Abstract
BACKGROUND AND PURPOSE: Brain pulsation is a well-known observation in neurosurgery, but methods for its visualization on MR imaging, like phase imaging, do not provide a detailed structural view. We prospectively investigated electrocardiographic (ECG)-gated cine true fast imaging with steady-state precession (FISP) sequence on volunteers to test a sequence for demonstrating brain pulsation and movements of intracranial structures related to CSF dynamics.
MATERIALS AND METHODS: Eleven healthy volunteers were investigated with prospectively ECG-gated cine true-FISP in the midsagittal plane. A total of 50 phases were recorded per cardiac cycle and per volunteer. The lamina terminalis was chosen to study the pulsatility of the brain, and the optic recess diameter was chosen for means of objective quantification of the degree of pulsatility.
RESULTS: Pulsatile motion of the lamina terminalis was apparent in all volunteers on the cine mode. The mean diameter of the optic recess was 2.5 mm. The greatest change in diameter in 1 volunteer was 1.5 mm. The mean change in diameter was 40% during 1 cardiac cycle.
CONCLUSIONS: Cine true-FISP sequence is a well-suited method for investigations of passive movements of the ventricular system. It shows pulsations of the brain as well as passive changes caused by CSF dynamics with high temporal and spatial resolution.
Brain pulsation and related CSF dynamics, which are observed by neurosurgeons, have so far been investigated by phase-contrast MR imaging,1 cine echo-planar imaging,2 or complementary spatial modulation of magnetization (CSPAMM).3 Although these methods allow quantification of movement, they do not provide detailed anatomic information in a cine view throughout the cardiac cycle. Electrocardiographic (ECG)-gated true fast imaging with steady-state precession (FISP)4 sequences, which can be viewed as cine sequences, are widely used to investigate cardiac motion5 with high temporal and spatial resolution. The contrast of cine true-FISP is similar to constructive interference in steady state, which gives a high signal intensity from CSF and a uniform low signal intensity from the brain, bone, cranial nerves, or ventricular walls.6
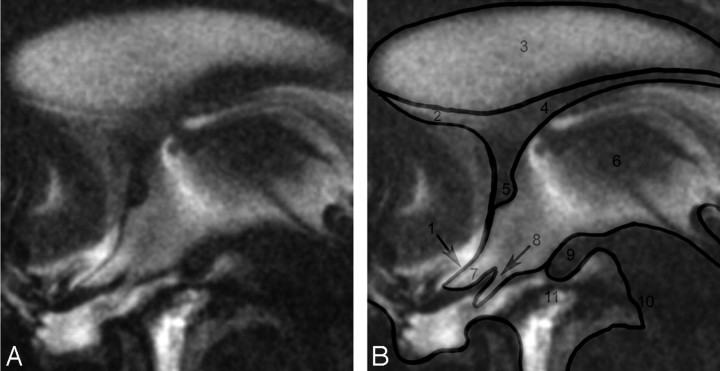
The aim of this study was to investigate if cine true-FISP is capable of demonstrating brain pulsations and movements of intracranial structures related to CSF dynamics. The lamina terminalis (Fig 1), which moves passively by CSF dynamics and is surrounded by CSF and therefore well suited to be imaged by the contrast characteristics of cine true-FISP, was chosen for studying the pulsatility of the brain. The optic recess diameter was chosen for means of objective quantification of the degree of pulsatility.
Fig 1.
On the left side, midsagittal image through the anterior part of the third ventricle and its surroundings. There is a hypointense jet seen upcoming from the aqueduct into the third ventricle. The CSF is partly reduced in signal intensity due to phase dephasing (eg, in the anterior part of the third ventricle). Close to the foramen of Monro is a hyperintense area probably caused by flow going upwards to the lateral ventricles. The sella is partially empty. On the right side are labels overlayed on the same image to give better anatomic orientation: 1, Lamina terminalis; 2, Lamina rostralis; 3, Septum pellucidum; 4, Fornix; 5, Anterior commissure; 6, Third ventricle; 7, Optic recess; 8, Optic chiasm; 9, Mammillary bodies; 10, Brain stem; 11, Interpeduncular fossa.
Materials and Methods
The movement of the lamina terminalis at the midsagittal level caused by CSF dynamics was examined in 11 healthy subjects by cine true-FISP MR imaging on a 1.5T unit (Avanto; Siemens, Erlangen, Germany). The midsagittal plane was determined by a 3D sequence (MPRAGE), as well as with the help of transversal (T2 TSE) and coronal planes completed first. There were 6 women and 5 men aged 21 to 29 years (mean age, 24 years). All volunteers were in good health and without any neurologic pathologic disorders. A commercially available, built-in, prospectively cardiac-gated cine true-FISP sequence was used.7–9 A TR of 80 ms and a TE of 5 ms were used throughout the study. In this sequence, there were 3 section positions with 50 phases acquired. Because of different heart rates, the time between consecutive images of 1 series differed from subject to subject. Other sequence data were 230 × 230-mm2 FOV, band width 130 Hz/Px, 320 × 320 matrix, and 2.5-mm section thickness.
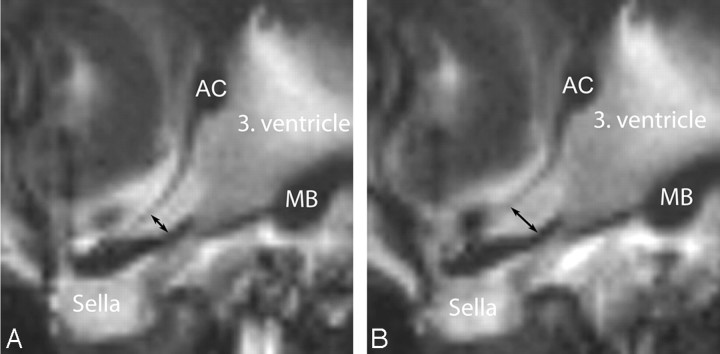
The images were transferred to an MR imaging workstation (Siemens) running Argus. The investigator studied the acquired images in a cine mode to assess the pulsatile motion of the lamina terminalis and third ventricular margins. For the following measurements, the Argus distance-tool was used. Figure 2 represents the examined region including the lamina terminalis and explains where the optic recess was used for the measurements.10 On the basis of the visual assessment on the cine mode, the point of maximal pulsatility was measured at this level for all phases of the cardiac cycle, as shown in Fig 2.
Fig 2.
Midsagittal images through the anterior part of the third ventricle, giving the location for the measurements of the diameter of the optic recess. Left: minimal diameter, right: maximal diameter during the cardiac cycle. Arrows indicate location of measurement in optic recess; AC, anterior commissure; MB, mamillary body. The sella is partially empty.
Together with the registered RR-interval and corresponding timings, the 50-diameter measurements per subject were submitted to Excel 2003 (Microsoft, Redmond, Wash) for additional statistical evaluation. Mean maximum and minimum during heart cycle were calculated for each volunteer. Means and SDs were also calculated for the group of subjects. The point in time related to the first R-wave when the maximal diameter appears was evaluated in absolute values and as a fraction of the RR-interval duration.
Results
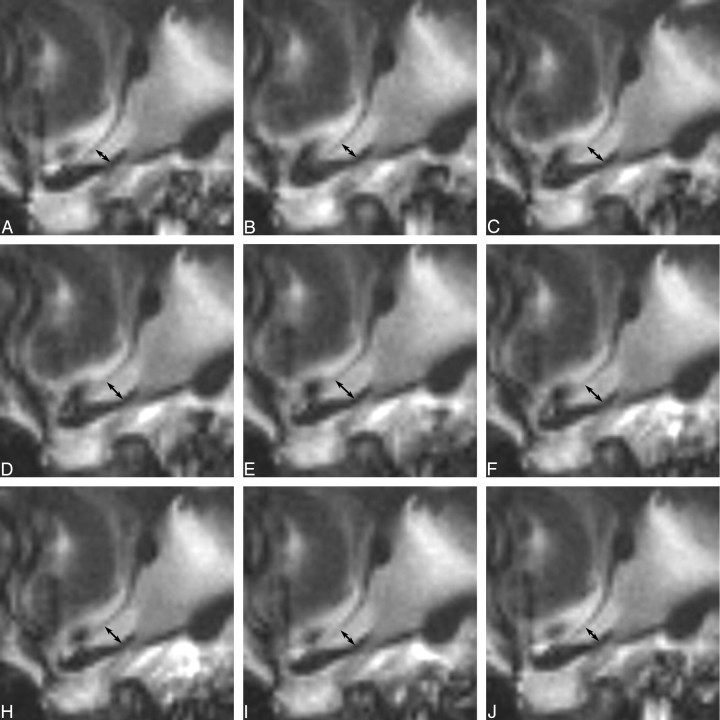
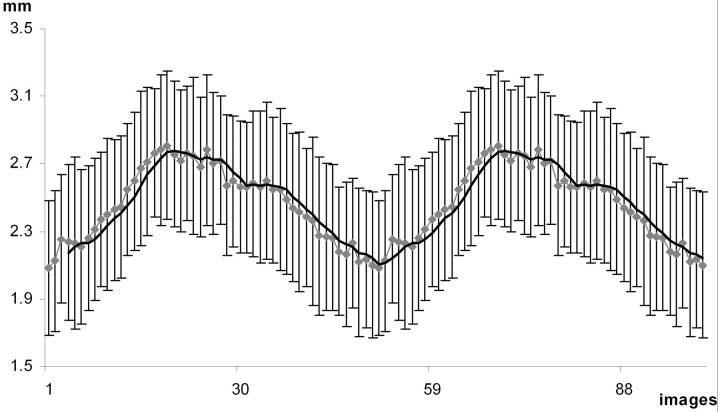
On visual inspection, all subjects demonstrated qualitative motion of the lamina terminalis (on-line videos 1 and 2, Fig 3), brain pulsation (on-line video 3), and CSF dynamics. Quantitative measurements of the optic recess (Table and Fig. 4) confirmed the movement of the third ventricular walls. In summary of all 11 cases without any consideration of movement, the average diameter of the optic recess was 2.5 mm but varied from 1.9 to 3.1 mm depending on individual anatomic variations. During the cardiac cycle, the average change of the optic recess diameter through all 11 volunteers was 1.0 mm. The largest measured difference between the minimal and maximal optic recess diameter was 1.5 mm, increasing from 1.8 mm to the maximum of 3.3 mm (subject H in accompanying Table). ECG-recorded RR-intervals varied from 765 to 1300 ms (mean, 950 ms). On average, the maximal diameter of the optic recess was reached at a time of 361 ms after the R-wave, corresponding to 38% of the RR interval from the beginning of the RR-cycle (Table).
Fig 3.
Series of cine true-FISP images for a full cardiac cycle demonstrating the movement of the lamina terminalis. The location of the images is the same as in Figs 1 and 2.
Details on volunteers (A-K) regarding duration of RR-intervals and measurements of the optic recess diameter
| Volunteer | A | B | C | D | E | F | G | H | I | J | K | Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR-interval (ms) | 783 | 890 | 1138 | 865 | 893 | 965 | 765 | 1020 | 1045 | 1300 | 785 | 950 |
| Time of maximal diameter after R-wave (ms) | 204 | 303 | 355 | 294 | 268 | 270 | 291 | 388 | 376 | 676 | 251 | 361 |
| Time of maximal diameter as fraction of RR-interval | 0.26 | 0.34 | 0.31 | 0.34 | 0.30 | 0.28 | 0.38 | 0.38 | 0.36 | 0.52 | 0.32 | 0.38 |
| Maximum (mm) | 2.5 | 3.0 | 3.4 | 2.8 | 2.0 | 2.2 | 3.0 | 3.3 | 3.6 | 3.1 | 2.9 | 3.0 |
| Minimum (mm) | 1.7 | 1.7 | 2.6 | 1.9 | 2.8 | 1.6 | 2.3 | 1.8 | 2.6 | 1.7 | 1.6 | 1.95 |
Fig 4.
Diameter of the optic recess during 2 cardiac cycles. Dotted line gives the mean diameter for the group of subjects with SDs; continuous line is the moving average. Image 1 on the x-axis refers to the first R-wave, the next R-wave locates at image 50.
Discussion
Brain pulsation resulting from pulsatile blood flow is very well known by neurosurgeons and has been measured by quantitative MR methods such as phase imaging, which shows a general cardiac-cycle-dependent movement of all brain structures.1 Shortly after carotid systole, a caudal motion of central brain structures in a range of 0.1 to 0.5 mm concurs with upward motion of peripheral structures. Simultaneously to this motion, Enzmann and Pelc7 observed caudal motion of CSF in the cervical subarachnoid space. Schroth and Klose11 measured that the CSF flow from the third to the fourth ventricle begins 200 ms after the R-wave. The maximal dilation of the optic recess observed in our study was observed at 361 ms, giving approximately 161 ms to reach the maximal dilation of the optic recess after the beginning of craniocaudal flow.
The position where the optic recess diameter was measured was subjectively chosen by the investigator to find the location of where the change in diameter has its maximum. It was not the aim of the study to define a reproducible point for measurements (eg, in relationship to the rostral end of the optic recess). If future studies show changes in pulsatility of the optic recess in certain diseases (eg, possibly in chronic hydrocephalus with dilation of the optic recess), it would become interesting to more accurately define a position to carry out measurement of the movement of the lamina terminalis.
In our study, cine true-FISP is presented as an MR method that is capable of giving detailed anatomic information of brain pulsation and movement of the anterior borders of the third ventricle because of CSF dynamics. Although phase imaging is already widely used to study CSF dynamics and has applications, for example, in the diagnosis of normal pressure hydrocephalus, obstruction of the cerebral aqueduct, or demonstration of altered CSF dynamics after CSF shunt surgery,12,13 the potential diagnostic scope of cine true-FISP is different. In our study, it is shown that cine true-FISP is capable of delineating the movement of the lamina terminalis as a representative CSF-surrounded intracranial structure. Moving structures cannot be very well delineated by MR methods, which are not ECG triggered and are blurred because of insufficient temporal resolution in standard MR images. Therefore, cine true FISP should be especially useful in settings such as the diagnosis of arachnoid cysts with moving membranes, evaluation of the structural result of third ventriculostomy, study of the structural dynamics in Chiari malformation and syringomyelia, or for anatomic studies of small arachnoidal membranes14 such as the Liliequist membrane. These possible applications are subject to additional studies.
The dephasing, which is observed in cine true-FISP in areas with high CSF flow velocities such as the cerebral aqueduct, leads to hypointense labeling of the moving CSF and gives a direct impression of CSF flow and turbulence in these areas (on-line video 1).
Taken together, cine true-FISP, with its anatomic images, is complementary to phase imaging, with its potential to quantify flow, to study brain pulsation and CSF dynamics. In neuroradiology, cine true-FISP has its major advantage in the study of moving structures surrounded by CSF.
Conclusion
Cine true-FISP MR imaging represents an effective method to investigate pulsating intracranial structures surrounded by CSF, such as the lamina terminalis, with high temporal and spatial resolution. Therefore, cine true-FISP should be suited for the analysis of pathologic processes in the ventricular system (eg, after ventriculostomy, arachnoidal cysts, aqueductal stenosis, and similar diseases), which will be subject to additional studies. Measurements of the pulsatility of the optic recess could play a role in diseases with changes of the optic recess diameter, such as chronic hydrocephalus, or after CSF shunt surgery.
Footnotes
indicates supplemental on-line video.
References
- 1.Enzmann DR, Pelc NJ. Brain motion: measurement with phase-contrast MR imaging. Radiology 1992;185:653–60 [DOI] [PubMed] [Google Scholar]
- 2.Poncelet BP, Wedeen VJ, Weisskoff RM, et al. Brain parenchyma motion: measurement with cine echo-planar MR imaging. Radiology 1992;185:645–51 [DOI] [PubMed] [Google Scholar]
- 3.Soellinger M, Ryf S, Boesiger P, et al. Assessment of human brain motion using CSPAMM. J Magn Reson Imaging 2007;25:709–14 [DOI] [PubMed] [Google Scholar]
- 4.Oppelt A, Graumann R, Barfuss A. FISP: a new fast MRI sequence. Electromedica 1986. :15–18
- 5.Barkhausen J, Ruehm SG, Goyen M, et al. MR evaluation of ventricular function: true fast imaging with steady-state precession versus fast low-angle shot cine MR imaging: feasibility study. Radiology 2001;219:264–69 [DOI] [PubMed] [Google Scholar]
- 6.Schmitz B, Hagen T, Reith W. Three-dimensional true FISP for high-resolution imaging of the whole brain. Eur Radiol 2003;13:1577–82 [DOI] [PubMed] [Google Scholar]
- 7.Enzmann DR, Pelc NJ. Cerebrospinal fluid flow measured by phase-contrast cine MR. AJNR Am J Neuroradiol 1993;14:1301–07; discussion 1309–10 [PMC free article] [PubMed] [Google Scholar]
- 8.Bhadelia RA, Bogdan AR, Wolpert SM. Analysis of cerebrospinal fluid flow waveforms with gated phase-contrast MR velocity measurements. AJNR Am J Neuroradiol 1995;16:389–400 [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann KT, Lehmann TN, Baumann C, et al. CSF flow imaging in the management of third ventriculostomy with a reversed fast imaging with steady-state precession sequence. Eur Radiol 2003;13:1432–37 [DOI] [PubMed] [Google Scholar]
- 10.Kier EL, Truwit CL. The normal and abnormal genu of the corpus callosum: an evolutionary, embryologic, anatomic, and MR analysis. AJNR Am J Neuroradiol 1996;17:1631–41 [PMC free article] [PubMed] [Google Scholar]
- 11.Schroth G, Klose U. Cerebrospinal fluid flow. I. Physiology of cardiac-related pulsation. Neuroradiology 1992;35:1–9 [DOI] [PubMed] [Google Scholar]
- 12.Baledent O, Gondry-Jouet C, Stoquart-Elsankari S, et al. Value of phase contrast magnetic resonance imaging for investigation of cerebral hydrodynamics. J Neuroradiol 2006;33:292–303 [DOI] [PubMed] [Google Scholar]
- 13.Stivaros SM, Jackson A. Changing concepts of cerebrospinal fluid hydrodynamics: role of phase-contrast magnetic resonance imaging and implications for cerebral microvascular disease. Neurotherapeutics 2007;4:511–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu J, Zhu XL. Cranial arachnoid membranes: some aspects of microsurgical anatomy. Clin Anat 2007;20:502–11 [DOI] [PubMed] [Google Scholar]