Abstract
BACKGROUND AND PURPOSE: Wernicke encephalopathy (WE) is a severe neurologic disorder resulting from dietary vitamin B1 deficiency. This study was undertaken to analyze and compare MR imaging findings and neurologic manifestations at clinical presentations of patients with WE with and without a history of alcohol abuse.
MATERIALS AND METHODS: WE patients were identified using diagnostic neurologic data bases. Fifty-six patients (29 females, 27 males) diagnosed between 1999 and 2008 with WE who improved within 1 month from the onset of thiamine administration were included in the analysis. Patients’ records were reviewed for clinical manifestations and imaging studies’ findings. MR imaging was performed in the acute phase of the disease at a field strength of 1T (16 patients) and 1.5T (40 patients). All MR images were of acceptable to good quality and were retrospectively reviewed. We compared imaging findings and clinical presentation in the alcoholic (AL) group versus the non-alcoholic (NA) group using the 2-tailed Fisher exact test and the Phi coefficient as appropriate.
RESULTS: Forty-three percent of the patients were in the AL group, whereas 57% were in the NA group. Eighty-nine percent showed changes in consciousness, 75% had ocular manifestations, and 54% had ataxia. On MR imaging, 80% of the patients had evidence of symmetric lesions in the medial thalami and in the periventricular region of the third ventricle; 59%, in the periaqueductal area; 45%, in the mamillary bodies; 36%, in the tectal plate; and 7%, in the periventricular gray matter located anteriorly to the fourth ventricle. Signal-intensity alterations in areas considered atypical for the disease were noted only in the NA group and always in association with the typical findings. Contrast enhancement of the thalamus and mamillary bodies was significantly associated with alcohol abuse.
CONCLUSIONS: Contrast enhancement in the mamillary bodies and thalamus is a typical finding of the disease in AL patients. Atypical MR imaging findings characterize NA patients.
Wernicke encephalopathy (WE) is an acute neurologic disorder resulting from thiamine (vitamin B1) deficiency. The disease is characterized by changes in consciousness, ocular dysfunction, and gait disturbances. This classic triad has been reported in 16%–38% of patients.1–3 The incidence of WE is underestimated in both adult and pediatric patients. MR imaging usually shows symmetric signal-intensity alterations in the thalami, mamillary bodies, tectal plate, and the periaqueductal area.3 Signal-intensity alterations in the cerebellum, cerebellar vermis, cranial nerve nuclei (CNN), red nuclei, dentate nuclei, caudate nuclei, splenium, and cerebral cortex represent atypical MR imaging findings.3,4–19 The prognosis of patients with WE depends on the time of onset of thiamine supplementation. When WE is unrecognized, Korsakoff psychosis and even death may ensue; therefore, neuroimaging plays a central role in the early stage of the disease. A previous study from our group on 26 alcoholic (AL) and nonalcoholic (NA) patients with WE showed that 85% of the patients had symmetric lesions in the medial thalami and the periventricular region of the third ventricle; 65%, in the periaqueductal area; 58%, in the mamillary bodies; 38%, in the tectal plate; and 8%, in the dorsal medulla.3
The aim of this study was to analyze a larger population of patients from multiple centers to establish whether the results of our previous study could be replicated. A specific aim was to compare the clinical and neuroradiologic data of AL and NA patients, because our previous report had shown some differences between the findings of AL and NA patients.3 For instance, contrast enhancement of the mamillary bodies had been shown to correlate positively with alcohol abuse, whereas atypical MR imaging findings had been found more frequently in NA patients.3 These data suggest that in AL and NA patients with WE, partially different metabolic pathways may be involved, leading to different MR imaging lesions.
Materials and Methods
This study included patients already reported in a previous retrospective study followed up with time and new patients recruited from additional hospital centers.3 Similar to our previous study, it has been designed to compare neuroimaging findings and clinical features of AL and NA patients with WE at presentation. A multicenter study group retrospectively reviewed MR imaging findings and clinical records of 56 patients (29 females, 27 males) diagnosed between 1999 and 2008 with WE. The age range was 6–88 years (mean age, 50.3 ± 17 years). Patients were identified by searching neurologic and neuroradiologic diagnostic data bases. Patients’ records were reviewed for clinical history, symptoms at presentation, imaging techniques, and findings. Inclusion criteria consisted of a clinical diagnosis of WE and improvement at clinical presentation within 1 month from the onset of thiamine administration.
MR imaging examinations were performed during the acute phase of the disease at field strengths of 1T (16 patients) and 1.5T (40 patients). Eleven of 56 (20%) MR imaging examinations showed movement artifacts; nevertheless, the scans were included in the study because they were considered to have acceptable diagnostic quality. Imaging sequences of the brain included long-TR, short-TE spin-echo sequences, and contrast-enhanced short-TR images in multiple planes. We compared imaging findings and clinical presentation in the AL group versus the NA group by using the 2-tailed Fisher exact test. When the test showed a significant association, we calculated the Phi coefficient to determine the strength of the relationship (statistical application package: Statistical Package for the Social Sciences, Version 15; SPSS, Chicago, Ill).
Results
Clinical Histories
Twenty-four (43%) patients affected by WE had a history of chronic alcohol abuse. Thirty-two (57%) patients affected by WE did not. In the NA group, the most frequent cause of thiamine deficiency was malabsorption secondary to a neoplasm of the gastrointestinal tract (14/32 patients, 44%). Among these patients, 4 underwent surgery for gastric cancer. Eleven (34%) patients had hyperemesis (4 with hyperemesis gravidarum, 6 with hyperemesis due to chemotherapy, and 1 with hyperemesis due to a gastrointestinal tract neoplasm). Seven (22%) of 32 patients had severe malnutrition caused by prolonged voluntary starvation (5 patients), anorexia nervosa (1 patient), and socioeconomic poverty (1 pediatric patient).
Neurologic Findings at Clinical Presentation
The most frequent neurologic findings were changes in consciousness in 50/56 patients (89%, 20 AL versus 30 NA). These changes showed a wide spectrum of presentations ranging from mild disorientation to coma. Forty-four patients (79%, 22 AL and 22 NA) showed ocular dysfunction. Thirty patients (54%, 17 AL and 13 NA) showed ataxia. With regard to changes in consciousness, no significant difference was found comparing the AL with the NA group. Ocular dysfunction was significantly more frequent in the AL group (P = .039, Phi = 0.276). Ataxia was significantly more frequent in the AL group (P = .025, Phi = 0.300). Only 24/56 patients (43%, 13 AL and 11 NA) presented with the classic triad of the disease.
Imaging Features
All 56 patients underwent MR imaging. The findings were symmetric hyperintensity on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images; symmetric hypointensity or no abnormalities on T1-weighted images; and symmetric areas of contrast enhancement after gadolinium injection in the thalamus, periventricular region of the third ventricle, mamillary bodies, periaqueductal area, tectal region, periventricular gray matter of the fourth ventricle (typical findings) and in the CNN, cerebellum, and supratentorial brain cortex (atypical findings). Forty-five (80%) patients showed symmetric lesions of the medial thalami and of the periventricular region of the third ventricle (15 AL and 30 NA). Thirty-three (59%) patients showed alterations of the periaqueductal area (11 AL and 22 NA). Twenty-five (45%) patients showed alterations of the mamillary bodies (8 AL and 17 NA). Twenty (36%) patients showed alterations of the tectal plate (3 AL and 17 NA). Ten (18%) NA patients showed symmetric lesions of the CNN (VI, VII, VIII, XII) (Fig 1).
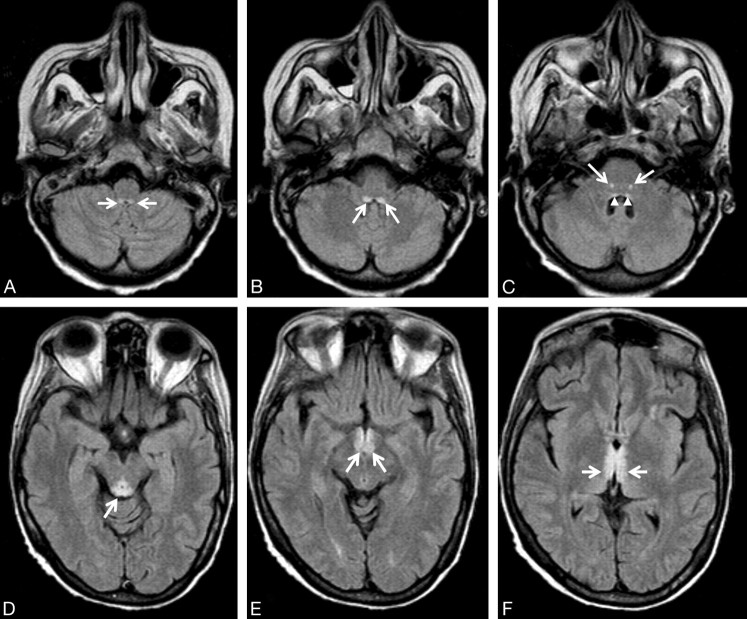
Fig 1.
A 54-year-old woman with leukemia, changes in consciousness, and ataxia. FLAIR axial images (11,000/140/2 [TR/TE/NEX]). A, The prepositus hypoglossal nuclei show symmetric high-signal-intensity alterations (arrows). B, The medial vestibular nuclei show symmetric hyperintense lesions (arrows). C, Symmetric high-signal-intensity alterations in the facial nuclei (arrows) are detected. Subtle signal-intensity alterations in the abducens nuclei are seen (arrowheads). D, The tectum of the midbrain and the periaqueductal gray matter shows signal-intensity alterations (arrow). E, The mamillary bodies (arrows) show signal-intensity alterations. F, Note signal-intensity alterations (arrows) of the medial thalami and periventricular region of the third ventricle.
Four (7%) patients showed signal-intensity alterations in the periventricular gray matter located anterior to the fourth ventricle (1 AL and 3 NA). Three (5%) NA patients showed symmetric alterations of the cerebellum. Two (4%) NA patients showed signal-intensity alterations in the vermis. One (1.8%) NA patient showed bilateral signal-intensity alterations in the dentate nuclei. One (1.8%) NA patient showed bilateral signal-intensity alterations of the pre- and postcentral cortex (Fig 2). Regarding the presence of signal-intensity alterations on unenhanced sequences involving anatomic regions considered typical and atypical for the disease, statistically significant positive associations were found between NA and signal-intensity alterations seen on long-TR spin-echo sequences at the level of thalami (P = .004, Phi = 0.389), tectal plate (P = .002, Phi = 0.420), and CNN (P = .003, Phi = 0.404), respectively.
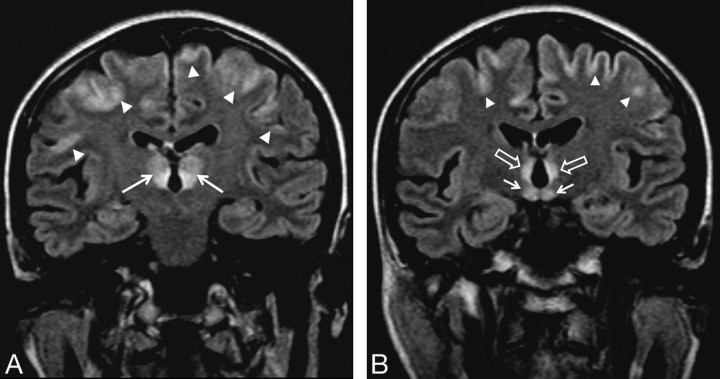
Fig 2.
A 54-year-old woman with a history of food refusal had changes in consciousness. FLAIR coronal images (11,000/140/2 [TR/TE/NEX]). A, Signal-intensity alterations with different intensity patterns are seen in the thalami (arrows). Diffuse signal-intensity alterations of the frontal cortex (arrowheads) are present. B, Note signal-intensity alterations in the mamillary bodies (arrows), periventricular region of the third ventricle (empty arrows), and brain cortex (arrowheads).
Contrast medium was administered in 41/56 (73%) patients (18 AL and 23 NA). Among these patients, 26/41 (63%, 17 AL and 9 NA) showed contrast enhancement. The anatomic structures that most frequently enhanced were the mamillary bodies (16 patients), followed by the tectal plate (8 patients), thalamus (8 patients), periaqueductal area (7 patients), and CNN (1 patient). Three AL patients showed contrast enhancement in the mamillary bodies as the only sign of the disease. Eight AL patients and 1 NA patient showed contrast enhancement but no signal intensity alterations on long-TR spin-echo sequences (P = .002, Phi = 0.481) (Fig 3). There was a significant positive association between AL and contrast enhancement in the mamillary bodies (P = .001, Phi = 0.501).
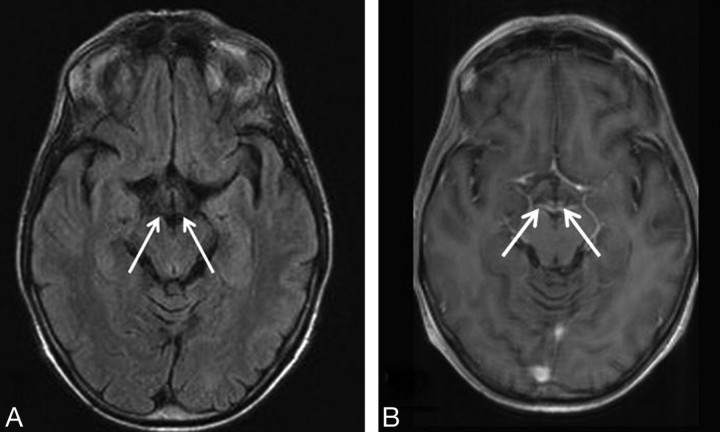
Fig 3.
A 47-year-old woman with a history of alcohol abuse presented with ataxia, changes in consciousness, and ocular abnormalities. A, FLAIR axial image (9000/114/1 [TR/TE/NEX]). No signal-intensity alteration are seen at the mamillary body level (arrows). B, T1-weighted axial image (551/14/2 [TR/TE/NEX]). Contrast enhancement is seen in the mamillary bodies (arrows).
A statistically significant positive association (P = .006, Phi = 0.433) between contrast enhancement in the thalami and AL was also found. A history of hyperemesis and chemotherapy showed a statistically significant positive association with CNN involvement (P = .006, Phi = 0.498; and P = .008, Phi = 0.474, respectively). No patient showed hydrocephalus. Data on histories, clinical manifestations, imaging techniques, and findings in both groups are summarized in On-line Tables 1 and 2. Table 1 shows the topographic distribution of lesions in both groups. Table 2 shows the neurologic symptoms typical for the disease in both groups.
Table 1:
Topographic distribution of the lesions in AL and NA patients with WE†
| Patient Group | Thal (%) | Periaq (%) | Mam Bodies (%) | Tectal Plate (%) | CNN (%) | Fvgm (%) | Cer (%) | DN (%) | Vermis (%) |
|---|---|---|---|---|---|---|---|---|---|
| AL | 63 | 46 | 33 | 13 | 0 | 4 | 0 | 0 | 0 |
| NA | 94* | 68 | 52 | 52* | 32* | 9 | 9 | 3 | 6 |
Note:—Thal indicates medial thalami and periventricular region of the third ventricle; Periaq, periaqueductal gray matter; Mam bodies, mamillary bodies; Fvgm, periventricular gray matter located anterior to the 4th ventricle; CNN, cranial nerve nuclei; Cer, cerebellum; DN, dentate nuclei.
Indicates positive statistical association.
Imaging sequences included long-TR and short-TE spin-echo sequences and contrast-enhanced short-TR images in multiple planes.
Table 2:
Neurologic symptoms at clinical onset in the AL and NA groups
Note:—CC indicates changes in consciousness; OA, ocular abnormalities; AT, ataxia; T, classic triad of the disease.
Indicates positive statistical association.
Discussion
Incidence
WE represents a medical emergency. When untreated, severe neurologic deficits like Korsakoff psychosis and even death may ensue. The incidence of WE is underestimated at all ages. Adult necroscopy studies have revealed a worldwide incidence ranging from 0.5% in Norway to 2.8% in Australia.2,20–23 The incidence observed in adults is approximately equal to that observed in children.24
Thiamine Deficiency
For healthy individuals, the daily thiamine requirement, which is related to carbohydrate intake, is between 1 and 2 mg. Because the reserves of thiamine in the body are on average only 30 to 50 mg, it can be estimated that they would be completely depleted within 4–6 weeks in the absence of adequate thiamine intake. Many clinical conditions may impair the correct absorption of a sufficient amount of thiamine, including chronic alcohol abuse,21,25 gastrointestinal surgery,26–28 prolonged vomiting, chemotherapy, systemic infectious and noninfectious diseases, and dietary imbalance.3 Alcoholism does not directly cause thiamine deficiency, though it may induce such deficiency because of its frequent association with malnourishment. More specifically, the low thiamine absorption rate at the mucosal level, the impaired hepatic function, and the raised alcohol-related thiamine metabolism may lead in combination to the development of chronic thiamine deficiency.25 Thiamine-deficient membranes are unable to maintain osmotic gradients; this inability results in the swelling of intra- and extracellular spaces. In the periventricular regions, the blood-brain barrier is physiologically less tight and there is a high rate of thiamine-related glucose and oxidative metabolism.29
Typical Imaging Findings
In our case series, enhancement (suggestive of damage) at the level of the blood-brain barrier was observed in the AL and NA groups. However, a statistically significant positive correlation was shown only between contrast enhancement in the mamillary bodies and thalami on the one hand and alcohol abuse on the other, supporting the hypothesis that alcohol may contribute to increased blood-brain barrier permeability.30 The correlation between alcohol abuse and damage to the blood-brain barrier is not supported by the results of the retrospective study by Fei et al,11 who concluded that “typical symmetric damage of the mammillary bodies and brain paraventricular regions may permit a specific diagnosis of nonalcoholic WE.” However, the study of Fei et al included only 3 patients who underwent contrast-enhanced MR imaging, and no formal comparison was made between AL and NA patients. In contrast, both this and our previous3 studies have shown that mamillary bodies and thalami are more frequently affected in AL patients; this finding suggests that these regions may be particularly susceptible to the toxic effects of alcohol.
Bilateral symmetric signal intensity alterations seen in WE can also be seen in a host of pathologies.3 However, their frequent association with other MR imaging alterations considered typical of WE may assist in the differential diagnosis.3
Atypical Imaging Findings
Among the neurologic manifestations at presentation, ataxia was positively associated with AL despite the lack of overt cerebellar lesions, whereas infratentorial signal-intensity alterations were only seen in NA patients. Cerebellar alterations were reversible and always associated with the other typical but also atypical findings, in agreement with previous reports.3,5–7,9 We, thus, speculate that in our AL patients, subtle damage to the cerebellum induced by chronic alcohol consumption may have pre-existed.30 Pathologic studies have demonstrated a higher prevalence of cerebellum involvement compared with that observed on imaging studies. More specifically, it has been reported that the cerebellum is involved in more than half of patients with WE.31 There are a few published cases on selective CNN involvement, which have described abducens, facial, vestibular, and hypoglossal nerve nuclei signal-intensity alterations only on long-TR images.3,5,6 These changes have invariably been found in NA patients and in association with the other typical alterations of the disease. So far, the question of whether CNN involvement represents a distinctive pattern of involvement in NA patients has remained unanswered. The results of our study lend support to the hypothesis that CNN involvement is a typical finding of NA patients, by showing a statistically significant association.
Wernicke Encephalopathy Shares Some MR Imaging Features with Metronoidazole-Induced Encephalopathy
The differential diagnosis of symmetric signal-intensity alterations of the dentate nuclei, vestibular, abducens, red nuclei, and splenium includes metronidazole-induced encephalopathy (MIE).32 NA patients with WE may show virtually the same MR imaging features typical of MIE, in addition to those typical of the disease.6,8,16 Therefore, the differential diagnosis may be difficult in malnourished patients treated with metronidazole. None of our NA patients showing alterations in the cerebellum, vermis, and dentate nuclei and CNN involvement had a history of metronidazole treatment, with only the exception of patient 1 (On-line Table 2). This finding raises the question of whether WE and MIE share similar metabolic pathways.
Does Chronic Alcohol Abuse Increase Blood-Brain Barrier Permeability?
In our patient population, we found a total of 8 patients (7 AL and 1 NA) showing contrast enhancement in areas typical of WE in the absence of alterations on long-TR sequences. This pattern has also been described in a pediatric patient, who showed signal-intensity alterations of the medial thalami and periaqueductal gray matter,33 and it may be due to the well-known “fogging effect”34 or to the increased detection of small lesions with contrast-enhanced T1-weighted images compared with T2-weighted ones.35 Weidauer et al36 described the aforesaid effect in 5 of their 7 MR imaging–positive patients with WE; among those showing positive contrast enhancement without alterations on long-TR images, the mamillary bodies were the most frequently affected structures (4 of 5 patients). Thus, it may also be speculated that this phenomenon, which has been frequently found at the level of the mamillary bodies, may be due, to some extent, to a partial volume effect related to the small size of the structures involved, such as the mamillary bodies. These findings support the hypothesis that alcohol may damage the blood-brain barrier. They also suggest that contrast-enhanced MR imaging should be performed when no signs of WE are seen on unenhanced MR images.
Limitations of the Study
The main limitation of our study is its retrospective design, because data have been collected from multiple centers during a 9-year period. In particular, the absence of predefined criteria for diagnosis may have introduced a selection bias. More specifically, some patients with WE may not have been identified due to missed clinical diagnoses, particularly if they had atypical clinical manifestations and no history of alcohol intake. Therefore, these 2 categories of patients may be under-represented. However, despite this limitation, we have still collected a large enough number of NA patients to allow meaningful comparison with AL patients. An additional bias is that MR images have been obtained from units with different field strengths and different diagnostic performances.
Conclusions
The results of our study confirm the usefulness of MR imaging in the early diagnosis of acute WE. Furthermore, we have been able to demonstrate that AL patients with WE appear to differ from NA patients in terms of MR imaging findings. Alcohol abuse may alter the blood-brain barrier, even in absence of long-TR signal-intensity alterations, as revealed by symmetric contrast enhancement in areas typically involved by WE. More specifically, we have observed more frequent contrast enhancement in the mamillary bodies and thalami in AL compared with NA patients, suggesting that these areas may be particularly susceptible to the toxic effects of alcohol. In contrast, we have demonstrated that MR imaging findings usually considered atypical for WE, seem, in fact, to be characteristic of NA patients. The reason why atypical lesions are seen only in NA patients is unclear, but we speculate that alcohol may have a protective effect on the brain areas that show atypical lesions in WE. On the other hand, atypical WE brain lesions resemble those of MIE, a finding that may suggest that WE and MIE share common metabolic pathways. In conclusion, our study provides evidence that MR imaging is useful in diagnosing early WE. MR imaging has a valuable diagnostic role, particularly in those patients with no history of alcohol abuse or presenting with atypical clinical manifestations, in whom the clinical diagnosis is more easily missed. In addition, MR imaging can help to distinguish AL from NA patients with WE.
Acknowledgments
We thank Drs Jaume Capellades, Jessica Mandrioli, Teresa Cabada Giadás, Luana Regnicolo, Renzo Manara, Luca Santelli, Bruno Tumiati, and Walter Bottari for their participation in the Wernicke Encephalopathy Working Group.
Footnotes
Paper previously presented in part at: Annual Meeting of the American Society of Neuroradiology, May 31–June 6, 2008; New Orleans, La.
Indicates article with on-line tables.
References
- 1.Victor M. The Wernicke-Korsakoff syndrome. In: Vinken PJ, Bruyn GW, eds. Handbook of Clinical Neurology. Vol 28, Part II. Amsterdam, the Netherlands: North-Holland Publishing Company;1976. :243–70
- 2.Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry 1986;49:341–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuccoli G, Gallucci M, Capellades J, et al. Wernicke encephalopathy: MR findings in 26 alcoholics and non-alcoholics patients. AJNR Am J Neuororadiol 2007;28:1328–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki S, Ichijo M, Fujii H, et al. Acute Wernicke's encephalopathy: comparison of magnetic resonance images and autopsy findings. Intern Med 1996;35:831–34 [DOI] [PubMed] [Google Scholar]
- 5.Bae SJ, Lee HK, Lee JH, et al. Wernicke's encephalopathy: atypical manifestation at MR imaging. AJNR Am J Neuroradiol 2001;22:1480–82 [PMC free article] [PubMed] [Google Scholar]
- 6.Zuccoli G, Motti L. Atypical Wernicke's encephalopathy showing lesions in the cranial nerve nuclei and cerebellum. J Neuroimaging 2008;18:194–97. Epub 2007 Oct 18 [DOI] [PubMed] [Google Scholar]
- 7.Lapergue B, Klein I, Olivot JM, et al. Diffusion weighted imaging of cerebellar lesions in Wernicke's encephalopathy. J Neuroradiol 2006;33:126–28 [DOI] [PubMed] [Google Scholar]
- 8.Liu YT, Fuh JL, Lirng JF, et al. Correlation of magnetic resonance images with neuropathology in acute Wernicke's encephalopathy. Clin Neurol Neurosurg 2006;108:682–87 [DOI] [PubMed] [Google Scholar]
- 9.Murata T, Fujito T, Kimura H, et al. Serial MRI and (1)H-MRS of Wernicke's encephalopathy: report of a case with remarkable cerebellar lesions on MRI. Psychiatry Res 2001;108:49–55 [DOI] [PubMed] [Google Scholar]
- 10.Zhong C, Jin L, Fei G. MR Imaging of nonalcoholic Wernicke encephalopathy: a follow-up study. AJNR Am J Neuroradiol 2005;26:2301–05 [PMC free article] [PubMed] [Google Scholar]
- 11.Fei GQ, Zhong C, Jin L, et al. Clinical characteristics and MR imaging features of nonalcoholic Wernicke encephalopathy. AJNR Am J Neuroradiol 2008;29:164–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolli M, Barbieri A, Pinna C, et al. Wernicke's encephalopathy in a malnourished surgical patient: clinical features and magnetic resonance imaging. Acta Anaesthesiol Scand 2005;49:1566–70 [DOI] [PubMed] [Google Scholar]
- 13.D'Aprile P, Tarantino A, Santoro N, et al. Wernicke's encephalopathy induced by total parenteral nutrition in patient with acute leukaemia: unusual involvement of caudate nuclei and cerebral cortex on MRI. Neuroradiology 2000;42:781–83 [DOI] [PubMed] [Google Scholar]
- 14.Doss A, Mahad D, Romanowski CA. Wernicke encephalopathy: unusual findings in nonalcoholic patients. J Comput Assist Tomogr 2003;27:235–40 [DOI] [PubMed] [Google Scholar]
- 15.Kim HA, Lee H. Atypical Wernicke's encephalopathy with remarkable cerebellar lesions on diffusion-weighted MRI. Eur Neurol 2007;58:51–53 [DOI] [PubMed] [Google Scholar]
- 16.Kang SY, Kang JH, Choi JC, et al. Wernicke's encephalopathy: unusual manifestation on MRI. J Neurol 2005;12:1550–52 [DOI] [PubMed] [Google Scholar]
- 17.Loh Y, Watson WD, Verma A, et al. Restricted diffusion of the splenium in acute Wernicke's encephalopathy. J Neuroimaging 2005;15:373–75 [DOI] [PubMed] [Google Scholar]
- 18.Blanco-Múñez O, Suárez-Gauthier A, Martín-García H, et al. Unusual cortical compromise in a case of Wernicke's encephalopathy [in Spanish]. Rev Neurol 2006;42:596–99 [PubMed] [Google Scholar]
- 19.Bonucchi J, Hassan I, Policeni B, et al. Thyrotoxicosis associated Wernicke's encephalopathy. J Gen Intern Med 2008;23:106–09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindboe CF, Løberg EM. Wernicke's encephalopathy in nonalcoholics: an autopsy study. J Neurol Sci 1989;90:125–29 [DOI] [PubMed] [Google Scholar]
- 21.Torvik A, Lindboe CF, Rodge S. Brain lesions in alcoholics: a neuropathological study with clinical correlations. J Neurol Sci 1982;56:233–48 [DOI] [PubMed] [Google Scholar]
- 22.Harper C. Wernicke's encephalopathy: a more common disease than realized—a neuropathological study of 51 cases. J Neurol Neurosurg Psychiatry 1979;42:226–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harper C. The incidence of Wernicke's encephalopathy in Australia: a neuropathological study of 131 cases. J Neurol Neurosurg Psychiatry 1983;46:593–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasconcelos MM, Silva KP, Vidal G, et al. Early diagnosis of pediatric Wernicke's encephalopathy. Pediatr Neurol 1999;20:289–94 [DOI] [PubMed] [Google Scholar]
- 25.Thomson AD. Mechanisms of vitamin deficiency in chronic alcohol misusers and the development of Wernicke-Korsakoff syndrome. Alcohol Alcohol Suppl 2000;35:2–7 [DOI] [PubMed] [Google Scholar]
- 26.Haid RW, Gutmann L, Crosby TW. Wernicke-Korsakoff encephalopathy after gastric plication. JAMA 1982;247:2566–67 [PubMed] [Google Scholar]
- 27.Chaves LC, Faintuch J, Kahwage S, et al. A cluster of polyneuropathy and Wernicke-Korsakoff syndrome in a bariatric unit. Obes Surg 2002;12:328–34 [DOI] [PubMed] [Google Scholar]
- 28.Shuster MH, Vazquez JA. Nutritional concerns related to Roux-en-Y gastric bypass: what every clinician needs to know. Crit Care Nurs Q 2005;28:227–60 [DOI] [PubMed] [Google Scholar]
- 29.Harper C, Butterworth R. Nutritional and metabolic disorders. In: Graham DI, Lantos PL, eds. Greenfield's Neuropatholog. Vol 1. 6th ed. London, UK: Hodder Arnold;1997. :601–52
- 30.Karhunen PJ, Erkinjuntti T, Laippala P. Moderate alcohol consumption and loss of cerebellar Purkinje cells. BMJ 1994;25:1663–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders due to Alcoholism and Malnutrition: Contemporary Neurology Series. 2nd ed. Philadelphia: FA Davis; 1989
- 32.Kim E, Na DG, Kim EY, et al. MR imaging of metronidazole induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol 2007;28:1652–58. Epub 2007 Sep 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harter SB, Noker SR. Gadolinium-enhanced MR findings in a pediatric case of Wernicke encephalopathy. AJNR Am J Neuroradiol 1995;16:700–02 [PMC free article] [PubMed] [Google Scholar]
- 34.Asato R, Okomura R, Konishi J. “Fogging effect” in MR of cerebral infarct. J Comput Assist Tomogr 1991;15:160–62 [DOI] [PubMed] [Google Scholar]
- 35.Sze G, Milano E, Johnson C, et al. Detection of brain metastases: comparison of contrast-enhanced MR with unenhanced MR and enhanced CT. AJNR Am J Neuroradiol 1990;11:785–91 [PMC free article] [PubMed] [Google Scholar]
- 36.Weidauer S, Nichtweiss M, Lanfermann H, et al. Wernicke encephalopathy: MR findings and clinical presentation. Eur Radiol 2003;13:1001–09 [DOI] [PubMed] [Google Scholar]