Abstract
BACKGROUND AND PURPOSE: Xerostomia (dry mouth) is one of the serious complications of head and neck irradiation and has a strong influence on a patient's activities of daily living. MR sialography with salivary secretion stimulation provides additional functional information (salivary secretion reserve) and may contribute to the evaluation of the severity of xerostomia and predict the risk of developing a radiation-induced xerostomia. This aim of the study was to analyze MR sialography as an objective tool to evaluate radiation-induced salivary injury.
MATERIALS AND METHODS: MR sialography with salivary secretion stimulation was performed in 16 patients with head and neck malignancy before and after irradiation therapy. Multivariate (stepwise multiple regression) analysis was performed to analyze the nonstimulated and stimulated MR sialography findings and the clinical severity of xerostomia.
RESULTS: Multivariate analysis of the preirradiation study revealed no significant independent variables that could predict the clinical severity of xerostomia. In the postirradiation study, following regression with 2 independent variables (secretion response of the submandibular gland [rSG] and parotid gland visualization on stimulated MR sialography [sPG]) could explain 70% of the cases: xerostomia severity grade = 0.681 + 0.871 × rSG − 0.471 × sPG.
CONCLUSIONS: MR sialography is a useful method for visualization of salivary gland radiation injury and estimation of the severity of radiation-induced xerostomia. Insufficiency of secretion reserve at the irradiated submandibular gland has the strongest influence on xerostomia severity. Our investigation suggests that careful submandibular gland protection may lead to prevention and avoidance of radiation-induced xerostomia.
In the treatment of head and neck malignancies, irradiation therapy has provided a favorable therapeutic effect and several benefits to a patient's activities of daily living, including function and cosmetic preservation. However, this noninvasive and favorable therapy cannot avoid several serious complications.
Radiation-induced xerostomia (dry mouth) is one of the common complications of head and neck irradiation.1 Radiation-induced salivary gland injury often occurs because most of the salivary glands are included in the general irradiation fields for head and neck malignancy and regional lymph nodes. Salivary gland radiation injury leads to salivary secretion dysfunction and induces several clinical symptoms such as dysphagia (swallowing difficulty) and xerostomia (with speech difficulty, sleep disturbance, intraoral infection, and dental caries).2
There are some clinical procedures to evaluate the severity of salivary gland hypofunction. Common Terminology Criteria for Adverse Events (CTCAE) and Visual Analog Scale (VAS) are representative techniques for adverse event evaluation.3 However, these procedures are often affected by the subjectivity of the patient and listener.
Conventional sialography and a radioisotope examination have been adapted as an objective evaluation technique of salivary gland hypofunction.1,4 Conventional sialography can provide detailed morphologic information but requires an invasive maneuver such as cannulation. Salivary scintigraphy has been applied for functional imaging of salivary glands. Salivary secretion function can be evaluated on salivary scintigraphy with secretion stimulation (tartaric acid administration), but radioisotope examination cannot be performed without radiation exposure. These conventional procedures are unsuitable for chronologic observation of salivary gland hypofunction because these invasive maneuvers should not be performed repeatedly on the same patient.
Like conventional sialography, MR sialography is a current technique that can visualize the major salivary glands, yet it does not require any invasive procedures such as cannulation and contrast media infusion.5 The noninvasiveness of MR sialography is suitable for chronologic observation of salivary gland hypofunction. Several articles reported that morphologic information provided by MR sialography was useful for the diagnosis of sialolithiasis and Sjogren syndrome.6-8
To our knowledge, there is no previous report concerning MR sialography in the evaluation and prediction of radiation-induced xerostomia. In this study, we tried to establish the availability of MR sialography with salivary secretion stimulation as an objective tool to evaluate and predict radiation-induced salivary gland injury.
Materials and Methods
Our subjects were 16 patients (13 men and 3 women; average age, 64.4 years) treated with irradiation (44–77 Gy) for head and neck malignancy (11 oral cavity cancers, 3 oropharyngeal cancers, 1 laryngeal cancer, 1 malignant lymphoma). All patients had conventional opposing portal irradiation to the head and neck region. The bilateral parotid and submandibular glands were included in the radiation field; the irradiated dose was 46–64 Gy.
For clinical evaluation of xerostomia severity, we introduced an original grading scale modified from CTCAE, Version 3.0 (Table).3 In our evaluation, xerostomia severity was classified into 3 grades (grade 1, mild; grade 2, moderate; and grade 3, severe).
Clinical grading scale of xerostomia*
| Grade | Characteristics |
|---|---|
| 1, Mild | Symptomatic (dry or thick saliva) without significant dietary alteration |
| 2, Moderate | Symptomatic and significant oral intake alteration (eg, copious water, other lubricants, diet limited to purees and/or soft moist foods) |
| 3, Severe | Symptoms leading to inability to adequately aliment orally; IV fluids, tube feedings, or parenteral nutrition indicated |
Clinical severity of radiation-induced xerostomia is classified into 3 grades using CTCAE Version 3.0 modified criteria.
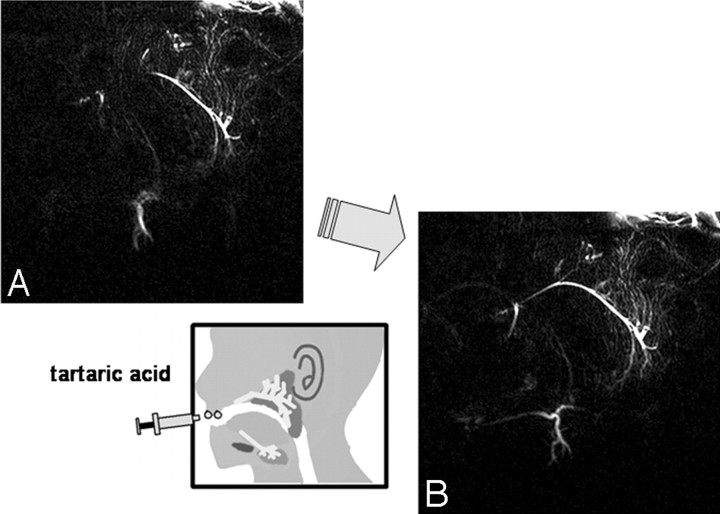
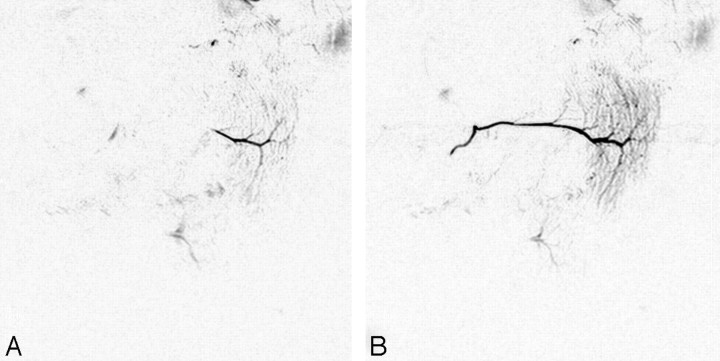
Initial MR imaging was performed before treatment, and the postirradiation study was performed within a week after the last irradiation. All MR imaging examinations were performed with a 1.5T superconductive MR imaging unit (Gyroscan ACS-NT; Philips Medical Systems, Best, the Netherlands) with bilateral 14-cm diameter surface coils for the temporomandibular joints. MR sialography was performed with 2D-thick sectioned heavy T2-weighted turbo spin-echo sequences with the following parameters: TR/TE, 10 000/1000 ms; number of averages, 6; turbo spin-echo factor, 6; spectral presaturation with inversion-recovery fat-suppression technique; FOV, 140 × 140 mm; matrix, 512 × 512; separate 27-mm section thickness of each side; and total imaging, 4 minutes 10 seconds. MR sialography was performed before and after salivary secretion stimulation with intraoral administration of a few drops of tartaric acid on the tongue (Fig 1.).
Fig 1.
MR sialography findings and response to salivary secretion stimulation. A and B, Oblique-sagittal projection MR sialography of a normal (preirradiated) salivary gland before (A) and after (B) secretion stimulation. Salivary secretion stimulation with tartaric acid administration on the tongue improves the depiction of the main duct and distal branches.
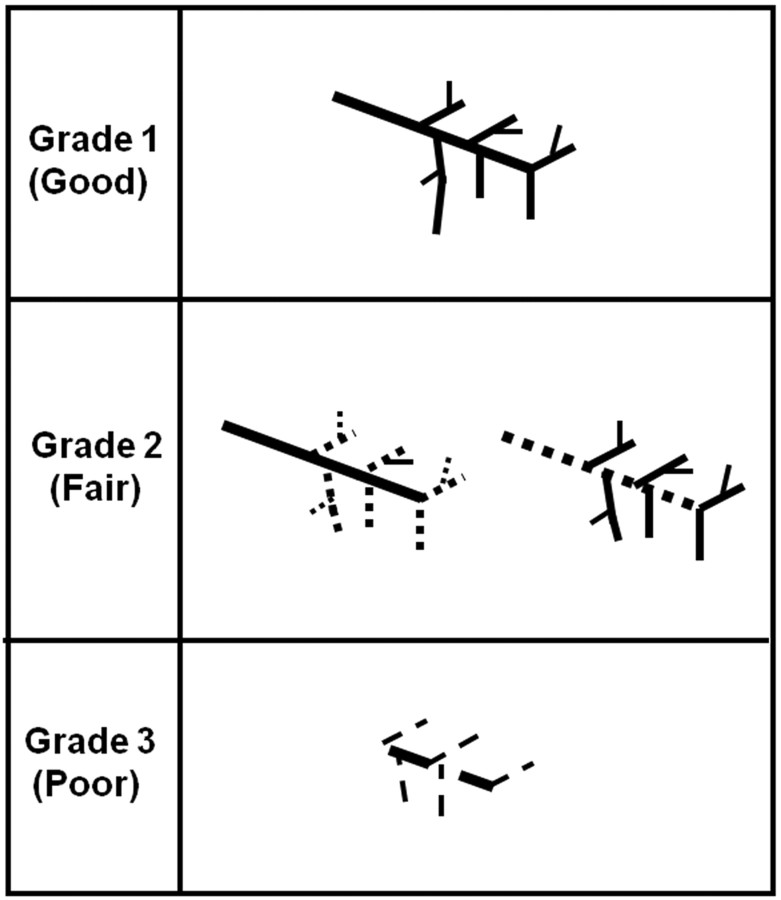
MR sialography grading was performed on each parotid and submandibular gland with both nonstimulated and stimulated status. The morphologic finding of each salivary gland was classified into 3 grades by using the following criteria: grade 1, a distinct depiction of both the main trunk and branches; grade 2, a distinct depiction of the main trunk or first- and second-order branches; grade 3, an indistinct depiction of the main trunk and first- and second-order branches (Fig 2). The salivary secretion function (reserve) was evaluated by comparison of salivary duct visualization at nonstimulated and stimulated MR sialography.
Fig 2.
Morphologic evaluation (grading) criteria of the salivary gland system on MR sialography. On nonstimulated and stimulated MR sialography, each salivary gland (parotid and submandibular gland) is classified into 3 grades according to the degree of lumen visualization: grade 1, a distinct depiction of both the main trunk and branches; grade 2, a distinct depiction of the main trunk or first- and second-order branches; and grade 3, an indistinct depiction of the main trunk and first- and second-order branches.
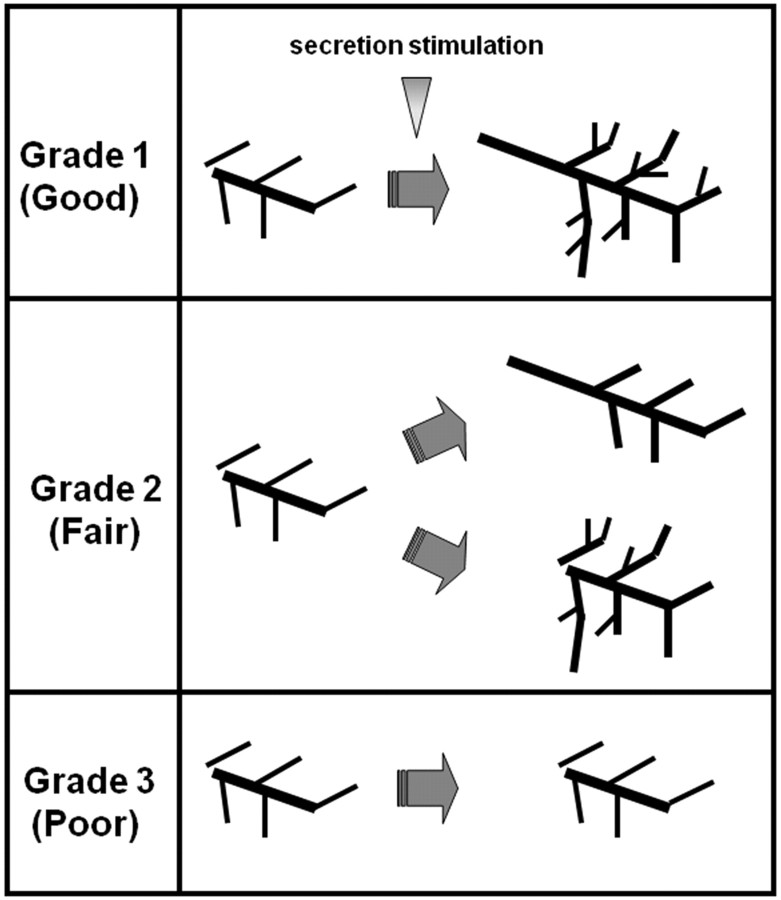
Response to salivary secretion stimulation was classified into 3 grades with the criteria shown in Fig 3. Grade 1 (good response) is a distinct depiction improvement at the main trunk and distal branches, grade 2 (fair response) is a distinct improvement at the main trunk or distal branches, and grade 3 (poor response) is no distinct response at either the main trunk or distal branches (Fig 3).
Fig 3.
Visual evaluation (grading) criteria after salivary secretion stimulation. Grade 1 (good response) is a distinct depiction improvement at the main trunk and each distal branch, grade 2 (fair response) is a distinct improvement at either the main trunk or the distal branches, and grade 3 (poor response) is no distinct response at either the main trunk or distal branches.
For MR sialography grading of duct visualization or secretion response, we were concerned that a laterality of grades sometimes occurs. In these situations, a lower (less severely) grade was adopted to avoid an overestimation of salivary gland disturbance.
In our investigation, 3 kinds of grading (nonstimulated [ns], stimulated [s] MR sialography images, and response [r] to stimulation) were obtained from the parotid gland (PG) and submandibular gland (SG). To evaluate the relation with radiation-induced xerostomia, 6 factors obtained from MR sialography (nsPG, nsSG, sPG, sSG, rPG, rSG) were introduced to multivariate analysis by using a stepwise multiple regression method (Stastical Package for the Social Sciences; SPSS, Chicago, Ill). In the analysis, a clinical grade of xerostomia was fixed to a target variable (dependent variable), and 6 factors from MR sialography were defined as explanatory variables (independent variables). In the stepwise method, break criteria for each explanatory variable injection were fixed at ≤5% of the significant level of the partial regression coefficient.
Results
The clinical severity of xerostomia for our 16 patients included 6 mild (grade 1) cases, 8 moderate (grade 2) cases, and 2 severe (grade 3) cases. The total irradiation dose to the whole salivary gland system revealed no significant positive correlation with the severity (clinical grade) of radiation-induced xerostomia (correlation coefficient, 0.437).
Conventional MR imaging of irradiated salivary glands showed variable volume reduction and high signal intensity on T2-weighted images compared with the initial (preirradiation) study. These findings were recognized in most cases and did not depend on the clinical severity of xerostomia.
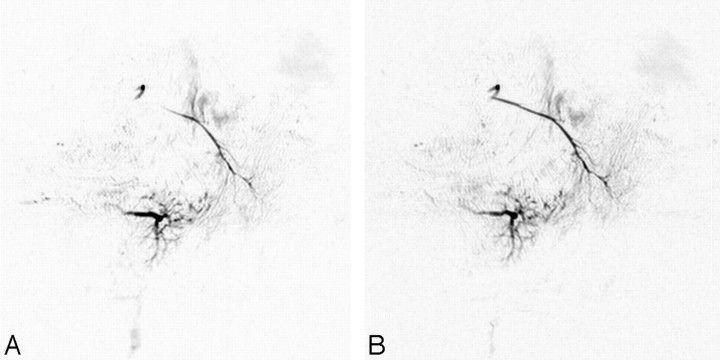
Initial (preirradiation) MR sialography depicted the main duct and branches of the parotid and submandibular glands. Intraoral administration of tartaric acid provided an imaging change that demonstrated a response to secretion stimulation (Fig 4A, -B). Irradiation to the salivary gland produced insufficient visualization of the main trunk and branches and attenuated the response to secretion stimulation (Fig 4C, -D). This morphologic and dynamic image change was recognized at both the parotid and submandibular glands and seemed to reflect direct injury from irradiation to the whole salivary gland system.
Fig 4.
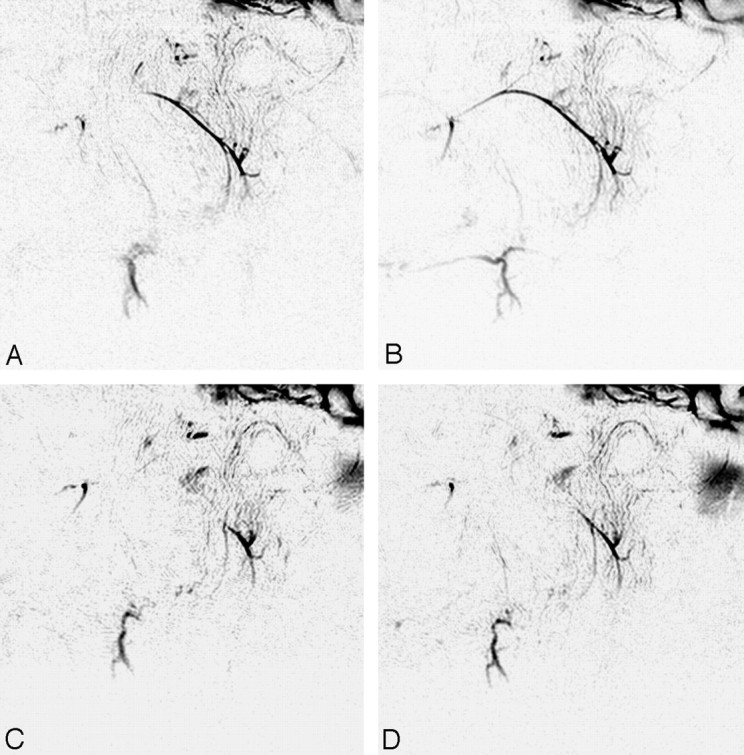
MR sialography (inverted images) before (A and B) and after (C and D) 46-Gy irradiation to the salivary gland. (A and C, nonstimulated; B and D; stimulated images). Initial (preirradiation) MR sialography of the right salivary system shows good depiction of parotid and submandibular gland ducts and response to secretion stimulation (A and B). Irradiation to the salivary gland induces insufficient visualization of the main trunk and distal branches and disturbs salivary secretion response (C and D).
Regarding response to secretion stimulation, there was a characteristic difference in irradiated parotid and submandibular glands. Most of the irradiated parotid glands showed better response to secretion stimulation than the irradiated submandibular glands (Fig 5). Insufficient depiction of the main duct/branches and a poor response to stimulation were recognized in irradiated submandibular glands, especially in patients with severe radiation-induced xerostomia (Fig 6).
Fig 5.
Nonstimulated (A) and stimulated (B) MR sialography of mild radiation-induced xerostomia. Images show nonstimulated (A) and stimulated (B) MR sialography of the 62-Gy irradiated right salivary system. Both parotid and submandibular glands show good depiction of the salivary duct and secretion response. The clinical xerostomia grade and MR sialography grade for this patient are 1 and 1.08.
Fig 6.
Characteristic MR sialography finding of the postirradiated submandibular gland in severe xerostomia. A, Nonstimulated MR sialography of the right salivary system shows poor depiction of both the parotid and submandibular glands. B, After secretion stimulation, the parotid gland shows good response, but the submandibular gland shows no significant response. The clinical grade and MR sialography grade of xerostomia in this patient are 3 and 2.82.
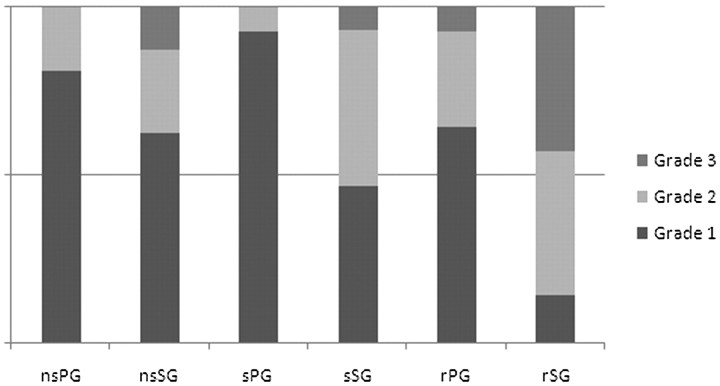
The results of MR sialography-based grading of pre- and postirradiation parotid and submandibular glands and their salivary secretion responses are shown in Figs 7 and 8. MR sialography findings of initial (nonirradiated) parotid and submandibular glands revealed individual differences in salivary gland visualization and response to salivary secretion stimulation (Fig 7). Multiple regression analysis with the initial (preirradiation) MR sialography findings and clinical xerostomia grade revealed no variables that could predict the clinical severity of radiation-induced xerostomia.
Fig 7.
MR sialography findings of preirradiation salivary glands and parotid and submandibular glands reveal individual differences in salivary gland visualization and secretion response.
Fig 8.
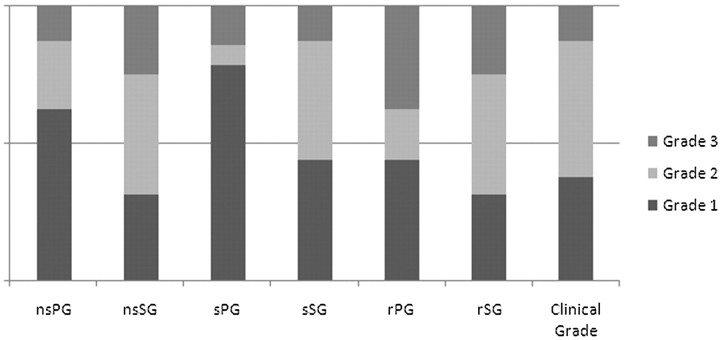
MR sialography findings of postirradiation salivary glands and clinical grade of radiation-induced xerostomia. Morphologic findings and secretion response of the submandibular gland seems to be more related to the clinical xerostomia grade than those of the parotid gland.
On the other hand, postirradiation MR sialography revealed 2 variables (sPG and rSG) as significant factors that could explain the clinical severity of radiation-induced xerostomia. The following regression equation with a postirradiation MR sialography could explain the clinical severity (grade) of xerostomia: xerostomia grade = 0.681 + 0.871 × rSG − 0.471 × sPG.
A multiple coefficient of determination was calculated at 0.698 (69.8%), and the analysis of variance was significant at the 1% standard. For the absolute value of the standardized partial regression coefficient, rSG (0.984) was superior to sPG (0.486).
Discussion
Salivary glands consist of major (parotid, submandibular, and sublingual) and minor glands. A major salivary gland has a main duct opening to the oral cavity for secreting saliva. Minor salivary glands are widely distributed over intraoral mucosa, and they secrete saliva directly from acini buried in the mucosa. The major function of secreted saliva is as a digestive juice. Other roles include intraoral moistening, antibacterial effect and sanitization, and buffering for prevention of dental caries. The submandibular gland is a serous-dominant mixed gland that continuously secretes saliva (resting saliva secretion). In humans, saliva secretion from submandibular glands accounts for >60% of the total amount of daily saliva secretion, and this resting saliva contributes to intraoral moistening and disinfection.9-11 The parotid gland is a serous gland and secretes digestive enzymes such as amylase for digestion, whereas the sublingual gland is a mucous gland that helps with food softening and mucosal protection.
Radiation-induced xerostomia, a serious complication of radiation therapy for head and neck malignancies, often occurs from the early stage of radiation therapy and strongly affects a patient's activities of daily living.12 As the radiation exposure dose to the salivary glands increases, the damage progresses and finally becomes irreversible.13 High-dose radiation exposure to the salivary gland increases the incidence of xerostomia and aggravates its severity.14-17 A computer-controlled irradiation technique called intensity-modulated radiation therapy (IMRT) has been introduced to reduce the radiation exposure of salivary glands and minimize radiation exposure to surrounding normal tissues. Eisbruch et al17 reported the utility of IMRT to avoid parotid gland impairment and to contribute to the prevention of xerostomia.17 However, several reports reviewed the relationship between the exposure dose of parotid and submandibular glands and the severity of radiation-induced xerostomia,10,18-20 and it was found that serious xerostomia easily occurred after a larger exposure dose to the submandibular gland. These reports postulated that the xerostomia severity was related to submandibular gland injury. The authors found that poor intraoral moistening lead to the onset of xerostomia, and they reported that when resting saliva secretion dropped to half of its baseline volume, patients experienced xerostomia.9
Seikaly et al21 and Al-Qahtani et al22 reported that the preliminary surgical transplantation of submandibular glands contributed to the prevention of radiation-induced xerostomia. The fact that most resting saliva is supplied from the submandibular gland supports the relationship between submandibular gland hypofunction and xerostomia. Our investigation results also support the relationship between submandibular gland hypofunction and the severity of radiation-induced xerostomia.
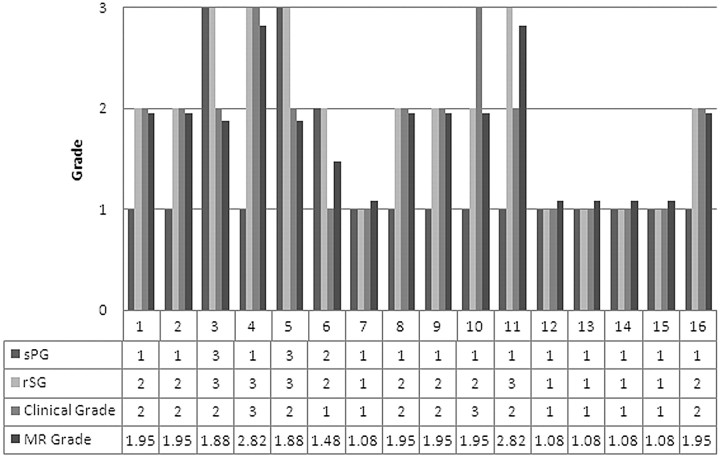
In our regression equation, another factor that explained xerostomia severity was sPG. Moreover, the coefficient showed a negative number (−0.471). In 3 of our 16 patients, the negative coefficient of sPG compensated for a grading error (overestimation) induced by a high grade of rSG (Fig 9). The negative number coefficient of sPG seemed to contribute to compensation for the grading error that came from individual differences of salivation function.
Fig 9.
Grading of the radiation-induced xerostomia of our 16 patients: clinical grade and MR sialography-based grade. In 70% of patients with radiation-induced xerostomia, MR sialography-based xerostomia grading with our regression equation is consistent with clinical grade (xerostomia grade = 0.681 + 0.871rSG − 0.471sPG). In patients 3, 5, and 6, the negative coefficient of sPG compensates the MR sialography grading error (overestimation) induced by the high grade of rSG.
In our investigation, preirradiation MR sialography with secretion stimulation could not help predict the severity of radiation-induced xerostomia. Regression equations obtained from postirradiated MR sialography revealed that submandibular gland secretion response is the most influential to radiation-induced xerostomia grading. Our results mean that hypofunction of the irradiated submandibular gland has the strongest influence on the clinical severity of xerostomia. In radiation therapy for head and neck malignancies, protection of the submandibular glands from excessive radiation exposure is suggested to be important for the prevention of radiation-induced xerostomia. To protect the submandibular glands from excessive irradiation, development of new irradiation techniques and new specific protection drugs are expected.
Conclusions
MR sialography with secretion stimulation can be a valuable tool to evaluate radiation-induced salivary gland disturbance. Hypofunction of the irradiated submandibular gland has the strongest influence on the clinical severity of xerostomia. For prevention of radiation-induced xerostomia, protection of the submandibular gland from excessive irradiation is highly important.
References
- 1.Ship JA. Diagnosing, managing, and preventing salivary gland disorders. Oral Dis 2002;8:77–89 [DOI] [PubMed] [Google Scholar]
- 2.Vissink A, Burlage FR, Spijkervet FK, et al. Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med 2003;14:213–25 [DOI] [PubMed] [Google Scholar]
- 3.Maes A, Colevas A, Setser A, et al. Common toxicity criteria (CTC) version 3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–78 [DOI] [PubMed] [Google Scholar]
- 4.Manashil GB. Clinical Sialography. Springfield, Ill: Charles C. Thomas;1978
- 5.Lomas DJ, Carroll NR, Johnson G, et al. MR sialography: work in progress. Radiology 1996;200:129–33 [DOI] [PubMed] [Google Scholar]
- 6.Jager L, Menauer F, Holzknecht N, et al. Sialolithiasis: MR sialography of the submandibular duct—an alternative to conventional sialography and US? Radiology 2000;216:665–71 [DOI] [PubMed] [Google Scholar]
- 7.Kalinowski M, Heverhagen JT, Rehberg E, et al. Comparative study of MR sialography and digital subtraction sialography for benign salivary gland disorders. AJNR Am J Neuroradiol 2002;23:1485–92 [PMC free article] [PubMed] [Google Scholar]
- 8.Tonami H, Ogawa Y, Matoba M, et al. MR sialography in patients with Sjogren syndrome. AJNR Am J Neuroradiol 1998;19:1199–203 [PMC free article] [PubMed] [Google Scholar]
- 9.Dawes C. Physiological factors affecting salivary flow rate, oral sugar clearance, and the sensation of dry mouth in man. J Dent Res 1987;(66 spec no):648–53 [DOI] [PubMed]
- 10.Berk LB, Shivnani AT, Small W Jr. Pathophysiology and management of radiation-induced xerostomia. J Support Oncol 2005;3:191–200 [PubMed] [Google Scholar]
- 11.Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci 1993;694:72–77 [DOI] [PubMed] [Google Scholar]
- 12.Wescott WB, Mira JG, Starcke EN, et al. Alterations in whole saliva flow rate induced by fractionated radiotherapy. AJR Am J Roentgenol 1978;130:145–49 [DOI] [PubMed] [Google Scholar]
- 13.Eisbruch A, Ten Haken RK, Kim HM, et al. Dose volume and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys 1999;45:577–87 [DOI] [PubMed] [Google Scholar]
- 14.Franzen L, Funegard U, Ericson T, et al. Parotid gland function during and following radiotherapy of malignancies in the head and neck: a consecutive study of salivary flow and patient discomfort. Eur J Cancer 1992;28:457–62 [DOI] [PubMed] [Google Scholar]
- 15.Roesink JM, Moerland MA, Battermann JJ, et al. Quantitative dose-volume response analysis of changes in parotid gland function after radiotherapy in the head-and-neck region. Int J Radiat Oncol Biol Phys 2001;51:938–46 [DOI] [PubMed] [Google Scholar]
- 16.Radfar L, Sirois DA. Structural and functional injury in minipig salivary glands following fractionated exposure to 70 Gy of ionizing radiation: an animal model for human radiation-induced salivary gland injury. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;96:267–74 [DOI] [PubMed] [Google Scholar]
- 17.Eisbruch A, Ship JA, Dawson LA, et al. Salivary gland sparing and improved target irradiation by conformal and intensity modulated irradiation of head and neck cancer. World J Surg 2003;27:832–37 [DOI] [PubMed] [Google Scholar]
- 18.Jellema AP, Doornaert P, Slotman BJ, et al. Does radiation dose to the salivary glands and oral cavity predict patient-rated xerostomia and sticky saliva in head and neck cancer patients treated with curative radiotherapy? Radiother Oncol 2005;77:164–71. Epub 2005 Oct 26 [DOI] [PubMed] [Google Scholar]
- 19.Coppes RP, Vissink A, Konings AW. Comparison of radiosensitivity of rat parotid and submandibular glands after different radiation schedules. Radiother Oncol 2002;63:321–28 [DOI] [PubMed] [Google Scholar]
- 20.Sullivan CA, Haddad RI, Tishler RB, et al. Chemoradiation-induced cell loss in human submandibular glands. Laryngoscope 2005;115:958–64 [DOI] [PubMed] [Google Scholar]
- 21.Seikaly H, Jha N, McGaw T, et al. Submandibular gland transfer: a new method of preventing radiation-induced xerostomia. Laryngoscope 2001;111:347–52 [DOI] [PubMed] [Google Scholar]
- 22.Al-Qahtani K, Hier MP, Sultanum K, et al. The role of submandibular salivary gland transfer in preventing xerostomia in the chemoradiotherapy patient. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:753–56. Epub 2006 Apr 21 [DOI] [PubMed] [Google Scholar]