Abstract
BACKGROUND AND PURPOSE: Endovascular therapy of intracranial arteriovenous malformations (AVMs) is increasingly used. However, it is still under discussion which embolic material is optimal. We report our experience in the treatment of AVMs with ethylene-vinyl alcohol copolymer (Onyx).
MATERIALS AND METHODS: Between July 2002 and January 2008, brain AVMs were embolized with Onyx in 82 consecutive patients in our department. There were 41 females and 41 males with a mean age of 44.2 years (range, 15–85 years). Clinical presentation included symptoms due to intracerebral hemorrhage (n = 37), seizures (n = 18), nonhemorrhagic neurologic deficits (n = 8), headaches (n = 9), or incidental symptoms (n = 10). According to the Spetzler-Martin scale, 59 AVMs were grades I–II, 16 were grade III, and 7 were grades IV–V.
RESULTS: Complete obliteration at the end of all endovascular procedures was achieved in 20/82 patients (24.4%), with an average of 75% (range, 30%–100%) volume reduction. A mean of 2.9 (range, 1–10) feeding pedicles was embolized per patient, whereas an average of 2.6-mL Onyx was used per patient. Procedure-related permanent disabling morbidity was 3.8%, whereas mortality was 2.4%.
CONCLUSIONS: The overall initial complete obliteration rate of intracranial AVMs with Onyx embolization is relatively high, compared with other embolic agents, with evidence of stability with time. Morbidomortality rates due to AVM embolization as a single treatment method or as a part of a multimodality treatment should be further assessed regarding the natural course of the disease.
Nidus reduction before surgery or radiosurgery, curative embolization, and palliative embolization of intracranial arteriovenous malformations (AVMs) are the different goals of endovascular treatment.1 Since the first report in the early 1960s about transcatheter embolization of cerebral AVMs with the use of steel spheres covered with methyl methacrylate to block feeding arteries,2,3 a considerable evolution of microcatheter tools, embolization materials, and techniques has improved the success of embolization. Currently, the target of embolization with liquid polymers is occlusion of the nidus, including the arteriovenous shunts. n-butyl 2-cyanoacrylate (n-BCA) was, up to now, the most frequently used embolic agent. It has been reported in the literature that initial complete occlusion of cerebral AVMs that persists at least after a 6-month angiographic follow-up is considered to be curative embolization.1 Nevertheless, the lack of long-term follow-up brings into question whether the angiographic obliteration at the time of embolization represents a long-term cure. Ethylene-vinyl alcohol copolymer (EVOH) (Onyx; ev3, Irvine, Calif) is a newer liquid polymerizing embolic material, which permits theoretically slower filling, better penetration, and obliteration of the nidus, providing a solid nidus cast due to its longer polymerization time and lack of adherence.
We evaluated the rates of initial obliteration of AVMs as well as the reperfusion rates after endovascular use of Onyx. Additionally, we assessed the clinical morbidity and mortality related to the periprocedural complications after embolization of 82 AVMs in our institution.
Materials and Μethods
Treatment Strategy
All patients were evaluated by an interdisciplinary conference consisting of neuroradiologists and neurosurgeons for assessment of the therapeutic options and the suitability of endovascular and/or surgical treatment or radiosurgery, based on MR imaging and digital subtraction angiography (DSA). Nidus reduction by means of endovascular embolization before surgery or radiosurgery and curative embolization are considered first-line treatment options in our institution. Surgery alone is considered for superficially located small AVMS not in eloquent areas with superficial venous drainage and a low possibility for complete endovascular occlusion (diffuse nidus and en passant, leptomeningeal, or tiny and elongated feeders). Radiosurgery is planned for deeply located AVMs with initial size or rest nidus after endovascular embolization of <3 cm.
Patients with intracerebral hemorrhage due to a ruptured AVM are submitted to angiographic control (DSA) to confirm the underlying pathology. In non-life-threatening cases, elective treatment of the AVM is performed after hematoma resorption, allowing more precise angiographic assessment of the fully expanded AVM nidus and better brain conditions (less perinidus edema, absence of blood, and lower intracranial pressure), thus facilitating a possible postembolization surgical excision.
Patient Demographics and Clinical Presentation
We retrospectively reviewed the records of 82 consecutive patients (41 males and 41 females; mean age, 44.2 years; range, 15–85 years) with intracranial AVMs who were treated by endovascular embolization with Onyx between July 2002 and January 2008. The most common clinical presentation included symptoms due to intracerebral hemorrhage in 37 patients. The remaining patients had nonhemorrhagic clinical presentations: seizures (n = 18), nonhemorrhagic neurologic deficits (n = 8), headaches (n = 9), or incidental symptoms (n = 10). According to the Spetzler-Martin (SM) scale, 59 AVMs were grades I- II; 16 AVMs, grade III; and 7 AVMs, grades IV–V (Table 1). Thirteen AVMs were located in the cerebellum, and 69 were located supratentorially (Table 2).
Table 1:
Patients’ characteristics at admission
| Characteristics | No. |
|---|---|
| Demographics | |
| Male | 41 |
| Female | 41 |
| Age (yr) (mean) | 44.2 (15–85) |
| Presenting symptoms | |
| Intracerebral hemorrhage | 37 |
| Seizure | 18 |
| Neurologic deficit | 8 |
| Headache | 9 |
| Incidental | 10 |
| SM scale | |
| I–II | 59 |
| III | 16 |
| IV–V | 7 |
Note:—SM indicates Spetzler-Martin.
Table 2:
Location of brain AVMs
| Location | No. |
|---|---|
| Frontal | 10 |
| Temporal | 19 |
| Parietal | 7 |
| Occipital | 13 |
| Frontotemporal | 2 |
| Frontoparietal | 5 |
| Temporo-occipital | 2 |
| Temporoparietal | 2 |
| Parieto-occipital | 5 |
| Intraventricular | 2 |
| Temporochoroidal | 1 |
| Basal ganglia | 1 |
| Cerebellar | 13 |
Note:—AVMs indicates arteriovenous malformations.
Onyx Liquid Embolics and Embolization Technique
Onyx is a new liquid embolic agent consisting of EVOH formed of 48-mol/L ethylene and 52-mol/L vinyl alcohol, dissolved in dimethyl-sulfoxide (DMSO) and mixed with micronized tantalum powder (35% weight per volume) for radiopaque visualization. Onyx Liquid Embolic System (Onyx LD, ev3) is available in ready-to-use vials of 1.5 mL in 3 different viscosities, Onyx 18, 20, and 34 (concentrations of EVOH, 6%, 6.5%, and 8% respectively). The Onyx viscosity 18, 20, and 34 are units of centipoise (the 100th of a poise, 1 poise = dynes/cm2 or 1.0 r/cm/s). The vials must be placed on a mixer and shaken for at least 20 minutes to obtain a homogeneous solution consisting of the embolic component and the tantalum powder.4
All procedures were performed with the patients under general anesthesia. Informed consent was obtained. A 6F sheath was placed in the femoral artery, and a 6F guiding catheter was then inserted in 1 of the main brain feeding arteries (internal carotid or dominant vertebral artery) that supplied the AVM. Continuous flushing of the sheath and the guiding catheter was performed with slightly heparinized saline (1000 IU of heparin per liter). Systemic heparin was not administered additionally because it is not routine in our department. A superselective catheterization with flow-directed microcatheters (Marathon or UltraFlow, ev3) and microguidewires (Mirage or SilverSpeed, ev3) was performed with the tip of microcatheter placed as close as possible to the AVM nidus. Angiographic series through the microcatheter with a 3-mL syringe proved to be especially useful for assessing the AVM architecture and adjusting the microcatheter tip in a safe and optimal position. Preinterventional identification of normal parenchymal branches arising from the pedicle distally or eloquent branches originating proximal to the microcatheter tip that could potentially be occluded due to reflux was of utmost importance. Microangiography was also useful in evaluating the local AVM flow and illustrating the anatomy of the draining vein.
The concentration of Onyx was selected according to the size of the feeding vessel. If a small feeding vessel (only slightly above normal vessel diameter) was present, Onyx 18 was used; if a large feeding vessel (at least 3 times the normal diameter) was present, Onyx 20 was used. Because there is very little difference between these 2 viscosities, we believe that using one or the other viscosity would not make much difference, with the exception of Onyx 34, which was selected for occlusion of a very high-flow fistulous nidus, partly because the risk of extended venous migration of the liquid embolic agent was estimated to be high. In case of very large fistulous vessels, a microballoon was used to block the flow proximally.
The microcatheter was flushed with normal saline and filled with DMSO. Afterward, 0.25-mL Onyx was injected slowly (≤0.1 mL/min) into the microcatheter. Injection beyond the catheter tip into the AVM was monitored fluoroscopically by road-mapping. In case of reflux around the catheter tip of >1 cm, we temporarily discontinued the injection of Onyx for 1–2 minutes to form a cast around the tip of the microcatheter and, thereafter, performed a second penetration of the nidus (plug and waiting technique). Every effort was made to occlude the arterial compartment of the AVM first to avoid bleeding complications associated with early occlusion of venous drainage. This was accomplished by redirection of Onyx through short breaks of Onyx application, lasting between 30 seconds and 1 minute, and, thereafter, performing a further injection. Injection of the Onyx into the draining veins was allowed only in their origin and only after occlusion of the arterial nidus compartment. Angiographic control was performed through the guiding catheter during the injection intervals to evaluate the residual flow to the AVM or nontarget embolization. In case of increased resistance of the injection, further application of Onyx was discontinued, to avoid rupture of the microcatheter or vessels. The microcatheter was removed by slowly increasing traction. In case of incomplete occlusion of the AVM and residual flow, another feeding pedicle was catheterized with a new microcatheter system and further Onyx application was attempted.
Assessment of Angioarchitecture in Completely Obliterated AVMs
We compiled the angiomorphologic characteristics of all the completely obliterated AVMs in detail concerning size (<3 cm, 3–6 cm, >6 cm), nidus morphology (compact or diffuse), shunt type (plexiform, fistulous, or mixed), arterial feeders (number, direct, en passant, leptomeningeal), and venous drainage (deep, superficial, number), to assess predominant anatomic characteristics and correlate them to complete AVM obliteration.
Postembolization Care
After the intervention, a cerebral tomography was performed to exclude hemorrhagic complications. Subsequently, the patient was transferred to the intensive care unit for 48-hour monitoring of vital functions. The systolic blood pressure should be kept within normal limits with a maximum of 150 mm Hg to avoid additional hemodynamic changes of the brain circulation and especially within the nonembolized part of the AVM. Steroids were given orally (4 mg × 4/day dexamethasone) for 4 days after the intervention to prevent or reduce perinidus edema.
Follow-Up
Angiographic evaluation of the obliteration results was scheduled at 6 months in patients with complete AVM obliteration after embolization or was performed earlier in case of recurrence of clinical symptoms, whereas in patients with surgical removal of the AVM after embolization, a DSA control was scheduled before discharge. Assessment of the clinical outcome, by using the modified Rankin Scale (mRS), was performed at patient's admission, at discharge, and scheduled at 6 and 12 months after the intervention.
Results
A total of 82 patients were treated by endovascular embolization alone or in combination with surgical excision and radiosurgery. A total of 119 embolization procedures were performed with a mean of 1.45 (range, 1–3) per patient. A mean of 2.9 (range, 1–10) feeding pedicles were embolized per patient, whereas an average of 2.6-mL Onyx was used per patient. Onyx 18 was used in 39 patients (48%), whereas Onyx 20 was used in 27 patients (33%). A combination of Onyx 20 and Onyx 18 was used in 15 patients (18%), and a combination of Onyx 34 and Onyx 18 was used in 1 patient (1%). n-BCA was additionally used in 1 procedure, whereas coil embolization was used in a case of a fistulous part of an AVM as adjunctive to Onyx embolization.
Initial complete obliteration at the end of all embolization procedures was achieved in 20/82 patients (24.4%). An average of 75% (range, 30%–100%) volume reduction was achieved at the end of the endovascular procedures. Evaluation of the AVM volume was performed by using the method of Pasqualin et al.5 After a mean follow-up of 8.8 months (range, 0–57 months) of the 20 initially completely embolized AVMs, 4 angiographic recurrences were evident between 2.5 and 4 months; 2 of them were minimal. Three of them underwent surgical excision, whereas 1 patient is scheduled for a further embolization treatment. The rate of complete embolization at follow-up was 19.5% (16/82 patients).
Neurosurgical removal of AVMs was performed in 49/82 patients after embolization. Postembolization radiosurgery was performed in 3 partially occluded AVMs. The rest of the patients are scheduled for angiographic control and evaluation of further treatment.
Angiomorphologic characteristics of the 20 initially completely obliterated AVMs were the following: Fifteen AVMs had a size <3 cm, 5 AVMs were 3–6 cm, whereas no AVMs were >6 cm. All 20 AVMs had compact nidi, whereas there were 18 plexiform AVMs and 2 mixed (plexiform-fistulous) shunt types. Nine AVMs had 3 or fewer arterial feeders, whereas 11 AVMs had >3 feeders. Furthermore, in 12/20 AVMs, en passant feeders were present. Concerning the venous drainage, 18 AVMs had superficial, whereas 2 had deep drainage. Ten AVMs had a single draining vein, and 10 had ≥2 draining veins. Seventeen AVMs were completely occluded in 1 session; 2 AVMs, in 2 sessions; whereas 1 AVM was treated 3 times. Complete embolization was achieved through a single pedicle in 6 AVMs, through 2 pedicles in 8 AVMs, and through ≥3 pedicles in 3 AVMs.
Postembolization Clinical Morbidity
According to the mRS, 10 patients (10/82) experienced nondisabling (mRS, 1–2) neurologic deficits (12.2%), whereas 6 patients (6/82) experienced disabling (mRS ≥3) neurologic deficits (7.3%) immediately after the embolization procedure. The overall periprocedural new clinical morbidity related to embolization was 19.5% (16/82 patients). Nondisabling neurologic deficits included 1 case of a slight arm paresis, 1 apraxia of the upper limb, 1 case of discrete ataxia, 1 case of third nerve palsy, and 6 visual field deficits, whereas disabling neurologic deficits included 5 cases of postinterventional hemiparesis and 1 case of lower limb paresis. Among the patients with deficits, 6 intracerebral hemorrhages, 1 intraventricular hemorrhage, 3 subarachnoid hemorrhages, and 3 infarcts/perfusion deficits were responsible for the neurologic changes, whereas in 3 cases, a clear cause was not identified. A microballoon that had been used for preventing coil migration into the venous side of a fistulous part of an AVM was stuck and could not be removed. This was the cause of 1 of the previously mentioned infarcts resulting in slight transient hemiparesis. One trapped microcatheter in the posterior circulation did not add clinical morbidity to our cohort.
The periprocedural mortality rate related to embolization was 2.4% (2/82 patients) due to postinterventional intracerebral bleeding. One of these patients experienced a large intracerebral hemorrhage 58 hours after the second session of a staged embolization for a grade II precentral AVM. We noted limited migration of Onyx into the venous side without obstruction of the superficial venous drainage. The second patient bled significantly immediately after the end of the second session of a staged embolization for a grade V cerebellar AVM. The patient immediately underwent surgery with evacuation of the hematoma and removal of the residual AVM. The patient bled again 8 hours after surgery and died. Blood pressure ranged within normal limits intra- and postinterventionally in both cases. Hemorrhagic clinical presentation before embolization was present in both patients. A clear reason for these bleedings was not identified. Possibly hemodynamic changes in the normal parenchyma led to a perfusion breakthrough hemorrhage. The surgeons did not reveal venous blockage with Onyx, but it is sometimes not obvious to them.
Procedure-Related Clinical Morbidity at Follow-Up
Long-term follow-up (mean, 14 months; range, 3–49 months) is available for the 12 among 16 patients who experienced new clinical morbidity immediately after the embolization. Four patients were not available for long-term follow-up; 1 of them with an initially disabling clinical complication is still in a rehabilitation program. However, concerning 3 patients with a nondisabling deficit, 1 was lost due to relocation, whereas 2 are scheduled for clinical assessment in 5 months.
Concerning the clinical course of the initially nondisabling neurologic deficits in 7 patients available for follow-up, 1 case of paresis of the arm, 1 case of upper limb apraxia, and 2 cases of visual field deficits showed complete resolution. The remaining initially nondisabling deficits (n = 3) showed partial or no improvement. At follow-up, the permanent nondisabling (mRS, 1–2) embolization-related clinical morbidity rate was 5.1% (4/78), including 1 patient with an initially disabling hemiparesis immediately after the embolization whose condition improved to a nondisabling residual hemiparesis at follow-up.
Concerning the initially disabling neurologic deficits in 5 patients available for follow-up, 1 case of hemiparesis showed complete resolution, 1 patient improved to a nondisabling residual hemiparesis, 2 patients improved but remained disabled, and 1 patient did not show any improvement, whereas 1 patient is still in a rehabilitation program and is not available for a follow-up beyond 3 months. At the end of the follow-up, disabling (mRS, ≥3) embolization-related clinical morbidity was 3.8% (3/78 patients).
The overall permanent morbidomortality rate related to the embolization procedure was 11.3%.
Discussion
The introduction of liquid polymers for AVM embolization allowed direct injection and obliteration of the nidus through flow-guided microcatheters. Up to now, n-BCA was the most frequently used embolic agent. Complete endovascular obliteration rates vary in the literature but are mostly estimated in the range of 10%.6 More specifically, in a series of 100 cases by Deruty et al,7 complete obliteration was achieved in 5% after embolization alone. Viñuela et al8 reported a 9.7% complete occlusion rate for embolization alone in small AVMs with few feeders. Among 125 patients, a cure rate of 11.2% was achieved by Gobin et al9 after embolization before radiosurgery. In another series of 150 patients by Lundqvist et al,10 13% of AVMs were completely embolized with n-BCA. Similar complete obliteration rates of 14% were mentioned by Fournier et al11 in a series of 49 patients. Complete embolization rates of 22% were reported by Yu et al12 among a smaller number of 27 patients. As an exception, Valavanis and Yasargyl13 reported a rate of 40% angiographic cure, by using n-BCA, among 387 patients selected according to their AVM size, location, and type of arteriovenous shunts constituting the nidus.
Onyx is a newer polymerizing agent consisting of EVOH dissolved in DMSO and mixed with micronized tantalum powder for radiopaque visualization.4 It allows more prolonged embolization injections and, therefore, better nidus penetration compared with n-BCA.14 Furthermore, during injections, small amounts of embolization material can be refluxed and used to block flow in the feeder vessel with the microcatheter threaded inside,15 allowing a second penetration of the nidus. The polymer solidifies slowly from outside to inside as it comes in contact with the blood, with limited influence by the AVM flow, allowing better control of penetration behavior and achieving more solid nidus cast compared with n-BCA. Another advantage of Onyx is the possibility for simultaneous angiographic control through the guiding catheter and assessment of the AVM occlusion percentage or nontarget embolization during the intervals.
Complete obliteration rates for AVMs reported in recent clinical series that used Onyx as an embolic agent are variable. In a clinical article by Pérez-Higueras et al,14 10 totally occluded AVMs (22.2%) were reported among 45 cases. In another clinical series of 48 cases by Pierot et al,15 2 AVMs (4.2%) were completely obliterated, whereas Leonardi et al16 reported 2 completely occluded cases (5.9%) among 34 AVMs, SM grades III–IV. Series with a smaller number of treated AVMs like Tevah and Huete17 reported 7 cases (29.2%) of complete embolization among 24, whereas Joseph et al18 described 2 cases of 5 of total occlusions. Concerning articles indexed in PubMed, Florio et al19 reported a total occlusion in 2 cases among 10 (20%) AVMs embolized with Onyx. In a series of 23 AVMs embolized with Onyx by Jahan et al,20 the cure rate after embolization was zero.
In more recent original research articles, van Rooij et al21 reported 7 cases (15.9%) of completely occluded AVMs, grades I and II, among 44, whereas Weber et al22 reported 19 patients (20%) with completely obliterated AVMs among 93. In a series of 70 patients by Song et al,23 13 AVMs (18.6%) were completely embolized with Onyx. In another series of 94 patients, Mounayer et al24 reported angiographic cure in 26 patients; in this series, the treatment was completed for 53 patients, by using a combination of Onyx and n-BCA.
In our retrospective study, a complete initial occlusion of 20 AVMs (24.4%) was achieved among 82 patients treated with Onyx, whereas a complete obliteration rate at follow-up was 19.5% (16/82 patients). According to the published experience up to now, complete obliteration rates of BAVMs with Onyx range obviously above the reported average of 10% of angiographic cure after embolization with n-BCA. This finding offers a new dimension in the endovascular management of these challenging lesions, not only as a preoperative adjunctive method to surgery but also as a sole minimally invasive treatment. Furthermore, our complete occlusion rates ranged between the upper limits of the previously reported percentages after Onyx embolization.
Concerning the anatomic features of AVMs included in series with high or complete obliteration after embolization with n-BCA, this was achieved mainly in small AVMs with a small number of feeding pedicles.8,9,11,25 Nevertheless Valavanis and Christoforidis26 identified direct or dominant feeding arteries, a monocompartmental nidus, and a dominant fistulous component of the nidus without perinidal angiogenesis as predictive characteristics of angiographic cure with n-BCA, whereas the AVM size or the number of feeding pedicles was not found to be a determinant predictive factor for complete endovascular occlusion. AVM characteristics reported to be associated with higher occlusion rates after Onyx embolization are a supratentorial and cortical location, a compact and plexiform nidus, a small number of supplying (direct) feeders, and 1 superficial draining vein.22 van Rooij et al21 reported that complete obliteration may be accomplished in smaller AVMs with 1 or 2 feeding pedicles.
We reviewed retrospectively the AVM angioarchitecture characteristics of the completely embolized AVMs. According to our previously mentioned results, complete embolization was mainly accomplished in small AVMs (<3 cm) with a compact nidus, plexiform shunt type, and superficial venous drainage. The number of draining veins did not show a special trend among the anatomic characteristics of the completely obliterated AVMs. The presence of multiple arterial feeders or en passant feeders did not preclude a complete endovascular occlusion. Furthermore, complete obliteration was achieved for most of the 20 initially completely embolized AVMs in 1 session through 1 or 2 pedicles. Figure 1 illustrates complete embolization of an infratentorial AVM through a single pedicle injection. This is probably attributed to the special embolization behavior of Onyx, which provides the opportunity of prolonged injections through a single pedicle and multidirectional nidus penetration with the possibility of retrograde filling of other feeders. This “steering” behavior of Onyx in experienced hands may result in complete occlusion, even in cases of big AVMs with multiple arterial feeders arising from different arteries (Fig 2).
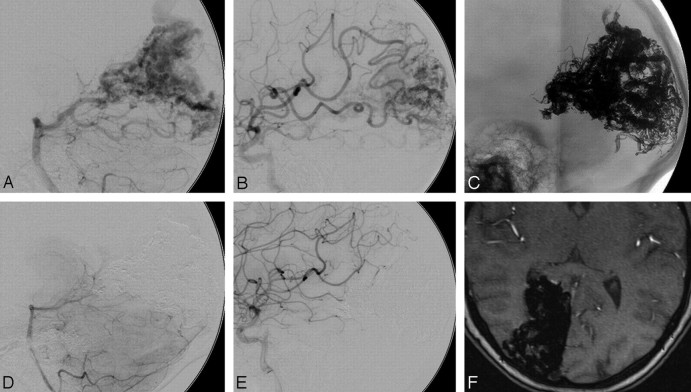
Fig 1.
A 48-year-old male patient with an SM grade II AVM in the left cerebellar lobe. A and B, Vertebral angiogram (lateral view, arterial and late venous phase) shows arterial supply to the AVM from feeders of the left posterior inferior cerebellar artery with superficial venous drainage. C, Unobstructed image illustrates a solid Onyx cast after the embolization. D, Left vertebral angiogram (lateral view, venous phase) illustrates complete AVM occlusion after embolization through a single pedicle.
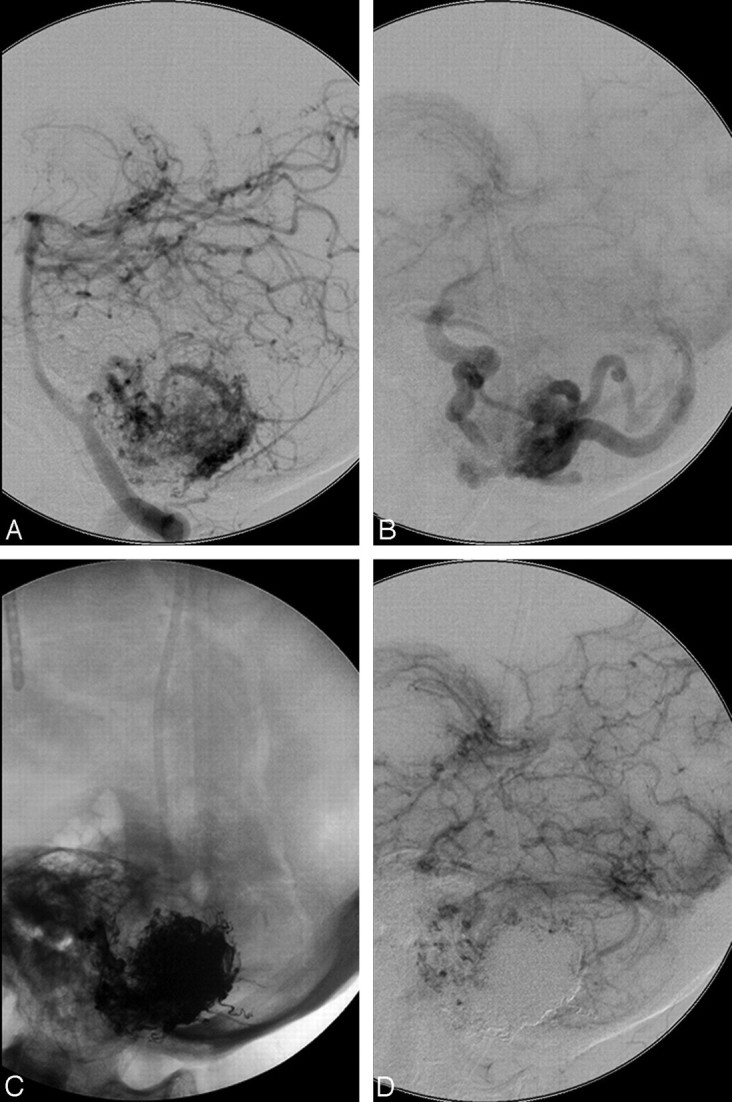
Fig 2.
A 29-year-old female patient with an SM grade IV AVM in the right occipital lobe. A, Vertebral angiogram (lateral view) shows arterial supply from multiple feeders arising mainly from the right posterior artery with superficial venous drainage. B, Right internal carotid artery angiogram (lateral view) shows additional arterial supply to the AVM from feeders of the middle cerebral artery. C, Unobstructed image illustrates a solid Onyx cast after the embolization. D and E, Control right vertebral and carotid artery angiograms (lateral view) at 6 months after embolization illustrate persistence of complete occlusion with no evidence of recanalization. F, MR image (time-of-flight, axial) at 6 months after embolization reveals complete occlusion of the AVM without evidence of reperfusion.
An initial total occlusion of the AVM nidus does not preclude a repermeation due to several mechanisms. One of the most important mechanisms reported in the literature is collateralization through dilation of pre-existent collateral vessels, usually in the perilesional tissue, and consequent reconstitution of blood flow to the AVM nidus.27 Additionally, intranodal cyanoacrylate resorption, though rare, has been reported in the literature.28 Sometimes, an angiographically total obliteration of the nidus is a result of partial lumen occlusion with polymerized glue, while the remaining lumen is filled with thrombus. In case of the absence of solid casting of the entire nidus, possible recapillarization of thrombus results in repermeation.29,30
Onyx seems quite stable because recanalization was not seen either in experimental studies up to 6 months after the procedure31 or in the histologic and radiologic images presented by Jahan et al,20 who performed surgery as late as 14 days after embolization. Nevertheless Pérez-Higueras et al14 reported 2 cases of reperfusion among 10 completely embolized AVMs after 2 and 4 years, respectively. More recently, Weber et al22 reported 2 early angiographic recurrences after complete obliteration of 19 AVMs that were evident at 3-months’ follow-up.
In our study, after a mean follow-up of 8.8 months (range, 0–57 months), of the 20 initially completely embolized AVMs, 4 angiographic recurrences were evident between 2.5 and 4 months, of which 2 were minimal. According to our experience, completely obliterated AVMs with solid casting of the AVM nidus, without angiographic evidence of intra- or postinterventional intranodal thrombus formation and no reperfusion in follow-up of ≥6 months, is an indicator of long-lasting stability. In 1 of our recurrence cases, an intranodal thrombus formation was evident. In another case, subtotal penetration of Onyx into the nidus did not prevent AVM recanalization, even after initial complete embolization (Fig 3). In the other cases, we estimated that pre-existent collateral vessels invisible in the initial postembolization angiogram were dilated and consequently led to a partial reconstitution of blood flow to the AVM nidus.
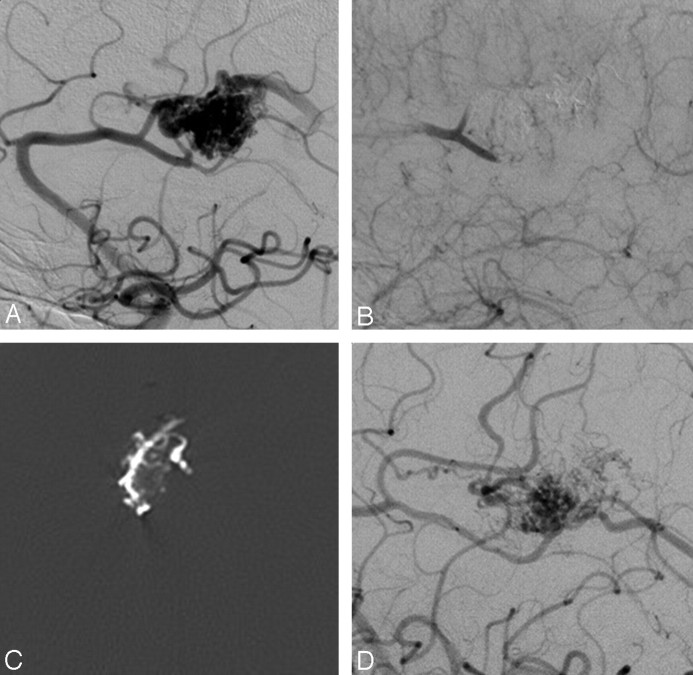
Fig 3.
A 53-year-old male patient with an SM grade I AVM in the right frontal lobe. A, Right internal carotid angiogram (lateral view) shows arterial supply to the AVM from feeders of the right pericallosal artery with superficial venous drainage. B, Right internal carotid angiogram (lateral view, early venous phase) illustrates complete AVM occlusion after embolization. C, Postembolization cerebral CT scan (axial) illustrates the Onyx cast with subtotal penetration inside the AVM nidus. D, Control right carotid artery angiogram (lateral view) at 4 months after embolization demonstrates partial recanalization of the AVM nidus with reconstitution of early venous drainage.
Estimated morbidity following brain AVM embolization has been reported to be 10% concerning temporary neurologic deficits and 8% concerning permanent neurologic deficits, whereas mortality was 1% in a review article of the literature between 1969 and 1993 by Frizzel and Fisher.32 Morbidity rates reported later in the literature, in the era before introduction of Onyx, ranged between 5.6% and 14% concerning overall morbidity and 2%–12.8% concerning permanent morbidity, whereas mortality rates ranged between 0.6% and 3.7%.3,6,9,33–39
Several authors have reported the clinical outcome in patients after AVM embolization with Onyx. In their clinical article, Pérez-Higueras et al14 reported an overall morbidomortality rate of 18% among 45 patients, whereas permanent morbidity was 15.5% and mortality, 2%. In another clinical series of 48 cases by Pierot et al,15 the morbidity rate was 10%, whereas the mortality rate was 2%. Leonardi et al16 reported 3% transient morbidity and 3% mortality among 34 AVMs, SM grades III–V. Series with smaller numbers of treated AVMs like that of Tevah and Huete17 reported 8.3% final morbidity (2/24 patients) and 0% mortality. Concerning literature indexed in PubMed, Florio et al19 reported 1 mild permanent (10%) and 2 transient (20%) neurologic deficits among 10 AVMs embolized with Onyx. Jahan et al20 reported a permanent morbidity rate of 4% and a mortality rate of 0% after Onyx embolization of 23 AVMs. In more recent original research articles, van Rooij et al21 reported permanent morbidity of 4.6% and mortality of 2.3% among 44 patients who underwent embolization with Onyx. Song et al23 reported a morbidity rate of 7.1% (5/70 patients) and a 1.4% mortality rate. In another series of 94 patients, Mounayer et al24 reported temporary morbidity of 3%, permanent morbidity of 9%, and a mortality rate of 3%; here the authors used a combination of Onyx and n-BCA for embolization. Weber et al22 reported a clinically significant deficit of 5% and a low-impact morbidity of 4% in a series of 93 patients embolized with Onyx. In a more recent article by Weber et al,40 which included 47 patients with compact intracranial AVMs who underwent preoperative embolization by using Onyx, nondisabling neurologic deficits were evident in 19% and disabling deficits, in 9% postinterventionally, whereas mortality after embolization was 0%.
In our study, the periprocedural new clinical morbidity related to embolization among 82 patients was 12.2% in patients with nondisabling neurologic deficits and 7.3% in patients with disabling neurologic deficits. At the end of the follow-up, the nondisabling embolization-related clinical morbidity was 5.1% and the disabling morbidity was 3.8%, whereas the mortality rate was 2.4%. Our results are in the range of previously reported morbidomortality rates concerning embolization with Onyx.
Several causes have been proposed in the literature to explain acute postembolization hemorrhage, such as inappropriate venous occlusion of a partially embolized AVM, increased pressure in feeding arteries as a result of embolization, normal perfusion pressure breakthrough, hyperemia of normal brain or redistribution of cerebral blood flow into adjacent regions, venous thrombosis secondary to stasis caused by substantial obliteration of the AVM, inflammatory reaction or mural necrosis induced by the embolic material, ischemic softening of tissue around an abnormal blood vessel that bled under pressure, and intranidal rupture of an aneurysm.41 Overall, the rate of periprocedural bleeding complications reported in the literature ranged between 2% and 16.7% after endovascular use of Onyx.11,14,15,17,21–24,40
In our study, 9 intracerebral/intraventricular hemorrhages (11%) with clinical impact occurred in our patients. Previous AVM rupture was identified as a related factor in 4/9 patients who experienced periprocedural bleeding complications. Although guidelines concerning whether an embolization should be performed in a few or multiple successive sessions have not yet been assessed, it has been suggested in the literature that AVM embolization should be performed in many partial steps to decrease the risk of hemorrhage technically.42
Ischemic procedure-related complications may be attributed to thrombotic emboli due to the catheter or embolization of normal vessels. Clinical symptoms depend on the location and extension of the lesion. According to our experience, intra-arterial administration of platelet glycoprotein IIb/IIIa antagonists is indicated in case of intraprocedural formation of thrombus in an eloquent brain vessel. This proved to be useful in 1 case of a thrombus in the left pericallosal artery causing a perfusion deficit that resolved quickly after intra-arterial administration of 5-mg abciximab.
Retention of the microcatheter is unlikely to happen, even in cases with extended reflux, due to the nonadhesive features of Onyx. Nevertheless, when trapped, it is better to leave it in place to prevent a potential rupture of a vessel. In our series, a trapped microcatheter in the posterior circulation was left in place without any clinical symptoms.
Concerning the risk of hemorrhage in patients with untreated brain AVMs, Stapf et al43 reported a rate of 6% among 622 patients (45% presented initially with intracranial hemorrhage) at a median pretreatment follow-up of 102 days. Nevertheless, the same authors reported that annual hemorrhage rates on follow-up ranged from 0.9% for patients without hemorrhagic AVM presentation, deep AVM location, or deep venous drainage to as high as 34.4% for those harboring all 3 risk factors. This finding underlines the fact that randomized clinical trials are needed to assess the acceptability of the up-to-now reported morbidomortality concerning AVM embolization or combined treatment, with regard to the natural course of the disease. For the moment, our center participates in the ARUBA (A Randomized Trial of Unruptured Brain Arteriovenous Malformations) study, a worldwide randomized controlled trial that compares treatments concerning embolization, surgery, and radiation therapy with conservative management of unruptured brain AVMs at a follow-up of 5 years after diagnosis.
Conclusions
Our experience with Onyx used for embolizing AVMs shows that it is a nonadhesive agent with controllable endovascular behavior, which allows more precise nidus penetration creating a solid cast. The overall initial complete obliteration rate of intracranial AVMs with Onyx is relatively high compared with that of other embolic agents (the mean obliteration rate with n-BCA is reported to be 10%5), mainly accomplished in small AVMs with compact nidi, plexiform shunt type, and superficial venous drainage, providing evidence of stability with time. Nevertheless, the efficacy of the role of this new nonadhesive polymerizing agent, as a method for curative treatment of brain AVMs, needs further evaluation through larger studies with longer follow-up. Furthermore, morbidomortality rates due to AVM embolization as a single treatment method or as a part of a multimodality treatment should be further assessed regarding to the natural course of the disease, to provide the optimal management for these lesions.
References
- 1.Berenstein A, Lasjaunias P, Ter Brugge KG. Goals and objectives in the management of brain arteriovenous malformations. In: Surgical Neuroangiography. 2nd ed. Berlin, Germany: Springer-Verlag Berlin;2004;695–735
- 2.Luessenhop AJ, Presper JH. Surgical embolization of cerebral arteriovenous malformations through internal carotid and vertebral arteries: long-term results. J Neurosurg 1975;42:443–51 [DOI] [PubMed] [Google Scholar]
- 3.Luessenhop AJ, Spence WJ. Artificial embolization of cerebral arteries: report of use in a case of arteriovenous malformations. JAMA 1960;172:1153–55 [DOI] [PubMed] [Google Scholar]
- 4.Alexander MJ, Tolbert ME. Targeting cerebral arteriovenous malformations for minimally invasive therapy. Neurosurgery 2006;59 (5 suppl 3):S178–83, discussion S3–13 [DOI] [PubMed] [Google Scholar]
- 5.Pasqualin A, Barone G, Cioffi F, et al. The relevance of anatomic and hemodynamic factors to a classification of cerebral arteriovenous malformations. Neurosurgery 1991;28:370–79 [DOI] [PubMed] [Google Scholar]
- 6.Fiorella D, Albuquerque FC, Woo HH, et al. The role of neuroendovascular therapy for the treatment of brain arteriovenous malformations. Neurosurgery 2006;59 (5 suppl 3):163–77, discussion S3–13 [DOI] [PubMed] [Google Scholar]
- 7.Deruty R, Pelissou-Guyotat I, Mottolese C, et al. The combined management of cerebral arteriovenous malformations: experience with 100 cases and review of the literature. Acta Neurochir (Wien)1993;123:101–12 [DOI] [PubMed] [Google Scholar]
- 8.Viñuela F, Duckwiler G, Guglielmi G. Contribution of interventional neuroradiology in the therapeutic management of brain arteriovenous malformations. J Stroke Cerebrovasc Dis 1997;4:268–71 [DOI] [PubMed] [Google Scholar]
- 9.Gobin YP, Laurent A, Merienne L, et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg 1996;85:19–28 [DOI] [PubMed] [Google Scholar]
- 10.Lundqvist C, Wikholm G, Svendsen P. Embolization of cerebral arteriovenous malformations. Part II Aspects of complications and late outcome. Neurosurgery 1996;39:460–67 [DOI] [PubMed] [Google Scholar]
- 11.Fournier D, TerBrugge KG, Willinsky R, et al. Endovascular treatment of intracerebral arteriovenous malformations: experience in 49 cases. J Neurosurg 1991;75:228–33 [DOI] [PubMed] [Google Scholar]
- 12.Yu SC, Chan MS, Lam JM, et al. Complete obliteration of intracranial arteriovenous malformation with endovascular cyanoacrylate embolization: initial success and rate of permanent cure. AJNR Am J Neuroradiol 2004;25:1139–43 [PMC free article] [PubMed] [Google Scholar]
- 13.Valavanis A, Yasargyl MG. The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg 1998;24:131–14 [DOI] [PubMed] [Google Scholar]
- 14.Pérez-Higueras A, Rossi Lopez R, Quinones Taria D. Endovascular treatment of cerebral AVM: our experience with Onyx. Interventional Neuroradiology 2005;11:141–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierot L, Januel AC, Herbreteau D, et al. Endovascular treatment of brain arteriovenous malformations using Onyx: preliminary results of a prospective multicenter study. Interventional Neuroradiology 2005;11:159–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonardi M, Simonetti L, Cenni P, et al. Brain AVM embolization with Onyx: analysis of treatment in 34 patients. Interventional Neuroradiology 2005;11:185–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tevah J, Huete I. Endovascular treatment of cerebral AVMs with a new material: Onyx. Interventional Neuroradiology 2005;11:165–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph S, Chadaga HC, Murali K. Endovascular treatment of cerebral AVMs with Onyx: initial experience. Interventional Neuroradiology 2005;11:171–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Florio F, Lauriola W, Nardella M, et al. Endovascular treatment of intracranial arterio-venous malformations with Onyx embolization: preliminary experience. Radiol Med (Torino)2003;106:512–20 [PubMed] [Google Scholar]
- 20.Jahan R, Murayama Y, Gobin YP, et al. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery 2001;48:984–97 [DOI] [PubMed] [Google Scholar]
- 21.van Rooij WJ, Sluzewski M, Beute GN. Brain AVM embolization with Onyx. AJNR Am J Neuroradiol 2007;28:172–77, discussion 178 [PMC free article] [PubMed] [Google Scholar]
- 22.Weber W, Kis B, Siekmann R, et al. Endovascular treatment of intracranial arteriovenous malformations with Onyx: technical aspects. AJNR Am J Neuroradiol 2007;28:371–77 [PMC free article] [PubMed] [Google Scholar]
- 23.Song DL, Leng B, Xu B, et al. Clinical experience of 70 cases of cerebral arteriovenous malformations embolization with Onyx, a novel liquid embolic agent [in Chinese]. Zhonghua Wai Ke Za Zhi 2007;45:223–25 [PubMed] [Google Scholar]
- 24.Mounayer C, Hammami N, Piotin M, et al. Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. AJNR Am J Neuroradiol 2007;28:518–23 [PMC free article] [PubMed] [Google Scholar]
- 25.Wikholm G, Lundqvist C, Svendsen P. Embolization of cerebral arteriovenous malformations. Part I Technique, morphology, and complications. Neurosurgery 1996;39:448–59 [DOI] [PubMed] [Google Scholar]
- 26.Valavanis A, Christoforidis G. Endovascular management of cerebral arteriovenous malformations. Neurointerventionist 1999;1:34–40 [Google Scholar]
- 27.Fournier D, Terbrugge K, Rodesch G, et al. Revascularization of brain arteriovenous malformations after embolization with bucrylate. Neuroradiology 1990;32:497–501 [DOI] [PubMed] [Google Scholar]
- 28.Cognard C, Spelle L, Pierot L. Pial arteriovenous malformations. In Forsting M, ed. Intracranial Vascular Malformations and Aneurysms. 2nd ed. Berlin, Germany: Springer-Verlag;2006. :39–100
- 29.Zanneti P, Sherman F. Experimental evaluation of a tissue adhesive as an agent for the treatment of aneurysms and arteriovenous anomalies. J Neurosurg 1972;36:72–76 [DOI] [PubMed] [Google Scholar]
- 30.Vinuela F, Fox AJ, Pelz D, et al. Angiographic follow-up of large cerebral AVMs incompletely embolized with isobutyl 2-cyanoacrylate. AJNR Am J Neuroradiol 1986;7:919–25 [PMC free article] [PubMed] [Google Scholar]
- 31.Murayama Y, Viñuela F, Ulhoa A, et al. Nonadhesive liquid embolic agent for cerebral arteriovenous malformations: preliminary histopathological studies in swine rete mirabile. Neurosurgery 1998;43:1164–75 [DOI] [PubMed] [Google Scholar]
- 32.Frizzel RT, Fisher WS 3rd. Cure, morbidity, and mortality associated with embolization of brain arteriovenous malformations: a review of 1246 patients in 32 series over a 35-year period. Neurosurgery 1995;37:1031–40 [DOI] [PubMed] [Google Scholar]
- 33.Hartmann A, Pile-Spellman J, Stapf C, et al. Risk of endovascular treatment of brain arteriovenous malformations. Stroke 2002;33:1816–20 [DOI] [PubMed] [Google Scholar]
- 34.Taylor CL, Dutton K, Rappard G, et al. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg 2004;100:810–12 [DOI] [PubMed] [Google Scholar]
- 35.Viñuela F, Dion JE, Duckwiler G, et al. Combined endovascular embolization and surgery in the management of cerebral arteriovenous malformations: experience with 101 cases. J Neurosurg 1991;75:856–64 [DOI] [PubMed] [Google Scholar]
- 36.Kim LJ, Albuquerque FC, Spetzler RF, et al. Postembolization neurological deficits in cerebral arteriovenous malformations: stratification by arteriovenous malformation grade. Neurosurgery 2006;59:53–59 [DOI] [PubMed] [Google Scholar]
- 37.Haw CS, terBrugge K, Willinsky R, et al. Complications of embolization of arteriovenous malformations of the brain. J Neurosurg 2006;104:226–32 [DOI] [PubMed] [Google Scholar]
- 38.Ledezma CJ, Hoh BL, Carter BS, et al. Complications of cerebral arteriovenous malformation embolization: multivariate analysis of predictive factors. Neurosurgery 2006;58:602–11 [DOI] [PubMed] [Google Scholar]
- 39.Jayaraman MV, Marcellus ML, Hamilton S, et al. Neurologic complications of arteriovenous malformation embolization using liquid embolic agents. AJNR Am J Neuroradiol 2008;29:242–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber W, Kis B, Siekmann R, et al. Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery 2007;61:244–54 [DOI] [PubMed] [Google Scholar]
- 41.Picard L, Da Costa E, Anxionnat R, et al. Acute spontaneous hemorrhage after embolization of brain arteriovenous malformation with N-butyl cyanoacrylate. J Neuroradiol 2001;28:147–65 [PubMed] [Google Scholar]
- 42.Heidenreich JO, Hartlieb S, Stendel R, et al. Bleeding complications after endovascular therapy of cerebral arteriovenous malformations. AJNR Am J Neuroradiol 2006;27:313–16 [PMC free article] [PubMed] [Google Scholar]
- 43.Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated arteriovenous malformations. Neurology 2006;66:1350–55 [DOI] [PubMed] [Google Scholar]