Abstract
BACKGROUND AND PURPOSE: Percutaneous sacroplasty has recently gained attention as a potential treatment for sacral insufficiency fractures. We describe a readily identifiable fluoroscopic landmark that facilitates needle placement and validate this with virtual needle placement by using CT data and fluoroscopically guided treatment in 13 patients.
MATERIALS AND METHODS: From CTs of 100 consecutive patients, the optimal target zone for needle placement in the sacral ala was defined at the intersection of lines from each of the corners of the first sacral segment, which is readily identifiable on lateral fluoroscopy. We then measured the distance from that virtual target point to the anterior sacral cortex by using the CT data for 3 specific trajectories: 1) parallel to the L5-S1 disk, 2) axial with respect to the patient, and 3) along the long axis of the sacrum. Case records of 13 consecutive patients treated by using this technique were also reviewed.
RESULTS: The mean distances for the 3 trajectories were 11.3 mm, 11.2 mm, and 12.8 mm, respectively. Needle placement would have been outside the anterior sacral cortex in 3 patients. Review of preprocedure imaging easily identified this potential breach. During treatment, needle placement by using the landmark was successful in all patients, and there were no complications.
CONCLUSIONS: A safe target for sacroplasty needle placement in the superolateral sacral ala can be defined by using the intersection of lines drawn from the corners of the first sacral segment. We validated this landmark by using it for treatment in 13 patients. Further studies evaluating clinical outcomes following sacroplasty will be necessary.
Percutaneous sacroplasty has recently gained attention as a treatment option for patients with sacral insufficiency fractures, similar in concept to vertebroplasty for vertebral compression fractures.1-7 However, the complex anatomy of the sacrum makes needle placement more difficult using traditional fluoroscopy alone as compared with vertebroplasty in the thoracic or lumbar spine. Several recent reports have advocated the use of CT including CT fluoroscopy, whereas others have used a combination of CT and a portable C-arm fluoroscopy unit,1-3,8 with CT guidance for needle placement and fluoroscopy for evaluation of cement filling. Fluoroscopy alone can be used, but breach of the anterior sacral wall (how deep the needle should be placed) is often the greatest concern when using this approach.
Fluoroscopic guidance has advantages with respect to superior visualization of cement during injection, but needle placement landmarks for the sacrum are not well defined. A recent report described the technique of orienting the needle parallel to the sacroiliac joint and the L5-S1 disk space,9 but it did not address the issue of how far anteriorly the needle could be safely advanced, to avoid extending beyond the anterior sacral cortex. Techniques along the long axis of the sacrum have also been reported, with a similar goal of placing cement primarily in the superior sacral segments and extending inferiorly.10 In this report, we describe a simple anatomic landmark that can be used to guide needle placement for fluoroscopically guided percutaneous sacroplasty, and we validate this landmark with data from retrospective review of thin-section CT scans. This landmark is also independent of the craniocaudal angle chosen and can be equally well used with the long-axis injection technique and the short-axis (parallel to the L5-S1 disk) technique. We also validated that landmark by using it as our fluoroscopic target in 13 consecutive cases.
Materials and Methods
Imaging Analysis
Institutional review board approval was obtained for this retrospective analysis. One hundred consecutive CT scans of adult patients in whom thin-section images of the pelvis were obtained were selected by review of the hospital PACS system. These were transferred to an Advantage Windows workstation (GE Healthcare, Milwaukee, Wis) for multiplanar reformatted image analysis. We defined the ideal target zone for needle placement as the intersection of lines from the corners of the first sacral segment (Fig 1). Virtual needle placement was performed for each hemisacrum by using 3 possible trajectories: 1) parallel to the L5-S1 disk, 2) axial with respect to the patient, and 3) along the long axis of the sacrum. For all 3 trajectories, it was anticipated that the medial-to-lateral orientation of the needle would be parallel to the sacroiliac joint, which avoids the joint and facilitates placement into the superolateral sacral ala.9
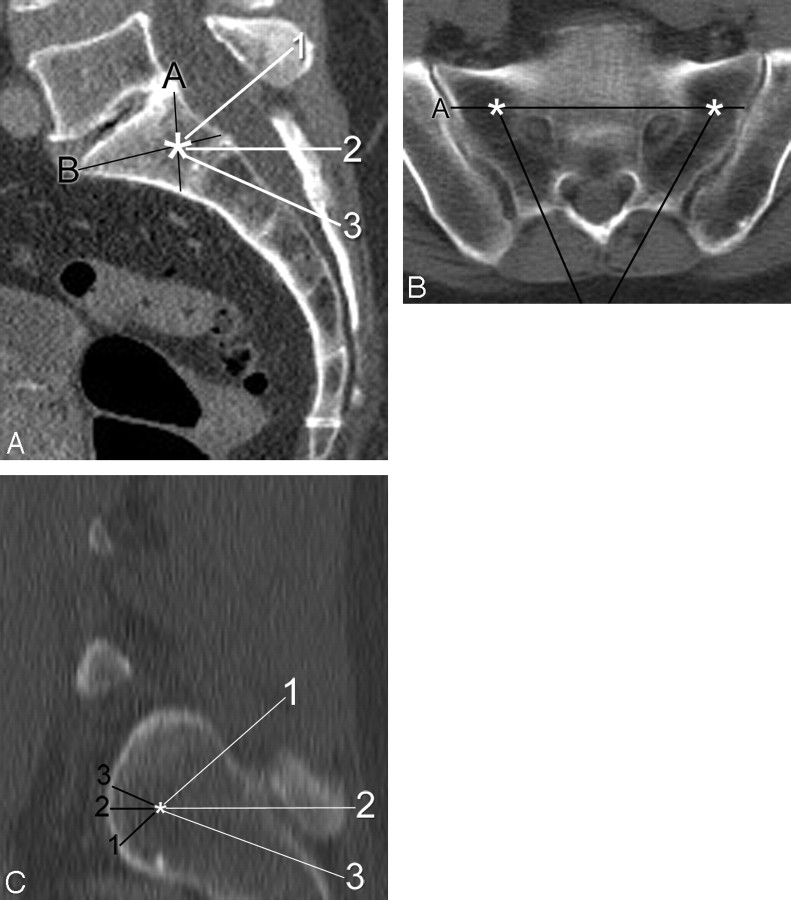
Fig 1.
Demonstration of measurements made during virtual needle placement on a CT scan of the pelvis in a 65-year-old woman. A, On a sagittal midline reconstructed image, note the target (asterisk) by the intersection of line A (from the posterosuperior corner of S1 to the anteroinferior corner of S1) with line B (from the anterosuperior corner of S1 to the posteroinferior corner of S1). Three possible needle trajectories have been described and are indicated by the numbered white lines: 1) parallel to the L5-S1 disk space, 2) neutral or axial with respect to the patient, and 3) along the sacral long axis. B, Axial image taken along line 2 (axial plane) from the same patient demonstrates the target zones (asterisks) for each sacral ala. Line A represents the projection of line A from the sagittal image (A). Note how the 2 needle trajectories (black lines) are both parallel to their respective sacroiliac joints. C, Sagittal oblique image obtained along a line parallel to the sacroiliac joint shows the relative location of the target (asterisk) within the lateral sacrum. The distance from the target point to the anterior sacral cortex was obtained for the 3 needle trajectories described previously.
From the target point, the distance to the anterior sacral cortex was measured, continuing along the chosen trajectory (Fig 1C). This generated 3 measurements per hemisacrum, for a total of 6 measurements per patient. All of these measurements were recorded for both sides of the sacrum. In addition, the presence of any congenital lumbosacral anomalies was also noted, such as lumbarization of the first sacral segment or other transitional anatomy. We also recorded the number of patients in whom the target point would be anterior to the anterior sacral cortex (in the presacral soft tissue). All the data were entered into an Excel (Microsoft, Redmond, Wash) spreadsheet for analysis.
Treatment Data
For patient-specific data, the records of 13 consecutive patients treated with percutaneous sacroplasty during a 24-month period were reviewed. Demographics and any complications that occurred during the procedure or any noticeable extraosseous cement deposition were noted. An example of using this landmark is highlighted in Fig 2.
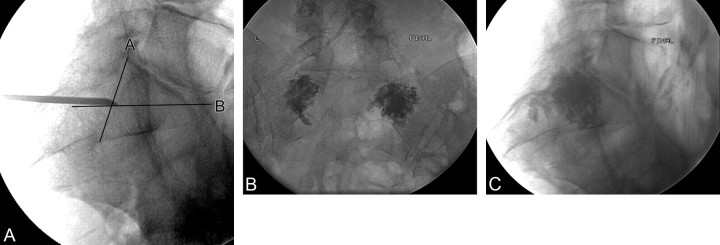
Fig 2.
Use of a fluoroscopic landmark in performing sacroplasty in a 76-year-old woman. A, Initial lateral image shows the needle in position at the intersection of lines as defined in Fig 1. B and C, Posttreatment frontal (B) and lateral (C) projections demonstrate polymethylmethacrylate cement in both sacral alas with no presacral extravasation.
Results
Imaging Analysis
We identified 51 male and 49 female patients, with a mean age of 67 years. The maximum, minimum, mean, and SD for each of the trajectories are summarized in Table 1. In 3 patients, needle placement in the target zone as described above would have been beyond the anterior sacral cortex. Transitional lumbosacral anatomy was present in 11 patients (11%). Six patients had lumbarization of the first sacral segment, whereas 5 patients had some element of sacralization of L5 (3 left, 1 right, 1 bilateral). Two of the 3 patients in whom the needle placement was anterior to the sacral cortex had lumbarization of S1. In addition, 5 of the 6 patients with lumbarization of S1 had measured distances below the respective mean values.
Table 1:
Distance measurements from a predefined target point to the anterior sacral cortex for each of the 3 trajectories described (Fig 1)*
| Distance to Anterior Sacral Cortex from Target Point | Trajectory 1 | Trajectory 2 | Trajectory 3 |
|---|---|---|---|
| Mean ± SD | 11.3 ± 4.6 mm | 11.1 ± 9.2 mm | 12.8 ± 5.2 mm |
| <3 mm | 7 (3.5%) | 8 (4%) | 7 (3.5%) |
| 3–10 mm | 123 (61.5%) | 138 (69%) | 117 (58.5%) |
| >10 mm | 70 (35%) | 54 (27%) | 76 (38%) |
Data from both the left and right hemisacrum were pooled, resulting in 200 measurements total.
Treatment Data
Patient demographics, sides treated, needle sizes, and any clinical or radiologic complications are summarized in Table 2. No complications were noted in any patients. All had subjective pain relief following the procedure, though objective measures were not studied.
Table 2:
Demographics of 13 consecutive patients treated with percutaneous sacroplasty using fluoroscopic guidance only
| Age (yr) | Sex | Sides Treated | Needle Size (Gauge) | Extraosseous Cement Seen on Imaging? | Clinical Complication or Worsening of Symptoms? |
|---|---|---|---|---|---|
| 73 | M | Bilateral | 13 | No | No |
| 65 | F | Bilateral | 11 | No | No |
| 65 | F | Bilateral | 11 | No | No |
| 68 | F | Bilateral | 11 | No | No |
| 61 | F | Bilateral | 11 | No | No |
| 74 | F | Bilateral | 11 | No | No |
| 76 | F | Bilateral | 11 | No | No |
| 83 | F | Right | 11 | No | No |
| 81 | F | Bilateral | 13 | No | No |
| 67 | F | Right | 13 | No | No |
| 78 | F | Bilateral | 11 | No | No |
| 81 | F | Bilateral | 13 | No | No |
| 76 | F | Bilateral | 11 | No | No |
Discussion
Several recent case reports and small series have described excellent pain relief with sacroplasty for painful sacral insufficiency fractures. Due to the complex anatomy of the sacrum, safe needle placement can be more difficult than with vertebroplasty. Existing reports have used CT, fluoroscopy, or both for guidance of needle placement. Advantages of using fluoroscopy include better visualization of the needle trajectory in 3D, especially with biplane fluoroscopy, and easier visualization of cement dispersion during sacroplasty. Among the reported advantages of using CT for guidance is visualization of the tip of the needle in relation to the anterior sacral cortex. However, with CT, it may be more difficult to observe cement distribution during injection, given the limitations in following it in 3D with axial CT.
The optimal location for cement distribution for pain relief from sacroplasty is yet to be determined. A recent finite-element analysis showed that cement placement in the superolateral sacral ala decreased maximal principal stress by 83% and fracture gap micromotion by 48%.9 Therefore, our goal was to place cement in this location to maximize stabilization.
Needle placement for sacroplasty is defined by angles along both the craniocaudal and medial-lateral axis. The medial-lateral angulation can be defined along a line parallel to the sacroiliac joint. Various techniques for choosing the craniocaudal angulation have been described, including along the long axis of the sacrum or parallel to the L5-S1 disk space. Regardless of which trajectory is chosen, one final determinant of needle placement is the depth to which the needle should be placed. Given the difficulty in visualizing the anterior edge of the sacrum by using lateral or anterior projections, a readily identifiable landmark for depth of needle placement would greatly simplify the procedure.
There are limitations to an imaging analysis of anatomic landmarks. First, unexpected anatomic variants can exist, such as transitional lumbosacral segments. In our study, this was seen in 11% of patients. There might have been a trend toward having a reduced distance between the anterior sacral cortex and the target distance in patients with lumbarization of S1. However, it is straightforward to test this simple landmark preprocedurally in any patient in whom sacroplasty is considered. Sagittal images from CT or MR imaging studies performed preprocedurally are usually readily available, and this landmark can be verified before needle placement by placing the mouse pointer on the desired target zone and scrolling to either side to ensure that this point is always within the sacral confines (Fig 3).
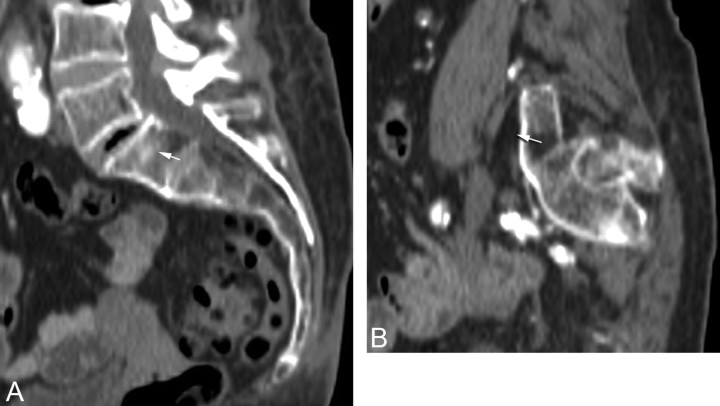
Fig 3.
Example of a potential breach of the anterior sacral cortex in an 88-year-old woman with lumbarization of S1. A, Midline sagittal reformatted image from a CT scan shows the target zone (arrow) within S1 as described in Fig 1. B, Left parasagittal reformatted image shows that needle placement (arrow) would be anterior to the sacral cortex.
Conclusions
We described an anatomic landmark that is readily identifiable by using lateral fluoroscopy, which can facilitate needle placement for sacroplasty. The safety of using this landmark in patients can be quickly verified by using preprocedural imaging, and we demonstrated the clinical utility by using this as a landmark in 13 patients. Further studies regarding the efficacy of sacroplasty in patients with painful insufficiency fractures will be necessary.
References
- 1.Layton KF, Thielen KR, Wald JT. Percutaneous sacroplasty using CT fluoroscopy. AJNR Am J Neuroradiol 2006;27:356–58 [PMC free article] [PubMed] [Google Scholar]
- 2.Strub WM, Hoffmann M, Ernst RJ, et al. Sacroplasty by CT and fluoroscopic guidance: is the procedure right for your patient? AJNR Am J Neuroradiol 2007;28:38–41 [PMC free article] [PubMed] [Google Scholar]
- 3.Binaghi S, Guntern D, Schnyder P, et al. A new, easy, fast, and safe method for CT-guided sacroplasty. Eur Radiol 2006;16:2875–78 [DOI] [PubMed] [Google Scholar]
- 4.Garant M. Sacroplasty: a new treatment for sacral insufficiency fracture. J Vasc Interv Radiol 2002;13:1265–67 [DOI] [PubMed] [Google Scholar]
- 5.Pommersheim W, Huang-Hellinger F, Baker M, et al. Sacroplasty: a treatment for sacral insufficiency fractures. AJNR Am J Neuroradiol 2003;24:1003–07 [PMC free article] [PubMed] [Google Scholar]
- 6.Frey ME, DePalma MJ, Cifu DX, et al. Efficacy and safety of percutaneous sacroplasty for painful osteoporotic sacral insufficiency fractures: a prospective, multicenter trial. Spine 2007;32:1635–40 [DOI] [PubMed] [Google Scholar]
- 7.Frey ME, Depalma MJ, Cifu DX, et al. Percutaneous sacroplasty for osteoporotic sacral insufficiency fractures: a prospective, multicenter, observational pilot study. Spine J 2008;8:367–73 [DOI] [PubMed] [Google Scholar]
- 8.Masala S, Konda D, Massari F, et al. Sacroplasty and iliac osteoplasty under combined CT and fluoroscopic guidance. Spine 2006;31:E667–69 [DOI] [PubMed] [Google Scholar]
- 9.Whitlow CT, Yazdani SK, Reedy ML, et al. Investigating sacroplasty: technical considerations and finite element analysis of polymethylmethacrylate infusion into cadaveric sacrum. AJNR Am J Neuroradiol 2007;28:1036–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith DK, Dix JE. Percutaneous sacroplasty: long-axis injection technique. AJR Am J Roentgenol 2006;186:1252–55 [DOI] [PubMed] [Google Scholar]