Abstract
BACKGROUND AND PURPOSE: Cerebral blood volume (CBV) is an important parameter in estimating the viability of brain tissue following an ischemic event. We tested the hypothesis that C-arm CT measurements of CBV would correlate well with those made with perfusion CT (PCT).
MATERIALS AND METHODS: CBV was measured in 12 canines by using PCT and C-arm CT. Two measurements with each technique were made on each animal; a different injection protocol was used for each of these techniques. PCT was performed by using a 64-section V-scanner. C-arm CT was performed by using a biplane Artis dBA system. PCT images were transferred to a commercially available workstation for postprocessing and analysis; C-arm CT images were transferred to a commercially available workstation for postprocessing and analysis by using prototype software. From each animal, 2 sections from each technique were selected for analysis.
RESULTS: There was good agreement of both the color maps and absolute numbers between the 2 techniques. The maximum and mean deviations of values between the 2 techniques for the first 5 animals were 30.20% and 7.82%; for the second 7 animals, these values were 26.79% and 7.40%. The maximum and mean deviations between the 2 C-arm CT studies performed on the first 5 animals were 33.15% and 12.24%; for the second 7 animals, these values were 41.15% and 10.89%.
CONCLUSIONS: In these healthy animals, measurement of CBV with C-arm CT compared well with measurements made with PCT.
Since publication of the PROACT II study in 1999, improvements in imaging have enhanced the ability to select patients who are most suitable for therapeutic intervention. These, along with improvements in therapeutic techniques and devices, have also increased the incidence of vascular recanalizations and improved posttreatment clinical outcomes.1-3 The assessment of the impact of an ischemic insult on brain viability is best done by using physiologic rather than morphologic criteria; this is especially so in the acute interval when anatomic changes are minimal and the options for interventions aimed at tissue salvage are greatest and most effective.4-6 Until now, such assessment has required examination either with perfusion CT (PCT) or perfusion- and diffusion-weighted MR imaging (PWI/DWI). Although both techniques have demonstrated utility in defining the status of cerebral hemodynamics following an ischemic insult, both require time and the use of contrast medium and are performed in an environment in which, except for the use of intravenous thrombolytics, therapeutic interventions are not possible.7-9
The ability to measure cerebral blood volume (CBV) in an angiographic suite where it is possible to use immediately all available therapeutic techniques might, simply by saving time from initial assessment to intervention, add value to the management of patients with ischemic strokes. Once a decision has been made to attempt revascularization, there is today no practical way to monitor the status of brain viability during the therapeutic intervention. Because these interventions often take hours to perform and adverse events increase significantly as the ratio of nonviable-to-viable brain increases, the ability, during treatment, to monitor some aspects of cerebral function indicative of tissue viability could add further value.10
During the last 2 years, early case-based reports of the use of flat-panel detector angiographic equipment having the capability of volumetric soft-tissue imaging have described the capabilities of these systems to detect intracranial hemorrhages and to visualize endoluminal devices and their relationship to the arterial wall and lumen.11-13 Although currently available C-arm CT lacks the temporal resolution to assess cerebral blood flow (CBF) or mean transit time, it does provide spatial resolution and contrast sensitivity that should be adequate to measure levels of blood in brain parenchyma (ie, CBV).14-16 Our purpose was to test the hypothesis that in normal canines, CBV could be measured by using C-arm CT and that these measurements would correlate with those made by using PCT.
Materials and Methods
Imaging Protocols
Under an institution-approved animal protocol, 12 canines were examined by using both conventional PCT and C-arm CT. PCT was performed on a 64-section V-scanner (GE Healthcare, Milwaukee Wis); C-arm CT was performed on a biplane flat-detector angiographic system (Artis dBA; Siemens, Forchheim, Germany). After induction with propofol (2–4 mg/kg), general endotracheal anesthesia was maintained with isoflurane 1%–3%. Constant monitoring of heart rate, O2 saturation, and end-tidal CO2 was done throughout the experiments. Contrast medium was injected into a peripheral vein by using a power injector (MEDTRON, Saarbrüken, Germany). This dual syringe injector allows control of the contrast concentration injected (eg, 100%, 50%, etc). Iohexol 300 mg I/mL (Omnipaque; Nycomed, Princeton, NJ) was used for the first 5 animals; Iohexol 375 mg I/mL was used for the second 7 animals. Two examinations were performed on each animal with each technique. In 5 of the animals, PCT and C-arm CT examinations were performed on separate days with at least a 5-day interval between examinations; in the other 7 animals, they were both performed on the same day.
For PCT, 8 adjacent 5-mm-thick sections from a level just anterior to the optic chiasm to just anterior to the torcula were selected from a scout image. Following the power injection of the contrast medium and after a 5-second prep delay, a continuous scanning was initiated with the following parameters: 80 kilovolt (peak), 200 mA, 1 second per rotation for 50 seconds. The 1-second images were reformatted at 0.5-second intervals. One acquisition was performed on all animals by using a biphasic contrast injection: 1 mL/s for 8 mL and then 0.5 mL/s for 8 mL, followed by a 15-mL saline chaser at 1 mL/s. In 5 animals, the second acquisition was done by using an injection protocol of 1.5 mL/s for 12 mL followed by a 12-mL saline chaser at 1.5 mL/s. In the other 7 animals, the second acquisition was performed by using an injection protocol of 1.5 mL/s for a total of 19 mL followed by a 15-mL saline chaser at a rate of 1.5 mL/s.
Postprocessing was performed retrospectively by an experienced technologist (K.A.P.) in conjunction with a senior neuroradiologist (C.M.S.). Vascular inputs were selected manually. Because of the very small size of the canine intracranial arteries and the proximity of the superior sagittal sinus to the very thick skull base, extracranial vascular structures were selected for arterial and venous input functions (Fig 1). All postprocessing of the PCT acquisitions was done by using CTP software (Version 3, GE Healthcare).
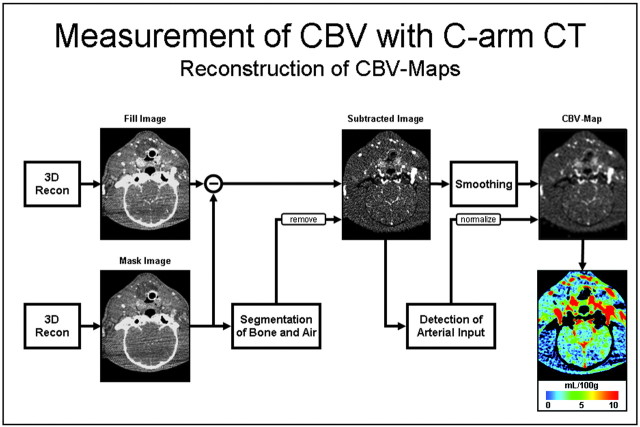
Fig 1.
Diagram showing the postprocessing scheme used to obtain the C-arm CT CBV maps. Recon indicates reconstruction.
C-arm CT was performed by using an Artis dBA (Siemens). Two different acquisition protocols were performed on each animal. The first protocol acquired 126 projection images during a 5-second rotation; the second acquired 275 projection images during a 10-second rotation. Each acquisition protocol consisted of 2 rotations: an initial rotation (mask run) followed by the power injection of the contrast medium, an appropriate x-ray delay, and then a second rotation (fill run). To select the proper time to begin the second rotation for the fill run, we performed a timing digital subtraction angiography (DSA) run. Simultaneously with the injection of contrast medium into a peripheral vein at the rate of 3.0 mL/s, a biplane DSA acquisition was started and continued until contrast medium was observed to have washed out of the intracranial circulation. The start of the fill run, to be used in the CBV calculation, was chosen to correspond to the time when the superior sagittal sinus was fully opacified. The timing of the fill run for the CBV measurement was then chosen so that there would be opacification of the superior sagittal sinus (eg, steady contrast level in the brain parenchyma) throughout the 5- or 10-second rotation.
Prototype software was used to generate a time-contrast curve from the DSA run, and from this, the appropriate delay could be determined. Typically, this entailed the power injection of contrast medium into a peripheral vein at the rate of 1.5 mL/s for 17–20 seconds, with an acquisition delay of 15–17 seconds after the contrast medium injection. CBV postprocessing was performed by using prototype software installed on a dedicated research syngo X-Workplace (Siemens). Because of limitations imposed by the lack of temporal resolution by using C-arm CT, CBV was obtained through use of an injection protocol that resulted in a steady state of contrast medium in the brain parenchyma during each acquisition.
The postprocessing algorithm used is shown in Fig 1. Initially, the mask run and the fill run are reconstructed and subtracted (similar to standard 3D DSA reconstruction). After this, an algorithm is applied to further segment out air and bone from the image volume. The steady state arterial input function value is then calculated from an automated histogram analysis of the vessel tree. A final scaling is then applied to the image volume to account for the arterial input value, and for other physiologic values (eg, hematocrit). In a final step, a smoothing filter is applied to reduce pixel noise.
Data Analysis
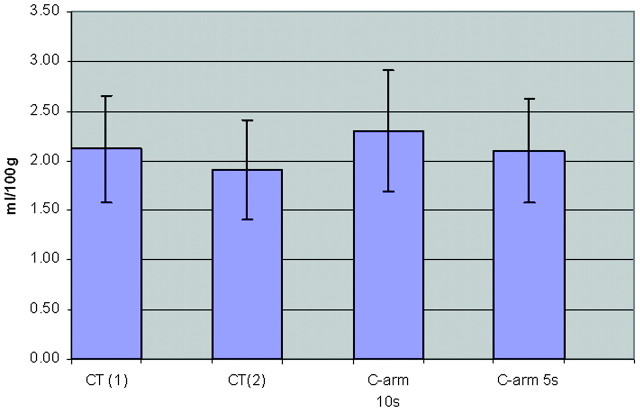
Two sections centered on the frontal horns of the lateral ventricles were selected from each of the 24 examinations; as closely as possible, sections were matched anatomically from animal to animal and from technique to technique. Six regions of interest were placed manually in each of the 2 sections selected for analysis by a researcher (K.A.P.) with more than a decade of experience performing these tasks. Regions of interest were chosen so as to encompass regions of white matter, gray matter, and basal ganglia. Because the “normal” baseline values for CBV in these regions have been shown to have significant variation, we chose to display not only the absolute CBV values across all regions of interest but also the relative deviations of these values across all regions of interest. For each of the 12 regions of interest, the mean and deviation between techniques were calculated. For each region of interest, these values were then used to calculate coefficients of variation (COV) within and between each technique. The COV values for all regions of interest were averaged to obtain a mean relative average deviation (RAD) for PCT and C-arm CT and between the techniques for each animal. Finally, the technique and intertechnique specific values for all subjects were then averaged to get a population-wide mean RAD for all regions of interest across the study population for each technique. The results from this comparison are shown in Fig 2.
Fig 2.
Average region of interest CBV values for C-arm and PCT studies.
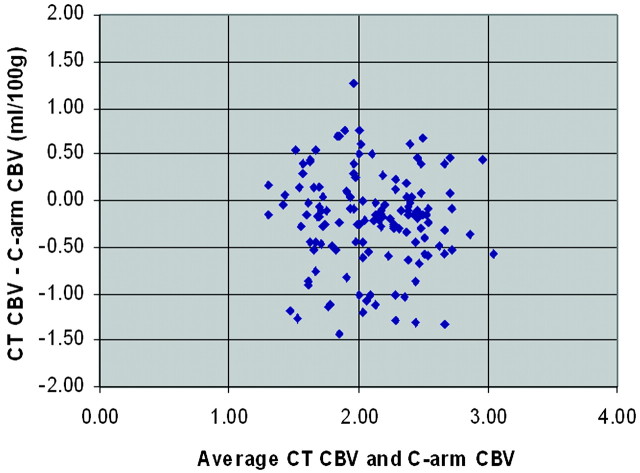
The Bland-Altman analysis of our mean CTP and C-arm CT CBV values across the complete set of regions of interest for all subjects is shown in Fig 3. This analysis shows a very small bias of −.20 mL/100 g, indicating that by using the C-arm CT technique, there is a small overestimation of the CBV compared with measurements made with CTP. The symmetry of the distribution of differences (CTP CBV–C-arm CT CBV) across the range of average CTP and C-arm CT CBV values also indicates that the C-arm CT technique works equally well (no over- or underestimation based on values of CBV) for all standard ranges of healthy canine CBV values across white matter, gray matter, and basal ganglia. The limits of agreement correspond to the 95% confidence intervals represented by 2 SDs of the differences. Finally, the measured COV (SD divided by the mean) for our mean C-arm CBV data and CT CBV data are 26.0% and 26.3%, respectively.
Fig 3.
Bland-Altman plot showing the relationship of the difference of CTP–C-arm CT CBV versus the average CT and C-arm CBV. Bias is −.20 mL/100 g. Limits of agreement are −.20 ± 1.03 mL/100 g.
Results
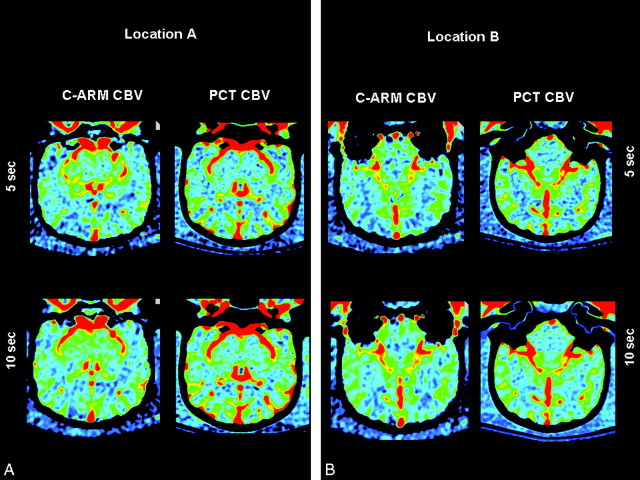
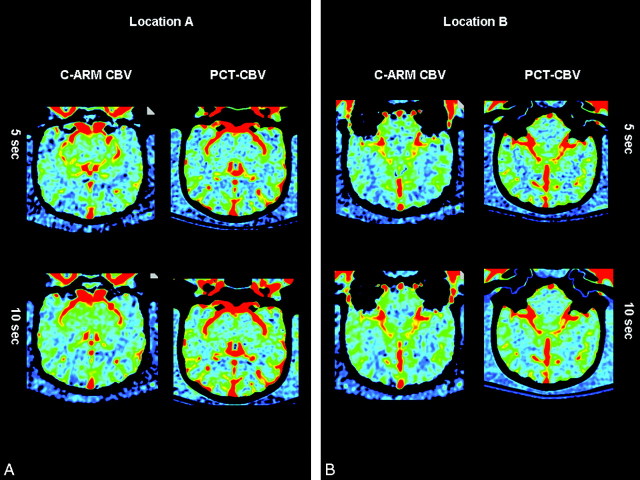
Representative CBV maps made with both techniques are shown in Figs 4 and 5. The regions of interest shown on these figures were, as closely as possible, used in all animals. Data from 1 of the 7 animals studied with both PCT and C-arm CT on the same day were not useable because of motion artifacts caused by a mechanical defect in the angiographic C-arm. The values for CBV from the other 11 animals made with both techniques correlated well with the mean variation of values between the 2 techniques. In the first 5 animals, the mean variation was 7.82%; in the second 6 animals, it was 7.40% (maximum deviations were 30.2% and 26.79%, respectively). The mean variation between the 2 PCT studies in the first 5 animals was 10.02%; that for the second 6 animals was 10.9% (maximum deviations were 40.71% and 37.53%, respectively). The mean variation between the 2 C-arm CT studies in the first 5 animals was 12.24%; that for the second 6 animals was 10.89% (maximum deviations were 33.155 and 41.5% respectively). When the 5-second and 10-second C-arm CT values were analyzed separately, there was also good correlation with values obtained from the PCT studies. The mean and maximum variations between the 2 techniques in the 5-second acquisitions were 8.85% and 38.01%, respectively. Those for the 10-second acquisitions were 9.72% and 38.96%, respectively. The means of the absolute CBV values for all regions of interest were 2.12 ± 0.53 and 1.91 ± 0.50 for the 2 PCT measurements and 2.30 ± 0.61 and 2.10 ± 0.52 for the 2 C-arm CT measurements.
Fig 4.
C-arm and PCT CBV maps from animal 1.
Fig 5.
C-arm and PCT CBV maps from animal 2.
Discussion
We have demonstrated the feasibility of performing functional (physiologic) imaging in the angiographic suite by using C-arm CT. Very good evidence shows that an assessment of cerebrovascular pathophysiology by using PCT or PWI/DWI is valuable in the triage of patients with acute ischemic stroke.10,17 The use of these techniques now makes it possible to distinguish those individuals who have large volumes of ischemic but potentially salvageable brain (who thus have a favorable risk-to-benefit ratio for recanalization therapy) from those whose brain tissue is largely not salvageable (who thus have an unfavorable risk-to-benefit ratio for recanalization therapy). It is also clear that the major determinants of tissue outcome following the occurrence of ischemia are the severity of the ischemia (degree of reduction in perfusion) and the duration of the ischemic event.6,17 It follows that the ability to perform patient selection in an environment in which treatment could be immediately started (ie, the interventional suite) would offer potential improvements both in safety and efficacy of endovascular treatments.6 Because the complications of revascularization therapy increase significantly when there is reperfusion of large volumes of nonviable brain, safety might also be enhanced because of the capacity to reassess tissue viability in instances in which there is a significant delay from initial diagnostic imaging to arrival in the angiographic suite. Finally, the ability to monitor tissue viability sequentially during the several hours often required for such treatments might also improve safety, by improving the ability to stop an intervention when there has been a significant increase in nonviable tissue since initial evaluation.
Currently, compared with standard multidetector CT scanners, C-arm CT does not provide image quality that is adequate for initial diagnostic evaluation of patients suspected of having an ischemic stroke. Improvements are ongoing, however, that will reduce scatter radiation, increase temporal resolution, and provide postprocessing algorithms that reduce motion artifact. Combined, these could result in image quality improvements of C-arm soft-tissue imaging that would eliminate this restraint.14,15,18
Using noncontrast CT (NCCT) in combination with CBV values from PCT, Parsons et al19 have demonstrated the ability to distinguish ischemic but salvageable tissue (eg, penumbra) from ischemic and nonviable tissue (eg, infarct core). In their study, infarct core appeared on NCCT as areas of parenchymal hypoattenuation, whereas penumbra appeared as areas of isolated focal swelling. Using PCT measurements of CBV, they were then able to divide tissue that appeared normal on the NCCT into that which was salvageable (normal or increased CBV) and nonsalvageable (decreased CBV).19 The combination of a standard NCCT performed for initial diagnosis and the ability to measure CBV with C-arm CT provides the potential to apply the same model in the angiographic suite by using currently available equipment. Murphy et al20 have also recently shown the potential value of CBV in distinguishing viable from nonviable brain tissue.
The low temporal resolution of C-arm CT makes it necessary to use a contrast medium injection protocol so that there is a steady level of contrast in the brain parenchyma throughout data acquisition. We were able to achieve this in the canines by using rates of injection and volumes of contrast medium that would, if scaled according to body weight, be acceptable for clinical use in humans. In scenarios in which CBV is measured in the angiographic suite, it is possible that contrast medium could be injected intra-arterially (aortic arch, internal carotid artery, vertebral artery), thereby significantly reducing the total amount of contrast medium needed for each measurement. There is very little experience in using intra-arterial injections for measurement of cerebral hemodynamics, particularly for measurement of what might be termed “regional” (eg, hemispheric) hemodynamics. We are currently exploring further the potential benefits and limitations of intra-arterial injections by using our canine model.
There is considerable variation in absolute values of CBV when measured in the same subject at different times and when acquisitions from the same subject are postprocessed by different individuals.21,22 The variations in our measurements made with the 2 techniques in these 10 healthy canines compare very favorably with these reports in humans. Also, both the variation and the absolute values of CBV obtained with C-arm CT and PCT in our study compare well with those previously reported for PCT measurements in canines.23 There were 2 variations in the methods used for the CTP studies. Five animals were studied with CTP and C-arm CT on different days, whereas 7 were studied on the same day. All animals were healthy, and there was no change in their condition during the interval between the 2 studies. Also, for 1 of the CTP studies, the volume of contrast medium and saline chaser was different in 5 animals from that used in the other 7. This change was minor and, on the basis of our clinical experience and variations of injection protocols reported in the literature, would not be expected to impact the observed results. Because of the small size of the canine intracranial arteries and the proximity of the superior sagittal sinus to the skull vault, external vascular structures were used for input functions. This has been previously done and validated in canine studies by Nabavi et al.23
The thresholds for infarction in canines have not, to our knowledge, been established. In humans; there is some evidence to indicate that the PCT value that most accurately describes the infarct core is the absolute CBV.5 More recently, a relative threshold value of 55% compared with the opposite side has been described.24 We believe that the results reported in our study indicate that CBV measurements made with C-arm CT are potentially of clinical relevance. Further study, both of animals with induced acute ischemic stroke and humans will be required to determine the ultimate utility of this technique.
There is increasing evidence of the value of CBV measurements in the evaluation of patients with a variety of neoplastic conditions. This would seem to be another application in which the ability to measure CBV, obtain high-spatial-resolution images of vascular structures, and perform a tumor biopsy could add value to patient management.24
Conclusions
In these healthy animals, measurement of CBV with C-arm CT compared well with measurements made with PCT. The ability to assess CBV in the angiographic suite enhances the environment in which patients with ischemic stroke are treated.
References
- 1.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study—a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 1999;282:2003–11 [DOI] [PubMed] [Google Scholar]
- 2.IMS II Trial Investigators. The Interventional Management of Stroke (IMS) II Study. Stroke 2007;38:2127–35. Epub 2007 May 24 [DOI] [PubMed] [Google Scholar]
- 3.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 2007;369:293–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamberg LM, Hunter GJ, Kierstead D, et al. Measurement of cerebral blood volume with subtraction three-dimensional functional CT. AJNR Am J Neuroradiol 1996;17:1861–69 [PMC free article] [PubMed] [Google Scholar]
- 5.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006;37:979–85 [DOI] [PubMed] [Google Scholar]
- 6.Saver JL. Time is Brain−Quantified. Stroke 2006;37:263–67 [DOI] [PubMed] [Google Scholar]
- 7.Schellinger PD, Fiebach JB, Hacke W. Imaging-based decision making in thrombolytic therapy for ischemic stroke: present status. Stroke 2003;34:575–83 [PubMed] [Google Scholar]
- 8.Warach S, Dashe JF, Edelmann RR. Clinical outcome in ischemic stroke predicted by early diffusion-weighted and perfusion magnetic resonance imaging: a preliminary analysis. J Cereb Blood Flow Metab 1996;16:53–59 [DOI] [PubMed] [Google Scholar]
- 9.Wintermark M, Fischbein NJ, Smith WE, et al. Accuracy of dynamic perfusion-CT with deconvolution in detecting acute hemispheric stroke. AJNR Am J Neuroradiol 2005;26:104–12 [PMC free article] [PubMed] [Google Scholar]
- 10.Heidenreich JO, Hsu D, Wang G, et al. Magnetic resonance imaging results can affect therapy decisions in hyperacute stroke care. Acta Radiol 2008;49:550–57 [DOI] [PubMed] [Google Scholar]
- 11.Heran NS, Song JK, Namba K, et al. The utility of DynaCT in neuroendovascular procedures. AJNR Am J Neuroradiol 2006;27:330–32 [PMC free article] [PubMed] [Google Scholar]
- 12.Benndorf G, Strother CM, Claus B, et al. Angiographic CT in cerebrovascular stenting. AJNR Am J Neuroradiol 2005;26:1813–18 [PMC free article] [PubMed] [Google Scholar]
- 13.Benndorf G, Claus B, Strother CM, et al. Increased cell opening and prolapse of struts of a Neuroform stent in curved vasculature: value of angiographic computed tomography—technical case report. Neurosurgery 2006;58 (4 suppl 2):ONS-E380. [DOI] [PubMed] [Google Scholar]
- 14.Orth RC, Wallace MJ, Kuo MD. C-arm cone-beam CT: general principles and technical considerations for use in interventional radiology. J Vasc Interv Radiol 2008;19:814–21 [DOI] [PubMed] [Google Scholar]
- 15.Kalender WA, Kyriakou Y. Flat-detector computed tomography (FD-CT). Eur Radiol 2007;17:2767–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zellerhoff M, Scholz B, Ruehrnschopf E-P, et al. Low contrast 3D-reconstruction from C-arm data. Proc SPIE 2005;5745:646–55 [Google Scholar]
- 17.Muir KW, Buchan A, vonKummer, R, et al. Imaging of acute stroke. Lancet Neurol 2005;5:755–68 [DOI] [PubMed] [Google Scholar]
- 18.Akpek S, Brunner T, Benndorf G, et al. Three-dimensional imaging and cone beam volume CT in C-arm angiograph with flat panel detector. Diagn Interv Radiol 2005;11:10–13 [PubMed] [Google Scholar]
- 19.Parsons MW, Pepper EM, Bateman GA, et al. Identification of the penumbra and infarct core on hyperacute noncontrast and perfusion CT. Neurology 2007;68:730–36 [DOI] [PubMed] [Google Scholar]
- 20.Murphy BD, Fox AJ, Lee DH, et al. White matter thresholds for ischemic penumbra and infarct core in patients with acute stroke: CT perfusion study. Radiology 2008;247:818–25. Epub 2008 Apr 18 [DOI] [PubMed] [Google Scholar]
- 21.Turk AS, Grayev A, Rowley HA, et al. Variability of clinical CT perfusion measurements in patients with carotid stenosis. Neuroradiology 2007;49:955–61. Epub 2007 Jul 24 [DOI] [PubMed] [Google Scholar]
- 22.Fiorella D, Heiserman J, Prenger E, et al. Assessment of the reproducibility of postprocessing dynamic CT perfusion data. AJNR Am J Neuroradiol 2004;25:97–107 [PMC free article] [PubMed] [Google Scholar]
- 23.Nabavi DG, Cenic A, Dool J, et al. Quantitative assessment of cerebral hemodynamics using CT: stability, accuracy and precision studies in dogs. J Comput Assist Tomogr 1999;23:506–15 [DOI] [PubMed] [Google Scholar]
- 24.Schaefer PW, Rarak ER, Kamalian S, et. al. Quantitative assessment of core/penumbra mismatch in acute stroke. Stroke 2008;39:2986–92 [DOI] [PubMed] [Google Scholar]
- 25.Covarrubias DJ, Rosen BR, Lev MH. Dynamic magnetic resonance perfusion imaging of brain tumors. Oncologist 2004;9:528–37 [DOI] [PubMed] [Google Scholar]