Abstract
BACKGROUND AND PURPOSE: A clinical-diffusion mismatch (CDM) among stroke patients presenting within 12–24 hours has been correlated with neurologic deterioration and infarct expansion. We sought to study the feasibility and safety of reperfusion therapy in a series of 11 consecutive patients fulfilling this criterion.
MATERIALS AND METHODS: Patients presenting with large vessel syndromes were considered for revascularization therapy. Of these patients, we identified those presenting beyond 8 hours who scored ≥8 on the National Institutes of Health Stroke Scale (NIHSS) and had limited abnormalities on diffusion-weighted MR imaging. One- and 7-day NIHSS scores were obtained. Rates of early neurologic deterioration (END, increase in NIHSS score by ≥4 points) and early neurologic improvement (ENI, decrease in NIHSS score by ≥4 points) at 1 week were determined. Follow-up imaging was obtained to evaluate intracranial hemorrhage (ICH).
RESULTS: Eleven patients were identified, 8 of whom were successfully revascularized. The mean age of all patients was 55 years with mean initial, 24-hour, and 1-week NIHSS scores of 14 ± 4, 11 ± 7, and 6 ± 5, respectively, with lower scores at 24 hours and 1 week (8 ± 5 and 4 ± 3, respectively) among patients successfully revascularized. Eight of the treated patients (72% of the total, 100% of those successfully revascularized) experienced ENI. No patient had END or ICH.
CONCLUSIONS: Endovascular treatment for acute ischemic stroke beyond 8 hours is feasible and may prevent END and promote ENI in patients fulfilling the criteria of a CDM. A prospective study is planned.
Current revascularization treatments for acute ischemic stroke (AIS) are generally restricted to within 3 hours for intravenous tissue plasminogen activator (tPA)1 or 6–8 hours for intra-arterial (IA) thrombolysis2 and mechanical thrombectomy.3–5 Patients with AIS, who present outside of these time windows, are precluded from therapy because of the increased risk of intracranial hemorrhage (ICH) associated with delayed revascularization efforts.6 The safety of established time windows is based on screening with cranial CT, known to be insensitive to early ischemia.1,6–8 MR imaging, particularly diffusion-weighted imaging (DWI), more accurately detects cerebral ischemia within minutes of its onset,7,9 and the use of DWI in conjunction with a clinical measure of stroke severity may identify patients presenting late, who may have salvageable tissue and still benefit from therapy.
In 2 large natural history studies,10,11 a clinical-diffusion mismatch (CDM) of an initial National Institutes of Health Stroke Scale (NIHSS) score ≥8 and a baseline DWI MR imaging lesion volume ≤25 mL identified a stroke subpopulation with increased rates of subsequent infarct expansion and early neurologic deterioration (END), defined as an increase in the NIHSS score of ≥4 points at 72 hours.10 This worsening was greatest among patients with CDM who did not receive reperfusion therapy.10 An additional study applying the Alberta Stroke Program Early CT Score (ASPECTS)12 to brain DWI of patients presenting within 24 hours of nonlacunar anterior circulation strokes also demonstrated a similar phenomenon of END and infarct expansion among patients with ASPECTS ≥8 and NIHSS score ≥8.13 We sought to study the rates of END and early neurologic improvement (ENI, decrease in NIHSS score of ≥4 points) at 7–10 days in a consecutive series of patients with CDM with AIS beyond 8 hours considered for endovascular revascularization therapy.
Materials and Methods
Subjects, Clinical Data Collection, and Definition of CDM
We reviewed records of patients in whom endovascular revascularization therapy was considered in 3 medical centers between February 2003 and March 2008. We then identified those presenting beyond 8 hours from symptom onset or from time last seen well, who had early DWI with limited areas of involvement (see MR Imaging Evaluation below) and an initial NIHSS score of ≥8. Other demographic variables including age and sex were also obtained. NIHSS scores at 24 hours and 7–10 days were collected for all patients. Because this was a retrospective analysis of patients treated according to clinical discretion, the institutional review board at each institution exempted this study from approval.
Definition of Time to Treat
“Time to treat” was defined as the time from either witnessed symptom onset or last witnessed normal condition to triage. In certain patients, fluctuating and worsened neurologic syndrome prompting consideration of revascularization therapy on compassionate grounds occurred up to 3 days after initial hospital admission, and this was taken as the “time to treat.” Subsequent endovascular therapy commenced within 1 hour.
MR Imaging Evaluation
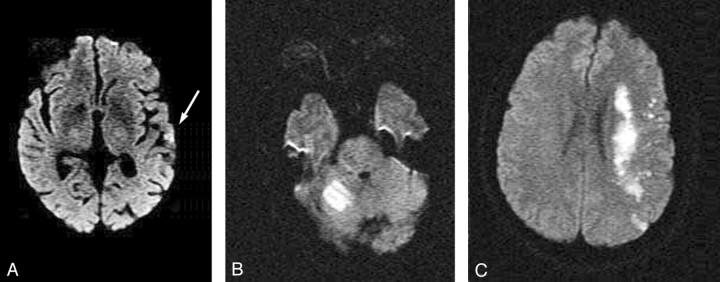
MR imaging was performed on 1.5T systems with echo-planar capabilities of 25-mT/m gradients and 300- to 350-ms rise time. Because of the variable availability of MR imaging for retrospective review in this analysis, in some cases DWI was recorded according to reported description or was grossly estimated by measuring a diameter in the largest area of abnormality. Where multiple small punctuate lesions were noted, the largest 2–3 lesions were summated. Overall sizes were divided into the following groups: none (no DWI abnormality), small (subcentimeter foci), moderate (1–10 cm), or large (10–25 cm, Fig 1).
Fig 1.
Diffusion weighted MR imaging showing examples of A, “small” (arrow); B, “moderate”; and C, “large” baseline DWI lesions. (See text for definition.)
Angiography and Revascularization Procedure
Patients or their families consented to cerebral angiography with possible pharmacologic and/or mechanical thrombolysis. Patients were sedated with intravenous fentanyl and/or midazolam. Paralysis with cisatracurium was performed in intubated patients. Digital subtraction angiography (DSA) was performed by using a 6F Envoy guide catheter (Cordis, Miami Lakes, Fla), or an 8F Merci balloon guide catheter (Concentric Medical, Mountain View, Calif) in the internal carotid or vertebral arteries. A Prowler Plus (Cordis) or Merci 18L microcatheter (Concentric Medical) was used to select the middle cerebral artery, vertebral artery, or basilar artery for infusion of the thrombolytic agent (either alteplase = 6 mg or reteplase ≤2 U). Thrombolytics were administered in bolus aliquots (alteplase, 2 mg in a total 10-mL volume of saline; reteplase 0.5 U in a total 5-mL volume of saline) with interval angiography assessing response to therapy. For patients undergoing mechanical thrombectomy, a 2- to 4-mm Amplatz goose neck snare (Microvena, White Bear Lake, Minn) or the Merci L5 retrieval device (Concentric Medical) was used. In certain cases, angioplasty with or without stent support of the extracranial carotid artery or basilar artery was performed. The vascular occlusions were quantified on the initial and final DSA images according to the Thrombolysis in Myocardial Infarction (TIMI) classification.14
Postangiography Management
Patients were admitted to the intensive care unit and were maintained at a blood pressure between 100/50 and 180/105 mm Hg and serum glucose levels between 4.4 and 6.6 mmol/L. Brain CT or MR imaging was performed within the next 24–72 hours to assess the presence of ICH. Subsequent infarction size on follow-up CT or MR imaging, obtained at 48 hours and beyond, was compared with the baseline MR imaging to quantify infarct growth as none to minimal (<50% initial volume), <2 times initial volume, and >2 times initial volume infarct expansion.
Statistical Analysis:
Descriptive statistics were used to calculate means and standard deviation (SD); for age, initial, 24-hour, and 7- to 10-day NIHSS scores, initial and final TIMI scores, and rates of END and ENI. All statistical analyses were performed by using Statistical Package for the Social Sciences, Version 16.0 (SPSS, Chicago, Ill).
Results
A total of 11 patients fulfilling the criteria of CDM to whom endovascular therapy was offered were identified for these analyses. Among these patients, 8 achieved angiographic recanalization. Five of the patients studied were men (Table 1). The mean age was 55 ± 15 years, and mean time to treat was 31 ± 20 hours. The initial, 24-hour, and 7-day NIHSS scores were 14 ± 4, 11 ± 7, and 6 ± 5, respectively. Among the subset in whom revascularization efforts were successful, initial, 24-hour, and 7-day NIHSS scores were 14 ± 4, 8 ± 5, and 4 ± 3, respectively. The mean initial TIMI score was 1 ± 1, with 9 of the 11 patients having scores of 0–1. Final TIMI scores were 2 ± 1, with 8 of the 11 patients having partial or complete recanalization (final TIMI scores of 2–3). Most the patients had baseline DWI lesions defined as moderate (1- to 10-cm DWI lesions), and 1 of the treated patients had subsequent infarct expansion >2 times the initial size (9%), with all others having subsequent infarct expansion limited to <2 times baseline. None of the treated patients had END; 8 of the 11 treated patients (72%) experienced ENI. Among patients in whom attempts at treatment were successful, all experienced ENI. None of the patients studied had ICH (Table 2).
Table 1:
Demographic, radiographic, and clinical data of all patients
| Age (yr), Sex | Time to Triage* (hours) | Affected Vessel | Base MRI | F/Uimg | Initial NIHSS Score | 24-Hour NIHSS Score | 7- to 10-DayNIHSS Score | Initial TIMI Score | Final TIMI Score | END | ENI | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 67, M | 36 | L ICA | Mod | No/min | 17 | 12 | 5 | 0 | 2 | No | Yes | CAS, IA tPA |
| 49, F | 48 | L ICA | Large | <2 | 13 | 11 | 3 | 1 | 3 | No | Yes | CAS |
| 62, F | 12 | BA | None | No/min | 17 | 11 | 8 | 2 | 2 | No | Yes | IA tPA |
| 45, F | 48 | R VA | Mod | No/min | 8 | 5 | 3 | 2 | 2 | No | Yes | PTA |
| 55, M | 17 | L MCA | Small | <2 | 16 | 22 | 14 | 0 | 0 | No | No | MTD, PTA |
| 65, F | 12 | L MCA | Mod | No/min | 10 | 10 | 2 | 0 | 3 | No | Yes | MTD, IA tPA |
| 52, M | 48 | BA | Mod | <2 | 21 | 19 | 15 | 0 | 0 | No | No | MTD, PTA |
| 41, M | 72 | L MCA | Large | >2 | 9 | 16 | 10 | 0 | 0 | No | No | MTD, IA tPA |
| 26, F | 24 | BA | Mod | <2 | 17 | 17 | 7 | 0 | 2 | No | Yes | Cr stent |
| 84, F | 13 | L M2 | Small | No/min | 11 | 1 | 0 | 1 | 2 | No | Yes | IA tPA |
| 61, M | 16 | BA | Mod | <2 | 17 | 1 | 0 | 1 | 2 | No | Yes | IA tPA |
Note:—MRI indicates MR imaging; F/Uimg, follow-up imaging; L, left; ICA, internal carotid artery; Mod, moderate; No/min, none to minimal; CAS, carotid artery stent; BA, basilar artery; MCA, middle cerebral artery; PTA, percutaneous transluminal angioplasty; MTD, mechanical thrombus disruption; <2, <2 times initial DWI volume (see text); >2, >2 times initial DWI volume (see text); N/A, not available; Cr stent, intracranial stent; M2, M2 intracranial division; NIHSS, National Institute of Health Stroke Scale; TIMI, Thrombolysis in Myocardial Infarction; END, early neurologic deterioration; ENI, early neurologic improvement; VA, vertebral artery; IA, ?; tPA, tissue plasminogen activator.
See text.
Table 2:
Comparisons of mean scores for demographic and clinical variables between the total revascularized group (A) and the successfully revascularized group (B)
| Group | Age (years) | Time to Treat (hours) | Initial NIHSS Score | 24-Hour NIHSS Score | 7-Day NIHSS Score | Initial TIMI Score | Final TIMI Score | END | ENI | ICH |
|---|---|---|---|---|---|---|---|---|---|---|
| Group A (n = 11) | 55 ± 15 | 31 ± 20 | 14 ± 4 | 11 ± 7 | 6 ± 5 | 1 ± 1 | 2 ± 1 | n = 0 (0%) | n = 8 | n = 0 (0%) |
| Group B (n = 8) | 57 ± 17 | 26 ± 16 | 14 ± 4 | 8 ± 5 | 4 ± 3 | 1 ± 0 | 2 ± 0 | n = 0 (0%) | n = 8 (100%) | n = 0 (0%) |
Note:—ICH indicates intracranial hemorrhage.
Discussion
This series of 11 patients demonstrates the potential feasibility of offering endovascular revascularization to patients presenting beyond 8 hours based on CDM, with no incidence of associated ICH. The common premise among these patients with delayed presentations is that their moderately severe clinical syndromes represent the ischemic penumbra, and limited areas of DWI abnormality suggest potentially lower risk of hemorrhage with revascularization efforts. Such endeavors at extended time windows must take into consideration safety and the likelihood of achieving any clinical benefit at late junctures.
It is conceivable that CDM may most benefit patients who very likely experienced the stroke directly before presentation, though lacking a witness to the event, cannot establish a time of onset, such as patients with “wake-up stroke,” who represent approximately 25% of all annual AIS admissions.15–17 Patients in this analysis were seen to generally fall into 1 of 3 categories: those with wake-up strokes, those with well-established long duration of symptoms of the posterior circulation, or those with stenotic nonocclusive lesions and fluctuating neurologic syndromes. These groups may be ideal focuses of intervention in further studies of therapies at later times. Additionally, in many patients, ischemic regions may be partially supported by collateral circulation (Fig 2), which maintains tissue viability though it does not prevent clinical neurologic dysfunction.18 Revascularization restores the preferential circulation and normal blood flow to the brain, potentially limiting ultimate infarct expansion.
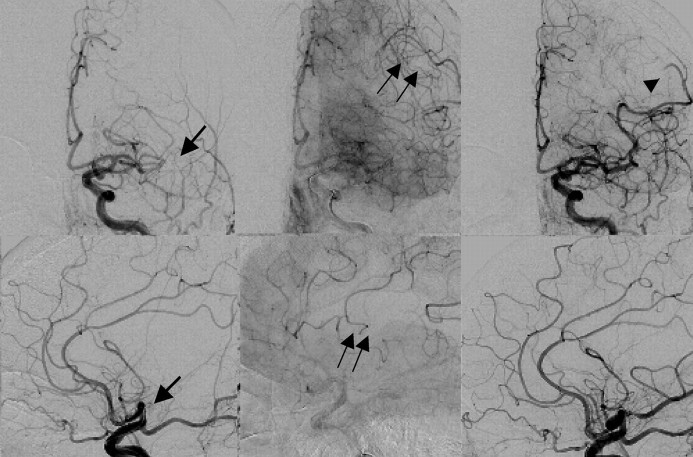
Fig 2.
Panel shows left internal carotid artery angiography of an 84-year-old woman 13 hours after she was last seen at normal neurologic baseline, with acute aphasia, found to have small areas of scattered diffusion abnormality (Fig 1A). Top panel shows angiograms in the anterior oblique projection, and the bottom panel, in lateral projections as seen in early arterial and late arterial phases pretreatment and final posttreatment. Initial divisional middle cerebral artery occlusion is seen in the early arterial phase (arrow). Retrograde pial collateralization (double arrows) can be seen in the late arterial phase. Posttreatment angiography shows recanalization of this division, though with persistent distal branch vessel occlusion (arrowhead).
Certain points merit further discussion, namely the choice of imaging technique serving as the clinical basis for guiding therapy and, second, the treatment options offered in these cases (endovascular therapy rather than systemic thrombolysis). Perfusion-weighted imaging (PWI) has been widely studied in conjunction with DWI (PWI-DWI mismatch [PDM]) as a means of identifying patients who may benefit from thrombolysis outside of standard time windows,19 but is limited by the time and expertise required for technical postprocessing. However, PDM may have a role in patients presenting with lower NIHSS scores who would not otherwise fulfill the above criteria of CDM. This may particularly be the case in strokes involving the nondominant hemisphere, which in fact are unrepresented among our patients, perhaps highlighting this very statement. Apart from this, we believe that the use of CDM is well grounded. In comparison with PDM and CDM, Prosser et al11 found that patients who did not meet the CDM criteria but were found to have PDM tended to have larger DWI lesions of injury early on and would likely benefit less from revascularization.
The choice for endovascular reperfusion strategies in these patients was predicated on evidence that with greater clot burden and larger vessel occlusion (eg, ICA, M1 stem, etc), systemic thrombolysis, though a more expeditious treatment, may be less effective.20–22 However, it is likely that in certain patients, should the site of occlusion a priori be known to exist in a small branch, CDM may also help triage candidates who might benefit from systemic thrombolysis alone.
Major weaknesses in this study relate to several imaging-related points. First, not all the patients by protocol underwent follow-up MR imaging or CT at a prespecified time point, making it difficult to comment on infarct expansion, a main study outcome in the articles of Davalos et al10 and Prosser et al.11 These studies also used volumetric analysis to quantify the area of DWI involvement. Due to above-cited reasons, we used crude methods for measurement. More rigorous modes of DWI measurement must be used in any future prospective study.
Another limitation of this analysis is the small number of patients. Although endovascular treatment seemed to protect against END and lower final NIHSS scores were found, the small sample size prevents drawing any firm conclusions. Finally, because data were reviewed retrospectively from hospital in-patient records alone, long-term follow-up data are not available for this analysis. This would be of prime importance in any future study. Still, we are encouraged by most of the treated patients who experienced ENI and by the absence of ICH among all patients. Whereas strict treatment windows in a general stroke population with limited selection criteria (noncontrast head CT) is important, a certain population of patients with stroke may still be treated safely and successfully in a delayed fashion. The identification of ideal patients for delayed therapy and validation of alternate triaging methods remain to be studied. The CDM may be useful to identify such patients. Further data are needed to more rigorously study delayed revascularization in AIS based on CDM. A phase I study is planned.
References
- 1.Tissue plasminogen activator for acute ischemic stroke: The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995;333:1581–87 [DOI] [PubMed] [Google Scholar]
- 2.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: The PROACT II study—a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 1999;282:2003–11 [DOI] [PubMed] [Google Scholar]
- 3.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–38. Epub 2005 Jun 16 [DOI] [PubMed] [Google Scholar]
- 4.Gobin YP, Starkman S, Duckwiler GR, et al. Merci 1: a phase 1 study of Mechanical Embolus Removal in Cerebral Ischemia. Stroke 2004;35:2848–54. Epub 2004 Oct 28 [DOI] [PubMed] [Google Scholar]
- 5.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 2008;39:1205–12. Epub 2008 Feb 28 [DOI] [PubMed] [Google Scholar]
- 6.Clark WM, Albers GW, Madden KP, et al. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g): results of a double-blind, placebo-controlled, multicenter study—thromblytic therapy in acute ischemic stroke study investigators. Stroke 2000;31:811–16 [DOI] [PubMed] [Google Scholar]
- 7.Davis DP, Robertson T, Imbesi SG. Diffusion-weighted magnetic resonance imaging versus computed tomography in the diagnosis of acute ischemic stroke. J Emerg Med 2006;31:269–77 [DOI] [PubMed] [Google Scholar]
- 8.Lansberg MG, Albers GW, Beaulieu C, et al. Comparison of diffusion-weighted MRI and CT in acute stroke. Neurology 2000;54:1557–61 [DOI] [PubMed] [Google Scholar]
- 9.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 2007;369:293–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davalos A, Blanco M, Pedraza S, et al. The clinical-DWI mismatch: a new diagnostic approach to the brain tissue at risk of infarction. Neurology 2004;62:2187–92 [DOI] [PubMed] [Google Scholar]
- 11.Prosser J, Butcher K, Allport L, et al. Clinical-diffusion mismatch predicts the putative penumbra with high specificity. Stroke 2005;36:1700–04 [DOI] [PubMed] [Google Scholar]
- 12.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: ASPECTS Study Group Alberta Stroke Programme Early CT Score. Lancet 2000;355:1670–74 [DOI] [PubMed] [Google Scholar]
- 13.Tei H, Uchiyama S, Usui T. Clinical-diffusion mismatch defined by NIHSS and ASPECTS in non-lacunar anterior circulation infarction. J Neurol 2007;254:340–46 [DOI] [PubMed] [Google Scholar]
- 14.Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase—clinical findings through hospital discharge. Circulation 1987;76:142–54 [DOI] [PubMed] [Google Scholar]
- 15.Chaturvedi S, Adams HP Jr, Woolson RF. Circadian variation in ischemic stroke subtypes. Stroke 1999;30:1792–95 [DOI] [PubMed] [Google Scholar]
- 16.Lago A, Geffner D, Tembl J, et al. Circadian variation in acute ischemic stroke: a hospital-based study. Stroke 1998;29:1873–75 [DOI] [PubMed] [Google Scholar]
- 17.Elliot WJ. Cyclic and circadian variations in cardiovascular events. Am J Hypertens 2001;14:291S–95S [DOI] [PubMed] [Google Scholar]
- 18.Bang OY, Saver JL, Buck BH, et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2008;79:625–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furlan AJ, Eyding D, Albers GW, et al. Dose escalation of desmoteplase for acute ischemic stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke 2006;37:1227–31 [DOI] [PubMed] [Google Scholar]
- 20.Lee K, Han SW, Kim SH, et al. Low efficacy of intravenous TPA in acute cerebral infarction involving the large cerebral arteries. Paper presented at: International Stroke Conference; February 16–18,2006; Kissimmee, Fla
- 21.Christou I, Burgin WS, Alexandrov AV, et al. Arterial status after intravenous TPA therapy for ischaemic stroke: a need for further interventions. Int Angiol 2001;20:208–13 [PubMed] [Google Scholar]
- 22.Linfante I, Llinas RH, Selim M, et al. Clinical and vascular outcome in internal carotid artery versus middle cerebral artery occlusions after intravenous tissue plasminogen activator. Stroke 2002;33:2066–71 [DOI] [PubMed] [Google Scholar]