Abstract
BACKGROUND AND PURPOSE: Atherosclerotic calcifications are present not only in the extracranial carotid bifurcation but also in the intracranial part of the internal carotid artery. We assessed the association between intracranial internal carotid artery calcifications and cardiovascular risk factors in patients with ischemic cerebrovascular disease and the association between calcifications and the presence of this disease.
MATERIALS AND METHODS: Patients undergoing multidetector CT (MDCT) angiography of the carotid arteries for assessment of stenosis degree were included in the study. A semiautomatic custom-made system to quantify calcifications was developed. The associations between the volume of calcifications and cardiovascular risk factors and the type of ischemic cerebrovascular symptoms were assessed with logistic regression.
RESULTS: MDCT angiography was performed in 406 patients (age, 62 ± 14 years; 242 men). Men had a significantly higher calcification volume (66 mm3) than women (33 mm3). Calcification volume was positively associated with age in both men and women. Smoking, hypercholesterolemia, and a history of cardiac disease were independently related to the presence of calcifications. A history of cardiac disease and ischemic cerebrovascular disease were independently related to the volume of calcifications. No association was found between calcifications and the presence or type of ischemic cerebrovascular disease in the vascular territory of the intracranial internal carotid artery.
CONCLUSIONS: Calcifications were associated with higher age and male gender. The presence and volume of calcifications were independently associated with cardiovascular risk factors. Calcifications were not related to the presence or type of ischemic cerebrovascular disease.
Coronary artery calcification, visualized with electron beam or multidetector CT (MDCT) and assessed with the Agatston score, has been the most frequently imaged atherosclerotic plaque feature. Coronary artery calcification reflects the total plaque burden,1 is associated with cardiovascular risk factors,2–5 and is an independent risk factor for future ischemic cardiac and cerebral events.2,6,7 Although atherosclerotic calcifications in the intracranial internal carotid arteries are very frequent, to our knowledge, their association with cardiovascular risk factors and their predictive value for ischemic cerebrovascular events have not been studied extensively.
The Agatston score is calculated as the product of the area of a calcified lesion, defined as the number of voxels with an attenuation value ≥130 Hounsfield units (HU), the size of 1 voxel, and a factor assigned according to the maximum attenuation value of the lesion.2 This score can be calculated semiautomatically when the atherosclerotic calcifications are surrounded by soft tissue, as in the coronary arteries and at the carotid bifurcation. However, the Agatston score cannot be applied semiautomatically to calcifications in the intracranial internal carotid artery because the close relationship between calcifications in the arterial wall and the bony structures of the skull base prohibits an easy segmentation of the calcifications based on HU. Consequently, previous studies made use of a qualitative grading system.8–12 Although Taoka et al13 used commercially available software to assess the Agatston score of intracranial calcifications, they had to eliminate the contamination of bone attenuation on wide-windowed CT images. We developed custom-made software for quantification of intracranial calcifications.
The purpose of this study was to evaluate the reproducibility of a semiautomatic system for quantification of intracranial internal carotid artery calcifications, to assess the association between these calcifications and cardiovascular risk factors in patients with ischemic cerebrovascular disease, and to assess the association between calcifications and the presence and type of ischemic cerebrovascular disease.
Materials and Methods
Study Population
Consecutive patients from the rapid transient ischemic attack (TIA) service and stroke unit of the neurology department, with ischemic cerebrovascular symptoms, underwent full neurologic examinations and recording of their medical history. Patients who were selected for MDCT angiography (MDCTA) of the carotid arteries for assessment of possible carotid artery stenosis were included in this study. Normally, all patients undergo an MDCTA, except those with a major stroke as judged by the treating physician, because they lack a clinical indication for extensive evaluation of the carotid arteries. All scanning was part of a research protocol that was approved by the institutional review board and for which patients had given written informed consent.
Scanning and Image Reconstruction
Scanning was performed on a 16-section MDCT scanner (Sensation 16; Siemens, Erlangen, Germany) with a standardized optimized contrast-enhanced protocol (120 kilovolt [peak]; 180 mAs; collimation, 16 × 0.75 mm; table feed, 12 mm/rotation; pitch, 1).14 The MDCTA scanning range reached from the ascending aorta to the intracranial circulation (2 cm above the sella turcica). All patients received 80-mL contrast material (iodixanol, 320 mg/mL, Visipaque; Amersham Health, Little Chalfont, UK), followed by a 40-mL saline bolus chaser, both with an injection rate of 4 mL/s. Synchronization between the passage of contrast material and data acquisition was achieved by real-time bolus tracking at the level of the ascending aorta.
Image reconstructions were made with an FOV of 100 mm; matrix size, 512 × 512 (real in-plane resolution, 0.6 × 0.6 mm); section thickness, 1.0 mm; increment, 0.6 mm, with an intermediate reconstruction algorithm.15
Analysis of Intracranial Internal Carotid Artery Calcifications
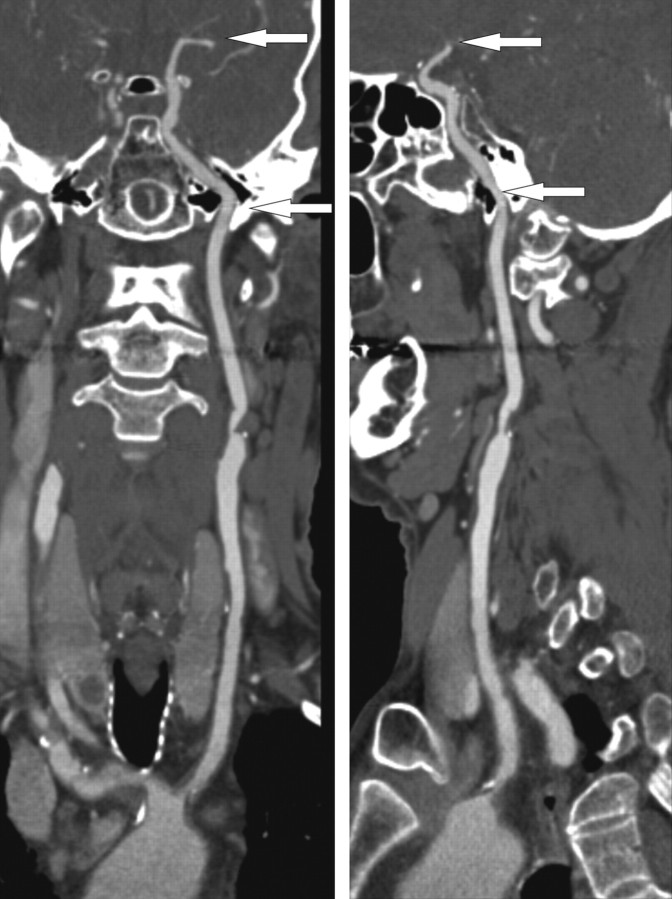
A trained reader (H.C.) who was blinded to the clinical data of the patients performed the quantification of the calcifications in both intracranial internal carotid arteries. A second trained reader (T.T.d.W.) evaluated independently 100 patients to assess interobserver variability. The intracranial internal carotid artery comprised the horizontal segment of the petrous internal carotid artery to the top of the internal carotid artery (Fig 1).
Fig 1.
A curved planar reformatted image from the aortic arch up to the top of the internal carotid artery. The arrows indicate the part of the internal carotid artery at which calcifications are segmented on axial sections. This part comprises the horizontal segment of the petrous internal carotid artery to the top of the carotid artery.
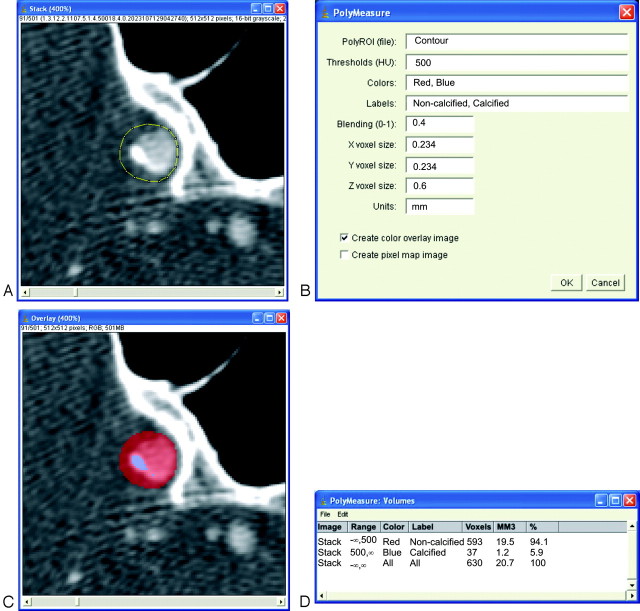
The volume of calcifications was measured with the custom-made polymeasure plug-in for the freely available software ImageJ (developed by Wayne Rasband; National Institute of Mental Health, Bethesda, Md; available at: http://rsb.info.nih.gov/ij). This plug-in made it possible for an observer to draw polygonal regions of interest in consecutive axial MDCTA images and to calculate automatically the total number of pixels above a predefined HU value within the region of interest (Fig 2). The threshold value for calcification was set at 500 HU, which is above the normally used value of 130 HU for the Agatston score.2 This threshold was chosen to enable an automatic differentiation between contrast material in the lumen, which was <500 HU,14 and calcifications in the vessel wall. In case a calcification was close to the skull base, care was taken to delineate the calcification and to include it in the region of interest (Fig 3). The volume of calcifications was calculated by multiplying the number of pixels above the threshold (500 HU), the pixel size, and the increment.
Fig 2.
A, Regions of interest (region of interest) are drawn on axial images that show calcifications. Care is taken to include the whole calcification and not to include any other high-attenuation structures (eg, skull base). Because a minimum attenuation of 500 HU is defined for the presence of calcifications, lumen can be included in this region of interest, for lumen will not reach such a high level of attenuation. All regions of interest of 1 patient are saved within 1 file. B, The polymeasure software uses the contour file, the 500-HU threshold, and the voxel dimensions (0.234 × 0.234 × 0.6 mm) to create a color overlay image and a statistics table. C, The color overlay image shows which pixels within the region of interest are above the predefined 500-HU threshold (blue) and which are below this threshold (red). D, The statistics table presents the amount of calcium pixels and the calcium volume (cubic millimeters).
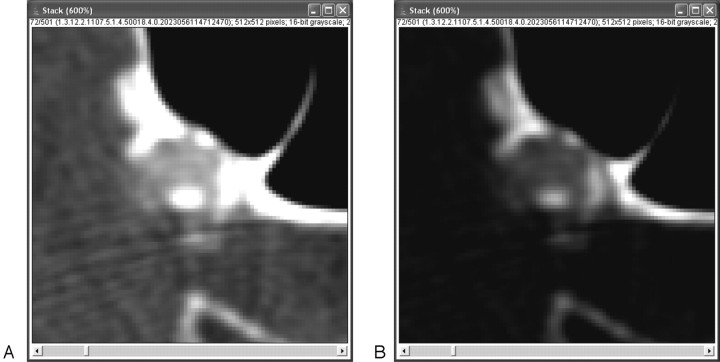
Fig 3.
A, Where calcifications merge with the skull base, it is hard to define the delineation line. B, Changing the window-level setting can then be of help, but observers might still disagree about the exact position of the delineation line. However, the low observer variability assessed in the present study shows that such disagreement only minimally influences total quantification results. Furthermore, the merging of calcifications and skull base prohibits, up to now, fully automated quantification.
Cardiovascular Risk Factors
Clinical measures and information on risk factors and medication were obtained at admission to the hospital or, in cases in which measurements at this time point could be biased (eg, blood pressure at admission), they were derived from clinical history or obtained at discharge. Subjects were categorized as ever (including present) or never smokers. Hypertension was defined as a mean systolic blood pressure >140 mm Hg and/or mean diastolic blood pressure >90 mm Hg during 2 episodes of at least 15 minutes of continuous noninvasive blood pressure measurement or as treatment with antihypertensive medication (ie, angiotensin converting enzyme inhibitors, calcium antagonists, beta-blockers, and diuretics). Hypercholesterolemia was defined as fasting cholesterol >5.0 mmol/L or as treatment with cholesterol-lowering drugs. Diabetes was defined as fasting serum glucose levels >7.9 mmol/L, nonfasting serum glucose levels >11.0 mmol/L, or the use of antidiabetic medication. Information on a history of cardiovascular disease (defined as a clinical diagnosis of myocardial infarction, atrial fibrillation, angina pectoris, chronic heart failure, or coronary artery bypass grafting) and a history of ischemic cerebrovascular disease (defined as a clinical diagnosis of amaurosis fugax, TIA, or ischemic stroke) was collected.
Symptoms
Amaurosis fugax was defined as a sudden loss of vision of presumed vascular origin and confined to 1 eye. TIA was defined as a sudden focal neurologic deficit that was presumed to be of vascular origin and was confined to an area of the brain perfused by a specific artery and that was resolved within 24 hours. In addition, no relevant infarct (1 that explains the deficit) was visible on the CT scan. An ischemic stroke was defined as a sudden focal neurologic deficit that lasted >24 hours or that was accompanied by a relevant infarct on the CT scan.
Statistics
Data are presented as mean ± SD. Baseline characteristics between men and woman were evaluated for differences. Interobserver differences in volume measurements were presented with an intraclass correlation coefficient, a coefficient of variation (defined by the SD of the paired difference divided by the mean of the absolute value), and a Bland-Altman plot. Differences between categoric data and continuous data were analyzed with a χ2 test and a Mann-Whitney U or Student t test, respectively. The Spearman correlation coefficient was used to analyze the association between age and calcifications for men and women. Logistic regression was used to evaluate the association between cardiovascular risk factors and calcifications. The presence of calcifications (left and right summated) was compared with the absence of calcifications, and the highest quartile of calcification was compared with the lower 3 quartiles. First, the regression analysis was adjusted only for age and sex. Second, additional adjustments were made for all risk factors.
The volume of intracranial calcifications in the left and right and in symptomatic and asymptomatic internal carotid arteries was compared with a paired t test. The association between the volume of intracranial calcifications in the symptomatic internal carotid artery and the type of cerebral vascular disease was assessed with logistic regression analysis with adjustment for age, sex, and cardiovascular risk factors.
Results
A total of 406 patients with amaurosis fugax, TIA, or minor ischemic stroke was analyzed. Patient characteristics are shown in Table 1. There were no significant differences between men and women in age, symptomatic artery, and ischemic cerebrovascular symptoms. However, men were more often smokers and had more often experienced cardiac disease; women had more often experienced hypercholesterolemia.
Table 1:
Baseline characteristics of the study population
| Patients | Men | Women | P Value | |
|---|---|---|---|---|
| No. | 406 | 242 (60%) | 164 (40%) | |
| Age (mean ± SD, yr) | 62 ± 14 | 62 ± 13 | 62 ± 14 | .57 |
| Symptomatic artery | ||||
| Carotid | 351 (86%) | 212 (88%) | 139 (85%) | .41 |
| Vertebrobasilar | 55 (14%) | 30 (12%) | 25 (15%) | |
| Cerebrovascular symptoms | ||||
| Amaurosis fugax | 84 (21%) | 50 (21%) | 34 (21%) | .99 |
| TIA | 122 (30%) | 72 (30%) | 50 (30%) | .87 |
| Minor stroke | 200 (49%) | 120 (50%) | 80 (49%) | .87 |
| Risk factors | ||||
| Smoking | 197 (49%) | 136 (56%) | 61 (37%) | <.01 |
| Hypertension | 290 (71%) | 177 (73%) | 113 (69%) | .35 |
| Diabetes | 61 (15%) | 38 (16%) | 23 (14%) | .64 |
| Hypercholesterolemia | 319 (79%) | 177 (73%) | 142 (87%) | <.01 |
| History of cardiac disease | 107 (26%) | 73 (30%) | 34 (21%) | .03 |
| History of cerebrovascular disease | 105 (26%) | 70 (29%) | 35 (21%) | .09 |
| Calcifications | ||||
| Presence | 263 (65%) | 171 (71%) | 92 (56%) | <.01 |
| Volume (mm3) | 53 ± 114 | 66 ± 124 | 33 ± 49 | <.01 |
Note:—TIA indicates transient ischemic attack.
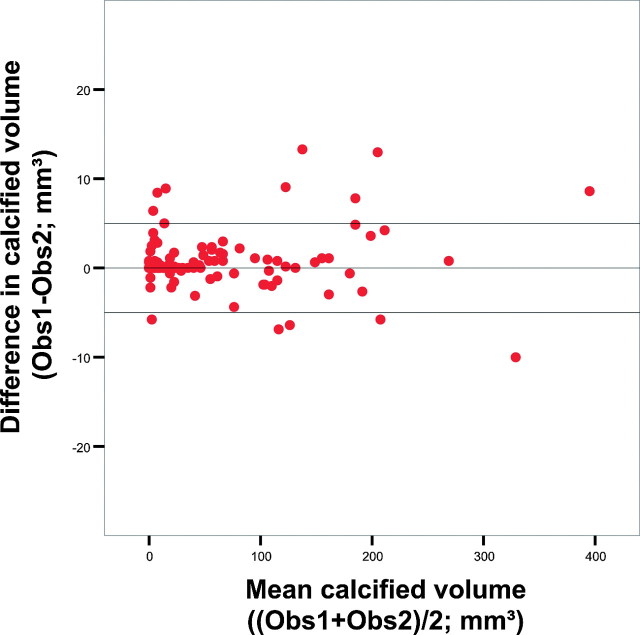
The intraclass correlation coefficient for the assessment of intracranial calcifications was excellent (0.99), and the coefficient of variation for the interobserver differences was very low (7%). Absolute differences between observers were low and did not depend on the size of the calcifications (Fig 4).
Fig 4.
Bland-Altman plots of calcium volume assessed by 2 observers (Obs1 and Obs2). The horizontal lines express the mean difference and the mean difference ± 2 SDs.
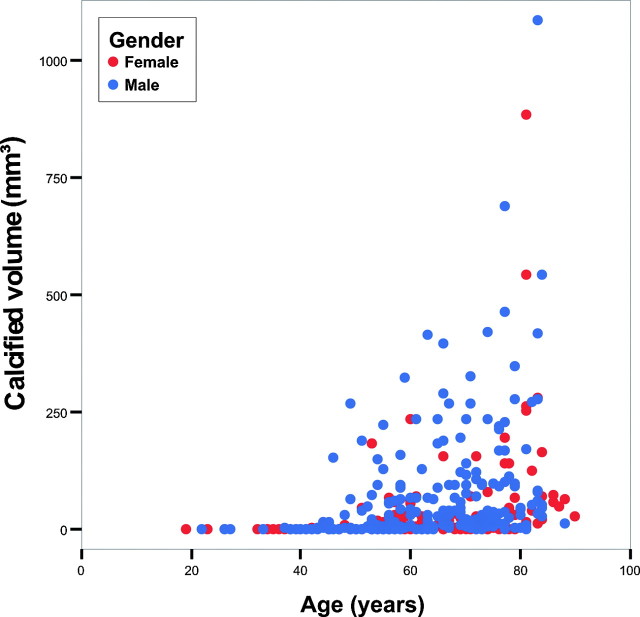
Intracranial internal carotid artery calcifications were more frequent in men than in women (71% and 56%, respectively; P = .003), and they were larger in men (66 ± 124 mm3 and 33 ± 91 mm3, respectively; P < .001). Figure 5 shows the association between age and calcifications for both men and women with a Spearman ρ of 0.548 (P < .001) for men and 0.501 (P < .001) for women and 0.531 (P < .001) for men and women together.
Fig 5.
Scatterplot of calcium volume versus age for men and women separately. The correlation coefficient for men is 0.548 (P < .001), and for women, 0.501 (P < .001).
Table 2 presents the age- and sex-adjusted and multivariate-adjusted odds ratios for the associations between cardiovascular risk factors and intracranial calcium volume. Smoking, hypercholesterolemia, and a history of cardiac disease were independently associated with the presence of intracranial internal carotid artery calcifications. A history of cardiac disease and a history of ischemic cerebrovascular disease were independently associated with the highest quartile of intracranial calcium volume.
Table 2:
The association between risk factors and the presence of calcifications in the intracranial internal carotid artery
| Variable | Odds Ratios Adjusted for Age and Sex |
Odds Ratios Adjusted for Age, Sex, and All Risk Factors |
||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| A | ||||
| Smoking (ever) | 1.7 (1.0–2.8) | .04 | 1.9 (1.1–3.3) | .01 |
| Hypertension | 1.8 (1.1–3.1) | .03 | 1.6 (0.9–2.8) | .11 |
| Hypercholesterolemia | 2.0 (1.1–3.8) | .02 | 1.9 (1.0–3.7) | .04 |
| Diabetes | 1.7 (0.8–3.4) | .15 | 1.6 (0.8–3.4) | .21 |
| History of cardiac disease | 2.3 (1.3–4.2) | <.01 | 2.1 (1.1–4.0) | .02 |
| History of cerebrovascular disease | 1.2 (0.7–2.0) | .59 | 1.0 (0.6–1.9) | .93 |
| B | ||||
| Smoking (ever) | 1.5 (0.9–2.5) | .14 | 1.4 (0.8–2.4) | .20 |
| Hypertension | 1.2 (0.6–2.1) | .63 | 1.0 (0.5–1.8) | .90 |
| Hypercholesterolemia | 1.7 (0.9–3.3) | .11 | 1.7 (0.9–3.4) | .13 |
| Diabetes | 1.4 (0.7–2.6) | .33 | 1.3 (0.7–2.6) | .44 |
| History of cardiac disease | 2.2 (1.3–3.7) | <.01 | 2.0 (1.2–3.5) | .01 |
| History of cerebrovascular disease | 2.0 (1.2–3.4) | .01 | 1.9 (1.1–3.3) | 0.03 |
Note:—CI indicates confidence interval; A, presence of calcifications versus absence; B, upper quartile versus lower 3 quartiles.
There was no significant difference between the intracranial calcium volumes of the left and right carotid arteries (P = .97). Table 3 provides the intracranial calcium volume for the symptomatic and contralateral asymptomatic internal carotid artery and the intracranial calcium volume in the symptomatic internal carotid arteries of patients with amaurosis fugax, TIA, and minor ischemic stroke. No significant differences were found in intracranial calcium volumes at the symptomatic and asymptomatic side (P = .73). In a logistic regression analysis adjusted for age, sex, and all risk factors, intracranial calcifications were not related to the type of symptoms (amaurosis fugax versus TIA or minor stroke).
Table 3:
Calcium volumes (mean ± SD) in the left and right internal carotid arteries, the symptomatic and asymptomatic internal carotid arteries, and the symptomatic artery of patients with amaurosis fugax, TIA, and minor ischemic stroke
| No. | Calcifications (mm3) | |
|---|---|---|
| Left internal carotid artery | 406 | 26 ± 60 |
| Right internal carotid artery | 406 | 26 ± 57 |
| Symptomatic internal carotid artery | 351 | 28 ± 64 |
| Asymptomatic internal carotid artery | 351 | 28 ± 59 |
| Vertebrobasilar symptoms | 2 × 55 | 16 ± 31 |
| Amaurosis fugax | 84 | 20 ± 48 |
| TIA | 97 | 23 ± 55 |
| Minor ischemic stroke | 170 | 34 ± 74 |
Discussion
This study shows that a dedicated custom-made software tool can reproducibly quantify intracranial internal carotid artery calcifications. Furthermore, it shows that the presence of intracranial calcifications is associated with smoking, hypercholesterolemia, and history of cardiac disease and that the severity of calcifications is related to a history of cardiac disease and a history of ischemic cerebrovascular disease.
No difference was found in the volume of intracranial calcifications between the symptomatic and asymptomatic internal carotid arteries and in the volume of intracranial calcifications in the symptomatic artery between patients with amaurosis fugax and those with TIA or minor stroke.
Previous studies8–12 had a binary or a qualitative (5-point scale) grading system for classification of intracranial calcifications, probably because quantification difficulties arose due to the close relationship between calcifications and the bony structures of the skull. Taoka et al13 were able to use commercially available software for quantification of intracranial calcifications, but they had to eliminate the contamination of bone attenuation on wide-windowed CT images. We dealt with this problem by developing a custom-made software tool that allowed manual segmentation of intracranial calcifications without contamination of the bony skull, and we assessed the volume of calcifications instead of the Agatston score. Because this quantification method was HU-based, the manual outline of the border between calcifications and the skull was the only time-consuming part. However, this border was, in most cases, clearly visible, enabling the segmentation per patient to be done relatively quickly (<15 minutes). Observer differences were caused by the differences in the manual outline of the border between calcifications and skull. The very low observer variability indicates that observers did recognize the same hyperattenuated areas as possible calcifications and that their manual outlines between calcifications and skull were very similar.
Our study in patients with ischemic cerebrovascular symptoms and a mean age of 73 years revealed a prevalence of intracranial internal carotid artery calcifications of 65%. Age, sex, smoking, hypercholesterolemia, a history of cardiac disease, and a history of ischemic cerebrovascular disease were independently related to the presence or volume of intracranial internal carotid artery calcifications. Associations between cardiovascular risk factors and intracranial calcifications have been studied in patients referred for brain CT.10–12 One study had a prevalence of 36% in a group of patients with a mean age of 51 years,11 whereas the second study found a prevalence of 67% in a group of patients with a mean age of 63 years.10 Both studies confirmed the association between age and calcifications, an association that was also found in coronary artery studies.2
The present study found a higher prevalence and volume of calcifications in men, a finding that is in agreement with studies on coronary arteries16,17 and carotid bifurcations.17 One study found an independent association between sex and intracranial internal carotid artery calcifications,11 whereas another study did not find this association.10
In the present study, we found that smoking and hypercholesterolemia are independently associated with the presence of intracranial calcifications. Other studies showed independent associations of smoking with coronary, aortic arch, carotid bifurcation calcifications4,5,18,19 and intracranial internal carotid artery calcifications.11 Hoff et al5 found, in a large study among asymptomatic individuals, an independent association of hypercholesterolemia with coronary calcification in both men and women. However, a study by Allison et al3 examining risk factors for coronary, proximal aorta, and carotid calcifications found no independent associations with hypercholesterolemia. An independent association between hypertension and coronary calcifications,3–5,18 aortic arch calcifications,18 and carotid bifurcation calcifications3 has been described; however, the present study did not find an association between hypertension and the presence of intracranial carotid calcifications or an association between the presence of calcifications and diabetes, as is known from coronary literature.6 A possible explanation for the absence of such associations could be the fact that we are dealing with a fundamentally different pathophysiology. In the intracranial arteries, atherosclerotic calcifications normally develop within the vessel wall, with little noncalcified atherosclerotic components and little luminal compromise, whereas in the coronary arteries, the calcified parts in the plaque are accompanied by extensive noncalcified components and considerable luminal narrowing.
Internal carotid artery calcifications might be an indicator of arterial stenosis.13,20 Stenosis in the intracranial internal carotid artery carries an increased risk of stroke.21,22 Therefore, we compared the volume of calcifications in the symptomatic and asymptomatic internal carotid arteries; however, no difference in intracranial calcium volume was found between the symptomatic and asymptomatic side. This is in agreement with Babiarz et al,8,9 who found no differences in the scores ipsilateral and contralateral to the stroke. In addition, in 2 cross-sectional studies,8,9 they found that intracranial internal carotid artery calcifications were not independently related to the extent of white matter lesions or the presence of stroke. In a follow-up study of 72 patients, Taoka et al13 did not find an association between the intracranial internal carotid artery calcifications score and the later occurrence of stroke. Therefore, they concluded that intracranial calcifications should not be considered as the cause of an ischemic event by being a source of embolism or thrombosis, but they should be considered as a marker of systemic atherosclerotic disease, which is closely related to stroke.
A limitation of the present study was that it is still labor-intensive to draw a region of interest on each image that shows an intracranial internal carotid artery calcification; therefore, a tool should be developed that locates the calcification and quantifies it automatically. A second limitation is the lack of a gold standard. The accuracy of MDCTA-based assessment of the presence and volume of intracranial internal carotid artery calcifications could, therefore, not be established. However, the ability to determine and quantify calcifications accurately has been proved in other vascular beds.2,23 Another limitation is that the calcium volume in the intracranial contralateral carotid artery of patients with ischemic cerebrovascular symptoms has been used in the analysis as the asymptomatic volume, instead of the calcium volume in the intracranial carotid arteries of completely asymptomatic patients. Finally, we could not, due to ethical reasons, include patients with severe stroke especially because their data would contain relevant information on who was at risk of developing a severe stroke.
This study showed that, with custom-made software, quantitative evaluation is reproducible. Therefore, follow-up studies should explore the association between the presence and volume of intracranial internal carotid artery calcifications and (recurrent) ischemic cerebrovascular events. Furthermore, these studies should assess the additional value of this measure over commonly used parameters in the prediction of ischemic cerebrovascular events, as well as its value as a marker of systemic atherosclerotic disease.
Conclusions
Our study showed that intracranial internal carotid artery calcifications can reproducibly be quantified with a dedicated custom-made software tool and that age, sex, smoking, hypercholesterolemia, a history of cardiac disease, and a history of ischemic cerebrovascular disease are independently related to the presence or volume of arterial calcifications. Furthermore, we showed that the degree of calcifications does not differ between the symptomatic and contralateral asymptomatic arteries and that calcifications are not related to presence or type of ischemic cerebrovascular disease.
Footnotes
Aad van der Lugt is recipient of a fellowship from the Netherlands Organization for Health Research and Development (NWO-KF Grant No. 907-00-122).
References
- 1.Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area: a histopathologic correlative study. Circulation 1995;92:2157–62 [DOI] [PubMed] [Google Scholar]
- 2.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32 [DOI] [PubMed] [Google Scholar]
- 3.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol 2004;24:331–36 [DOI] [PubMed] [Google Scholar]
- 4.Allison MA, Wright CM. Age and gender are the strongest clinical correlates of prevalent coronary calcification (R1). Int J Cardiol 2005;98:325–30 [DOI] [PubMed] [Google Scholar]
- 5.Hoff JA, Daviglus ML, Chomka EV, et al. Conventional coronary artery disease risk factors and coronary artery calcium detected by electron beam tomography in 30,908 healthy individuals. Ann Epidemiol 2003;13:163–69 [DOI] [PubMed] [Google Scholar]
- 6.Arad Y, Goodman KJ, Roth M, et al. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol 2005;46:158–65 [DOI] [PubMed] [Google Scholar]
- 7.Vliegenthart R, Hollander M, Breteler MM, et al. Stroke is associated with coronary calcification as detected by electron-beam CT: the Rotterdam Coronary Calcification Study. Stroke 2002;33:462–66 [DOI] [PubMed] [Google Scholar]
- 8.Babiarz LS, Yousem DM, Wasserman BA, et al. Cavernous carotid artery calcification and white matter ischemia. AJNR Am J Neuroradiol 2003;24:872–77 [PMC free article] [PubMed] [Google Scholar]
- 9.Babiarz LS, Yousem DM, Bilker W, et al. Middle cerebral artery infarction: relationship of cavernous carotid artery calcification. AJNR Am J Neuroradiol 2005;26:1505–11 [PMC free article] [PubMed] [Google Scholar]
- 10.Chen XY, Lam WW, Ng HK, et al. The frequency and determinants of calcification in intracranial arteries in Chinese patients who underwent computed tomography examinations. Cerebrovasc Dis 2006;21:91–97 [DOI] [PubMed] [Google Scholar]
- 11.Ptak T, Hunter GH, Avakian R, et al. Clinical significance of cavernous carotid calcifications encountered on head computed tomography scans performed on patients seen in the emergency department. J Comput Assist Tomogr 2003;27:505–09 [DOI] [PubMed] [Google Scholar]
- 12.Sohn YH, Cheon HY, Jeon P, et al. Clinical implication of cerebral artery calcification on brain CT. Cerebrovasc Dis 2004;18:332–37 [DOI] [PubMed] [Google Scholar]
- 13.Taoka T, Iwasaki S, Nakagawa H, et al. Evaluation of arteriosclerotic changes in the intracranial carotid artery using the calcium score obtained on plain cranial computed tomography scan: correlation with angiographic changes and clinical outcome. J Comput Assist Tomogr 2006;30:624–28 [DOI] [PubMed] [Google Scholar]
- 14.de Monye C, Cademartiri F, de Weert TT, et al. Sixteen-detector row CT angiography of carotid arteries: comparison of different volumes of contrast material with and without a bolus chaser. Radiology 2005;237:555–62 [DOI] [PubMed] [Google Scholar]
- 15.de Weert TT, Ouhlous M, Zondervan PE, et al. In vitro characterization of atherosclerotic carotid plaque with multidetector computed tomography and histopathological correlation. Eur Radiol 2005;15:1906–14 [DOI] [PubMed] [Google Scholar]
- 16.Janowitz WR, Agatston AS, Kaplan G, et al. Differences in prevalence and extent of coronary artery calcium detected by ultrafast computed tomography in asymptomatic men and women. Am J Cardiol 1993;72:247–54 [DOI] [PubMed] [Google Scholar]
- 17.Odink AE, van der Lugt A, Hofman A, et al. Association between calcification in the coronary arteries, aortic arch and carotid arteries: the Rotterdam study. Atherosclerosis 2007;193:408–13. Epub 2006 Aug 21 [DOI] [PubMed] [Google Scholar]
- 18.Iribarren C, Sidney S, Sternfeld B, et al. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA 2000;283:2810–15 [DOI] [PubMed] [Google Scholar]
- 19.Reilly MP, Wolfe ML, Localio AR, et al. Coronary artery calcification and cardiovascular risk factors: impact of the analytic approach. Atherosclerosis 2004;173:69–78 [DOI] [PubMed] [Google Scholar]
- 20.Woodcock RJ Jr, Goldstein JH, Kallmes DF, et al. Angiographic correlation of CT calcification in the carotid siphon. AJNR Am J Neuroradiol 1999;20:495–99 [PMC free article] [PubMed] [Google Scholar]
- 21.Craig DR, Meguro K, Watridge C, et al. Intracranial internal carotid artery stenosis. Stroke 1982;13:825–28 [DOI] [PubMed] [Google Scholar]
- 22.Marzewski DJ, Furlan AJ, St Louis P, et al. Intracranial internal carotid artery stenosis: longterm prognosis. Stroke 1982;13:821–24 [DOI] [PubMed] [Google Scholar]
- 23.de Weert TT, Ouhlous M, Meijering E, et al. In vivo characterization and quantification of atherosclerotic carotid plaque components with multidetector computed tomography and histopathological correlation. Arterioscler Thromb Vasc Biol 2006;26:2366–72 [DOI] [PubMed] [Google Scholar]