Abstract
SUMMARY: We report a case of a patient with an intradural hemangiopericytoma of the lumbar spine and the unusual MR angiography (MRA) and spinal angiography findings of arteriovenous shunting with spinal venous congestion. We highlight the concordance of the unusual MRA and angiographic findings and their relationship to combined endovascular and surgical therapy.
Hemangiopericytomas, initially described by Stout and Murray,1 are vascular tumors derived from pericytes, the smooth muscle cells that control the diameter of the capillaries. The mass itself is composed of numerous capillaries with an intact basement membrane, with the neoplastic pericytes in the extravascular space.2 Hemangiopericytomas may be considered benign or malignant, have been identified in all age groups, and demonstrate no sexual predisposition.3 Primary spinal hemangiopericytomas are rare, with only 39 reported cases in the literature, those being primarily extradural.
We describe the unique presentation of a lumbar intradural hemangiopericytoma that produced imaging findings consistent with an arteriovenous shunt, with congested spinal veins and concordant spinal MR angiography (MRA) and conventional angiographic findings. Endovascular embolization of the hemangiopericytoma was performed to treat the arteriovenous shunt and prevent excessive intraoperative blood loss. In addition, the enlarged radicular vein was ligated during surgery to definitively prevent any myelopathic complications.
Case Report
A 54-year-old man presented to our emergency department with left paraspinal, buttock, and anterolateral thigh pain that radiated down the anterior shin and left foot. Initial noncontrast MR examination revealed a lesion in the left L4-L5 neural foramen, which was soft tissue signal intensity on all sequences. Serpiginous, intradural signal intensity voids were identified in the lumbar spinal canal without change in signal intensity in the cord (Fig 1). Contrast-enhanced spinal MRA and postcontrast images were recommended to elucidate the nature of the intradural flow voids and better define the neuroforaminal mass.
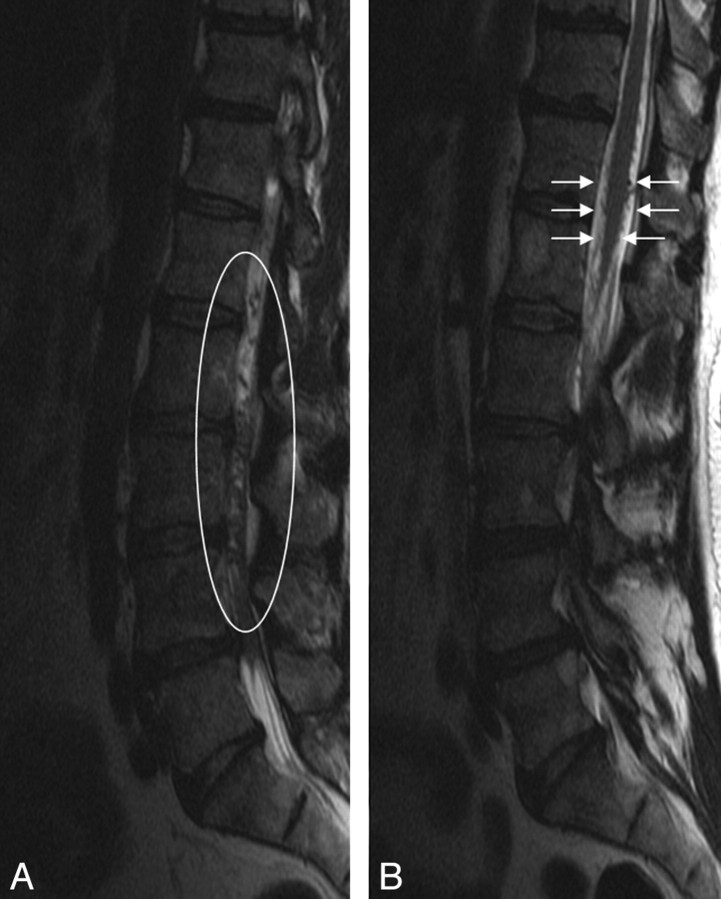
Fig 1.
Sagittal T2-weighted images of the lumbar spine demonstrating multiple intradural flow voids (left, white oval), which appear on the surface of the conus (right, white arrows). No change in signal intensity was identified within the cord.
The L4-L5 neuroforaminal mass enhanced with contrast (Fig 2). MRA (Fig 3, left) of the lumbar spine demonstrated prominent intradural vessels contiguous with the mass with congested pial veins, compatible with a spinal arteriovenous fistula or arteriovenous malformation. Spinal angiographic examination was recommended both to further elucidate the relationship between the soft tissue mass and the abnormal surface veins and for possible perioperative intervention.
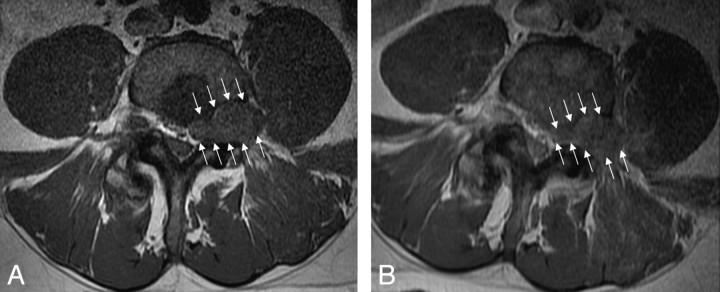
Fig 2.
Precontrast (left) and postcontrast (right) axial T1-weighted images demonstrate an enhancing mass in the left L4 neural foramen (white arrows). The low signal intensity within the posterior aspect of the vertebral body enhanced after contrast administration, consistent with atypical hemangiomas.
Fig 3.
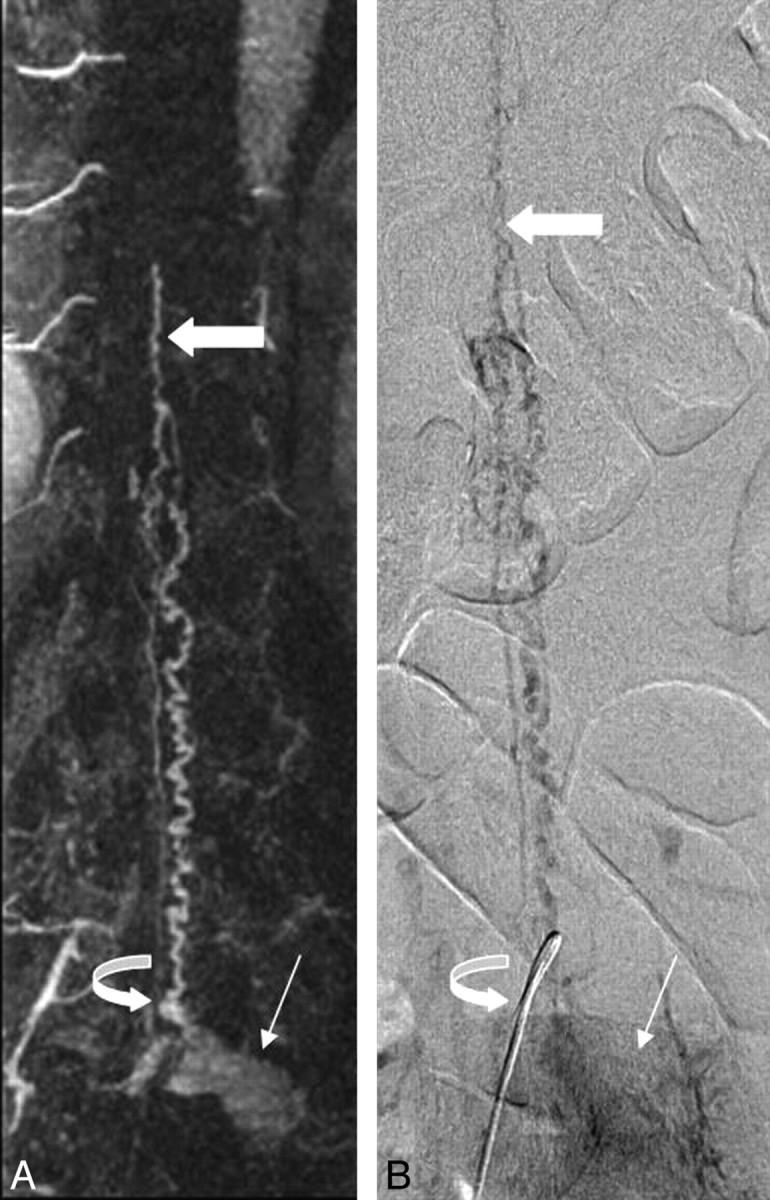
Correlative coronal contrast-enhanced spinal MRA maximum intensity projection image (left) and late-phase anteroposterior conventional spinal angiogram (right) demonstrate a hypervascular mass (thin arrow) with radicular venous drainage (curved arrow). Prominent intradural veins are present, which ascend to the level of the conus, where prominent anterior and posterior pial veins (thick arrow) are visualized.
Spinal angiography (Fig 3, right) revealed a hypervascular mass in the left L4 recess with radicular venous drainage and filling of congested intradural veins ascending to the level of the conus, where congested anterior and posterior spinal veins were apparent. Devascularization of the tumor was performed without residual tumor blush and disappearance of the apparent arteriovenous shunting accounting for spinal venous congestion. At surgery, the L4-L5 nerve sheath was incised, revealing a soft tumor that was adherent to both the overlying dura and the subjacent L4 nerve root. The large radicular draining vein, identified on the angiogram, was coagulated to prevent spinal venous hypertension.
Pathologic examination revealed a monomorphous tumor with closely packed, randomly oriented tumor cells, demonstrating numerous slitlike vessels, consistent with a hemangiopericytoma. The patient completed outpatient radiation therapy and made an uneventful recovery. Follow-up MRA demonstrated resolution of the spinal venous congestion.
Discussion
Intradural hemangiopericytomas are rare and do not demonstrate specific imaging features in the central nervous system. Typical imaging findings are a lobular, dural-based mass with heterogeneous signal intensity on T1-weighted and T2-weighted images. Hemangiopericytomas are hypervascular tumors that demonstrate marked enhancement with prominent flow voids on MR imaging. Like MR imaging, there are no specific findings of hemangiopericytoma on spinal angiography. Conventional angiographic findings typically reported for hemangiopericytomas are a hypervascular mass with irregular, “corkscrew” vessels with a prolonged tumor blush and slow circulation time.4 It is not surprising that, because hemangiopericytomas consist of dilated vascular channels, arteriovenous shunting with hemangiopericytomas on conventional angiography has been described.3
The presence of congested intradural veins, identified on spinal MRA, suggested the presence of a vascular shunt. Hemangiopericytomas are composed of capillary vascular spaces with an intact basement membrane and neoplastic pericytes within the interstitium. When present within the intradural space with multiple arterial feeders, the presence of a hemangiopericytoma may result in arteriovenous shunting from a lack of an intervening capillary bed within the mass. This results in venous hypertension, the presence of arterialized perimedullary veins, and ultimately myelopathy.
Because a hypervascular mass with dilated spinal surface veins was identified on the MRA images, preoperative embolization was suggested to both devascularize the hypervascular tumor (possibly preventing significant intraoperative blood loss) and treat the apparent arteriovenous shunting and spinal venous congestion to prevent myelopathy. The visualization of radicular venous drainage to the conus on conventional angiography predicated surgical ligation of the vein to definitively treat the venous hypertension.
Preoperative angiographic embolization of spinal hemangiopericytomas has been described in a small case series.4 Embolization was performed after significant intraoperative blood loss was identified in 2 cases of the series, which allowed for improved surgical therapy. The presence of intradural perimedullary vessels and arteriovenous shunting were not described. The diagnosis in this case, in addition to the other case reports of intradural hemangiopericytomas,5–7 was presumed to be a meningioma or neurofibroma intraoperatively, suggesting that no abnormal intradural vasculature was identified. Similar to this case, several other cases reported a primary pain component to the presentation of the tumor with lower extremity paresthesias, bladder dysfunction, and no defined motor weakness.
Conclusions
We present the case of a patient with a rare intradural mass, a lumbar hemangiopericytoma, which presented in a highly unusual fashion with markedly dilated spinal surface veins presumably from arteriovenous shunting through the lesion. Subsequent diagnostic conventional angiography with endovascular treatment of the mass and surgical resection with radicular vein ligation relieved the patient's radiculopathy and allowed for resolution of prominent spinal surface vasculature, thus avoiding the potential complication of myelopathy.
Footnotes
Case previously presented at: Annual Meeting of the American Society of Neuroradiology, June 5, 2008; New Orleans, La.
References
- 1.Stout AP, Murray MR. Hemangiopericytoma: a vascular tumor featuring Zimmerman's pericytes. Ann Surg 1942;116:26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris DJ, Fornasier VL, Livingston KE. Hemangiopericytoma of the spinal canal: report of three cases. J Neurosurg 49:914–20 [DOI] [PubMed] [Google Scholar]
- 3.Stella L, Cappabianca P, Pettinato, G, et al. Hemangiopericytoma of the meninges: report of two cases. Acta Neurologica 4:185–95 [PubMed] [Google Scholar]
- 4.Muraszko KM, Antunes JL, Hilal SK, et al. Hemangiopericytomas of the spine. Neurosurgery 1982;10:473–79 [DOI] [PubMed] [Google Scholar]
- 5.Ijiri K, Yuasa S, Yone K, et al. Primary epidural hemangiopericytoma of the lumbar spine. Spine 2002;27:E189–92 [DOI] [PubMed] [Google Scholar]
- 6.Betchen S, Schwartz A, Black C, et al. Intradural hemangiopericytoma of the lumbar spine: case report. Neurosurgery 2002;50:654–57 [DOI] [PubMed] [Google Scholar]
- 7.Ecker RD, Marsh WR, Pollock BE, et al. Hemangiopericytoma in the central nervous system: treatment, pathologic features, and long-term follow-up in 38 patients. J Neurosurg 2003;98:1182–97 [DOI] [PubMed] [Google Scholar]