 [PMC free article] [PubMed] [Google Scholar]
- 8. Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003; 34: 1398– 403 [DOI] [PubMed] [Google Scholar]
- 9. Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg 1999; 91: 284– 93 [DOI] [PubMed] [Google Scholar]
- 10. Castro E, Fortea F, Villoria F, et al. Long-term histopathologic findings in two cerebral aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1999; 20: 549– 52 [PMC free article] [PubMed] [Google Scholar]
- 11. Murayama Y, Tateshima S, Gonzalez NR, et al. Matrix and bioabsorbable polymeric coils accelerate healing of intracranial aneurysms: long-term experimental study. Stroke 2003; 34: 2031– 37 [DOI] [PubMed] [Google Scholar]
- 12. Gaba RC, Ansari SA, Roy SS, et al. Embolization of intracranial aneurysms with hydrogel-coated coils versus inert platinum coils: effects on packing density, coil length and quantity, procedure performance, cost, length of hospital stay, and durability of therapy. Stroke 2006; 37: 1443– 50 [DOI] [PubMed] [Google Scholar]
- 13. Cloft HJ. HydroCoil for Endovascular Aneurysm Occlusion (HEAL) study: periprocedural results. AJNR Am J Neuroradiol 2006; 27: 289– 92 [PMC free article] [PubMed] [Google Scholar]
- 14. Tamatani S, Ozawa T, Minakawa T, et al. Histological interaction of cultured endothelial cells and endovascular embolic materials coated with extracellular matrix. J Neurosurg 1997; 86: 109– 12 [DOI] [PubMed] [Google Scholar]
- 16. Abrahams JM, Forman MS, Grady MS, et al. Delivery of human vascular endothelial growth factor with platinum coils enhances wall thickening and coil impregnation in a rat aneurysm model. AJNR Am J Neuroradiol 2001; 22: 1410– 17 [PMC free article] [PubMed] [Google Scholar]
- 16. Murayama Y, Vinuela F, Suzuki Y, et al. Ion implantation and protein coating of detachable coils for endovascular treatment of cerebral aneurysms: concepts and preliminary results in swine models. Neurosurgery 1997; 40: 1233– 43, discussion 1243– 44 [DOI] [PubMed] [Google Scholar]
- 17. Kallmes DF, Williams AD, Cloft HJ, et al. Platinum coil-mediated implantation of growth factor-secreting endovascular tissue grafts: an in vivo study. Radiology 1998; 207: 519– 23 [DOI] [PubMed] [Google Scholar]
- 18. Marx WE, Cloft HJ, Helm GA, et al. Endovascular treatment of experimental aneurysms by use of biologically modified embolic devices: coil-mediated intraaneurysmal delivery of fibroblast tissue allografts. AJNR Am J Neuroradiol 2001; 22: 323– 33 [PMC free article] [PubMed] [Google Scholar]
- 19. Bendszus M, Bartsch AJ, Solymosi L. Endovascular occlusion of aneurysms using a new bioactive coil: a matched pair analysis with bare platinum coils. Stroke 2007; 38: 2855– 57 [DOI] [PubMed] [Google Scholar]
- 20. Sluzewski M, van Rooij WJ, Rinkel GJ, et al. Endovascular treatment of ruptured intracranial aneurysms with detachable coils: long-term clinical and serial angiographic results. Radiology 2003; 227: 720– 24 [DOI] [PubMed] [Google Scholar]
- 21. Wakhloo AK, Gounis MJ, Sandhu JS, et al. Complex-shaped platinum coils for brain aneurysms: higher packing density, improved biomechanical stability, and midterm angiographic outcome. AJNR Am J Neuroradiol 2007; 28: 1395– 400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tamatani S, Ito Y, Abe H, et al. Evaluation of the stability of aneurysms after embolization using detachable coils: correlation between stability of aneurysms and embolized volume of aneurysms. AJNR Am J Neuroradiol 2002; 23: 762– 67 [PMC free article] [PubMed] [Google Scholar]
- 23. Fiorella D, Albuquerque FC, McDougall CG. Durability of aneurysm embolization with Matrix detachable coils. Neurosurgery 2006; 58: 51– 59 [DOI] [PubMed] [Google Scholar]
- 24. Niimi Y, Song J, Madrid M, et al. Endosaccular treatment of intracranial aneurysms using Matrix coils: early experience and midterm follow-up. Stroke 2006; 37: 1028– 32 Epub 2006 Mar 2 [DOI] [PubMed] [Google Scholar]
- 25. Piotin M, Iijima A, Wada H, et al. Increasing the packing of small aneurysms with complex-shaped coils: an in vitro study. AJNR Am J Neuroradiol 2003; 24: 1446– 48 [PMC free article] [PubMed] [Google Scholar]
- 26. Butteriss D, Gholkar A, Mitra D, et al. Single-center experience of Cerecyte coils in the treatment of intracranial aneurysms: initial experience and early follow-up results. AJNR Am J Neuroradiol 2008; 29: 53– 56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Veznedaroglu E, Koebbe CJ, Siddiqui A, et al. Initial experience with bioactive Cerecyte detachable coils: impact on reducing recurrence rates. Neurosurgery 2008; 62: 799– 805, discussion 805– 06 [DOI] [PubMed] [Google Scholar]
- 27. Bendszus M, Solymosi L. Cerecyte coils in the treatment of intracranial aneurysms: a preliminary clinical study. AJNR Am J Neuroradiol 2006; 27: 2053– 57 [PMC free article] [PubMed] [Google Scholar]
- 29. Linfante I, Akkawi NM, Perlow A, et al. Polyglycolide/polylactide-coated platinum coils for patients with ruptured and unruptured cerebral aneurysms: a single-center experience. Stroke 2005; 36: 1948– 53 [DOI] [PubMed] [Google Scholar]