Abstract
BACKGROUND AND PURPOSE: A hyperintense putaminal rim, putaminal hypointensity, and putaminal atrophy on T2-weighted MR images are findings suggestive of parkinsonian-dominant multiple system atrophy (MSA-P). However, putaminal hyperintensity on T1-weighted images, which has not been discussed in previous reports, is also frequently observed in patients with MSA-P. Here, we investigated whether putaminal hyperintensity on T1-weighted images is helpful to diagnose MSA-P.
MATERIALS AND METHODS: Patients with MSA-P (n = 17), Parkinson disease (PD; n = 37) and progressive supranuclear palsy (PSP; n = 11), and healthy control subjects (n = 16) were enrolled in this study. Two examiners, who were blind to the diagnoses, independently rated the putaminal hyperintensity on T1-weighted images (T1H), hyperintense putaminal rim on T2-weighted images (T2H), putaminal hypointensity on T2-weighted images (T2 L), and putaminal atrophy by using a visual analog scale, and performed a receiver operating characteristic (ROC) analysis. The area under the curve (AUC; minimum, 0.5; maximum, 1.0) was automatically calculated as a positive parameter, indicating its usefulness to differentiate between diseases.
RESULTS: For differentiating patients with MSA-P from healthy control subjects, AUC values were 0.983 for T1H, 0.923 for T2H, 0.726 for T2 L, and 0.967 for putaminal atrophy. Between MSA-P and PD, the respective AUC values were 0.990, 0.921, 0.739, and 0.923; and between MSA-P and PSP, the respective AUC values were 0.984, 0.923, 0.727, and 0.967.
CONCLUSIONS: All putaminal findings except T2 L were useful for the diagnosis of MSA-P. T1H was superior to T2H to differentiate MSA-P from other diseases.
Multiple system atrophy (MSA) is a sporadic, progressive, adult-onset disorder associated with various degrees of dysfunction of the extrapyramidal, cerebellar, and autonomic systems. In neuropathologic examinations, the Parkinson variant of MSA (MSA-P) is characterized by selective neuronal loss and gliosis predominantly affecting the basal ganglia, substantia nigra, olivopontocerebellar pathways, and intermediolateral cell columns of the spinal cord.1 The clinical differentiation of MSA-P from Parkinson disease (PD) and other atypical parkinsonian disorders, such as progressive supranuclear palsy (PSP), is challenging, especially during the early stages of the disease.2–7 Early differentiation of these diseases is important, however, to establish differences in their natural history and treatment response.8,9 In clinical practice with patients diagnosed with MSA-P, clinicians should consider symptoms in addition to parkinsonism (eg, orthostatic hypotension, urinary retention, and respiratory disturbances). Proper diagnosis, thorough symptom review, and early intervention are critical because such symptoms can have grave consequences. For example, respiratory disturbances, including abductor paralysis of the vocal cord, often cause sudden death.
There is increasing evidence that MR imaging is useful to establish an accurate diagnosis. The abnormal putaminal findings that characteristically differentiate patients with MSA from those with other parkinsonian disorders and control subjects are well known: a hyperintense putaminal rim on T2-weighted images, putaminal hypointensity on T2-weighted images, and putaminal atrophy.7,10,11 In some patients, however, an unequivocal diagnosis cannot be made with MR imaging findings alone.7 In our daily practice, putaminal hyperintensity on T1-weighted images is frequently observed in patients with MSA-P, though this has not been discussed in previous reports.
In our study, we evaluated the diagnostic usefulness of high-intensity signals along the lateral putamen on T2-weighted images (T2H), low-intensity signals within the putamen on T2-weighted images (T2 L), high-intensity signals within the putamen on T1-weighted images (T1H), and putaminal atrophy in differentiating patients with MSA-P from those with PD, PSP, and healthy subjects by receiver operating characteristic (ROC) analysis.
Materials and Methods
Patients
The subjects were 17 patients with probable MSA-P, 11 patients with probable PSP, 37 patients with probable PD, and 16 control subjects. Informed consent was obtained from all subjects. This study was retrospective and included consecutive patients who were first referred to our hospital in the early stages of their disease. A clinical diagnosis of MSA-P was made according to the consensus statement by Gilman et al,12 a diagnosis of PSP was made according to the National Institute of Neurological Disorders and Stroke—Society for Progressive Supranuclear Palsy clinical criteria,13 and a diagnosis of PD was made according to the UK Parkinson's Disease Society Brain Bank clinical diagnostic criteria.14 The control subjects were consecutive patients who were referred to our hospital for complaints of headache or dizziness and who had no neurologic abnormalities. The demographic data of the subjects are listed in Table 1.
Table 1:
Patient characteristics
| MSA-P (n = 17) | PD (n = 37) | PSP (n = 11) | HC (n = 16) | |
|---|---|---|---|---|
| Age (y) | 62 ± 08 | 66 ± 7 | 71 ± 4 | 57 ± 12 |
| Sex (M/F) | 4/5 | 23/15 | 7/3 | 6/9 |
| Duration of disease (months) | 2 ± 1 | 4 ± 5 | 2 ± 1 |
Note:—MSA-P indicates the Parkinson variant of multiple system atrophy; PD, Parkinson disease; PSP, progressive supranuclear palsy; HC, healthy control.
MR Imaging Acquisition and Analysis
Brain MR imaging was performed with a 1.5 T MR scanner (Signa Horizon; GE Healthcare, Milwaukee, Wis). We acquired axial T2- and T1-weighted images using the following parameters: for T2-weighted images, TR was 4000 ms and TE was 96 ms; for T1-weighted images, TR was 500 ms and TE was 9 ms. The number of averages was 2; section thickness/gap, 6.0/1.5 mm; FOV, 230 × 230 mm; and matrix, 256 × 256. The axial sections were angled to lie parallel to the anteroposterior commissure (ACPC) line.
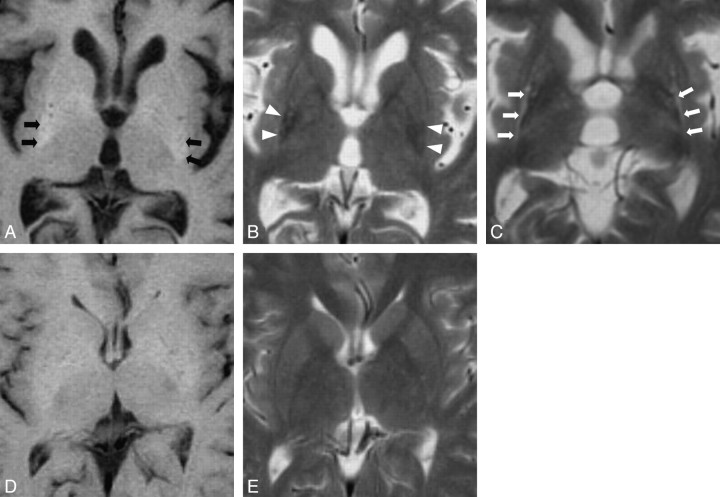
Axial sections of T2- and T1-weighted images in the largest area of the putamen were selected for evaluation. This section always contained the posterior limbs of the internal capsule and thalamic pulvinar and was usually rostral to the section showing the anterior commissure. On the selected section, 2 examiners (W.S. and S.I.), who were blind to the diagnosis and clinical data, evaluated T2H, T2 L, T1H, and putaminal atrophy (Fig 1). Because putaminal T2H, T2 L, and atrophy on the T2-weighted image could bias interpretation of T1H, T1-weighted and T2-weighted images were analyzed in a blinded, randomized fashion to control for this bias. T2H and T1H were defined as higher intensity than the adjacent insular cortex and white matter, respectively. T2 L was defined as lower intensity than adjacent white matter. Putaminal atrophy was assessed by visual inspection including evaluation of posterolateral linearization of the putaminal margin.15
Fig 1.
A T1-weighted axial image of a patient with MSA-P shows high-intensity signals within the putamen (A, black arrows). A T2-weighted axial image of the same patient also shows putaminal atrophy and low-intensity signals within the putamen (B, white arrows). Putaminal atrophy is assessed by linearization of posterolateral margin of the putamen and decreased width of the putamen. A T2-weighted axial image of a patient with MSA-P shows high-intensity signals along the lateral margin of the putamen (C, white arrows). T1-weighted and T2-weighted axial images of a healthy control subject show no abnormalities within and around the putamen (D and E).
We rated each finding on a 50-mm horizontal visual analog scale (left [0 mm], absolutely normal; middle [25 mm], equivocal; right [50 mm], absolutely abnormal). We specified the level of confidence to a statement by indicating a position along a continuous line between 2 end points. The length between the checked point and the left margin of the scale bar was measured as a marker of abnormality and was used for subsequent statistical analysis.16
Statistical Analysis
We evaluated the interrater agreement of each value by calculating Cronbach alpha as a common form of the intraclass correlation coefficient. Intraclass correlation coefficient of 0.60 to 0.80 was considered substantial to perform subsequent analysis.17 Analysis of variance (ANOVA) tests were performed to compare the differences for each putaminal finding (T2H, T2 L, T1H, and putaminal atrophy) among patients with MSA-P, PSP, PD, and healthy subjects, and then post hoc testing with the Scheffé F-test was performed. A Pearson correlation coefficient test was performed to analyze the correlation between ages, disease duration, and each putaminal finding.
Furthermore, to assess the diagnostic usefulness of each putaminal finding, we performed ROC analysis.18 We generated ROC curves by calculating the sensitivity and specificity of T2H, T2 L, T1H, and putaminal atrophy and plotting specificity against sensitivity, namely by varying the threshold value for the output units of the examiners. We automatically performed the ROC analysis on a PC with the Windows XP operating system by using ROCKIT version 0.9.1 beta and PLOTROC version 1.0.0, which were developed at the Department of Radiology at the University of Chicago (http://www-radiology.uchicago.edu/krl/index.htm). An ROC curve located in the upper left corner of the graph corresponded to both higher sensitivity and higher specificity. For differentiation of MSA-P from PSP, PD, and healthy control subjects, an area under the ROC curve (AUC) of more than 0.8 is considered good; an AUC of more than 0.9 is considered excellent.
Results
The evaluation of each abnormality revealed that the intraclass correlation coefficient was 0.739 in T1H, 0.692 in T2H, 0.715 in T2 L, and 0.669 in putaminal atrophy. The intraclass correlation coefficient was evaluated as acceptable. In the results of the ANOVA analysis (Table 2), the mean T1H on a visual analog scale (maximum, 50 mm) was significantly greater in patients with MSA-P (mean ± SD, 44.4 ± 6.1 mm) than in patients with PSP (21.8 ± 10.1 mm), patients with PD (21.6 ± 9.5 mm), and healthy control subjects (26.4 ± 5.3 mm) (P < .001, on-line Fig 1A). The mean T2H in patients with MSA-P (35.8 ± 8.6 mm) was significantly greater than that in patients with PSP (18.7 ± 7.7 mm), patients with PD (21.0 ± 8.4 mm), and healthy control subjects (19.3 ± 7.5 mm) (P < .001, on-line Fig 1B). The mean T2 L in patients with MSA-P (32.7 ± 11.8 mm) was significantly greater than that in patients with PD (26.1 ± 6.8 mm) and healthy control subjects (24.9 ± 6.0 mm) (P < .05, on-line Fig 1C), but it did not significantly differ from than in patients with PSP (29.2 ± 7.7 mm). The mean putaminal atrophy was significantly greater in the patients with MSA-P (38.4 ± 7.7 mm) than in patients with PSP (27.5 ± 5.1 mm), patients with PD (21.4 ± 8.3 mm), and healthy control subjects (16.6 ± 7.7 mm) (P < .001, on-line Fig 1D). It was also greater in patients with PSP and patients with PD than in healthy control subjects (P = .09 and P = .04, respectively).
Table 2:
Putaminal abnormalities on visual analog scale (maximum, 50 mm) in patients with MSA-P, PD, PSP, and healthy controls
| MSA-P (n = 17) | PD (n = 37) | PSP (n = 11) | HC (n = 16) | |
|---|---|---|---|---|
| T1H (mm) | 44.4 ± 6.1* | 21.6 ± 9.5 | 21.8 ± 10.1 | 26.4 ± 5.3 |
| T2H (mm) | 35.8 ± 8.6* | 21.0 ± 8.4 | 18.7 ± 7.7 | 19.3 ± 7.5 |
| T2 L (mm) | 32.7 ± 11.8† | 26.1 ± 6.8 | 29.2 ± 7.7 | 24.9 ± 6.0 |
| Atrophy (mm) | 38.4 ± 7.7* | 21.4 ± 8.3‡ | 27.5 ± 5.1 | 16.6 ± 7.7 |
Note:—T1H indicates T1 hyperintensity; T2H, T2 hyperintensity; T2 L, T2 hypointensity.
Significantly different from PD, PSP, and HC (P < .001).
Significantly different from PD and HC (P < .05).
Significantly different from HC (P < .05).
The ROC analysis revealed that in differentiation between the AUC in patients with MSA-P and healthy control subjects was 0.983 in T1H, 0.923 in T2H, 0.726 in T2 L, and 0.967 in atrophy (Table 3, on-line Fig 2A). In differentiation between MSA-P and PD, the AUC was 0.990 in T1H, 0.921 in T2H, 0.739 in T2 L, and 0.923 in atrophy (Table 3, on-line Fig 2B). In differentiation between MSA-P and PSP, the AUC was 0.984 in T1H, 0.923 in T2H, 0.727 in T2 L, and 0.967 in atrophy (Table 3, on-line Fig 2C). Sensitivity and specificity in the differentiation of patients with MSA-P from healthy control subjects are listed in Table 3.
Table 3:
Putaminal abnormalities in the differentiation of patients with MSA-P from healthy controls
| T1H | T2H | T2 L | Atrophy | |
|---|---|---|---|---|
| MSA-P from HC | ||||
| AUC | 0.983 | 0.923 | 0.726 | 0.967 |
| Sensitivity | 0.93 | 0.81 | 0.61 | 0.88 |
| Specificity | 0.94 | 0.93 | 0.98 | 0.93 |
| MSA-P from PD | ||||
| AUC | 0.990 | 0.921 | 0.739 | 0.923 |
| Sensitivity | 0.95 | 0.80 | 0.59 | 0.80 |
| Specificity | 0.98 | 0.93 | 0.96 | 0.95 |
| MSA-P from PSP | ||||
| AUC | 0.984 | 0.923 | 0.727 | 0.967 |
| Sensitivity | 0.94 | 0.81 | 0.61 | 0.89 |
| Specificity | 0.99 | 0.93 | 0.98 | 0.93 |
Note:—AUC indicates area under the receiver operating characteristic curve.
In patients with MSA-P, no parameter of putaminal MR imaging findings correlated with age or disease duration. There was a trend in the correlation between T2H and T1H (correlation coefficient (r) = 0.435 and P = .081), and between T2H and atrophy (r = 0.568 and P = .017). In healthy control subjects, age correlated positively with T2H (r = 0.507 and P = .015) and putaminal atrophy (r = 0.548 and P = .009). There was a trend in the correlation between T2 L and age, but this trend was not statistically significant (r = 0.318 and P = .099). T1H did not correlate with age.
Discussion
The aim of our study was to determine the usefulness of T1H in MR imaging for the diagnosis of MSA-P. In addition, we compared the usefulness of well-known abnormal putaminal findings such as T2H, T2 L, and putaminal atrophy with T1H by using ROC analysis. Our results show that T1H, T2H, and putaminal atrophy are excellent findings to discriminate patients with MSA-P from patients with PD, patients with PSP, and healthy control subjects in the early stages of disease, though our results failed to show the usefulness of T2 L, in contrast to previous studies.11 The usefulness of T2H and putaminal atrophy has been reported,7,10 but T1H has not been described for the diagnosis of MSA-P. In our results, T1H was superior to T2H to differentiate MSA-P, and correlation analysis showed that T1H was not influenced by age in patients or in healthy control subjects, whereas T2H and atrophy correlated with age in healthy subjects. In diseases mainly featuring parkinsonism, we suggest that T1H is one of the useful findings in the diagnosis of MSA-P.
With regard to T1H in patients with MSA-P, however, the corresponding histopathologic changes were not investigated. In general, hyperintensities on T1-weighted images can be caused by various tissue changes, such as hemorrhages, protein-rich lesions, fatty lesions, calcification, mineral accumulation, melanin-containing lesions, and other factors.19 It is assumed that mineral accumulation is one of the causes of T1H in MSA-P, but additional histopathologic studies are necessary to clarify those causes.
T2H was an excellent indicator of MSA-P, but it was observed also in patients with PSP and PD, especially in older subjects.20–22 T2H is thought to reflect putaminal degeneration, gliosis, and enlargement of the space between the putamen and the external capsule in patients with MSA-P,23 but it can be influenced by age and brain ischemia. In our results, T2 L correlated positively with age in healthy subjects, and it was not useful to discriminate MSA-P from other diseases. In a previous report, putaminal T2 L on T2- and T2*-weighted MR imaging have been reported as useful sequences to differentiate MSA from PD,6 but T2 L represents a susceptibility effect caused by iron deposition.24 It is thought that T2 L within the putamen is not a reliable finding in older subjects because iron deposition in the basal ganglia is common in elderly patients. In fact, a previous report indicated that T2 L alone was a nonspecific MR imaging sign of MSA-P, whereas T2 L combined with T2H was a highly specific sign.11
Although conventional MR imaging is inferior to volumetric, spectroscopic, or diffusion MR imaging in the detection of subtle tissue degeneration,4,5,25,26 it is important in clinical practice to know which findings on conventional MR imaging have the best diagnostic accuracy. It is certain that the visual analog scale used in our study is a subjective method and measurement of signal intensity is more objective and quantifiable than visual inspection, but in daily clinical practice, we usually perform visual inspection for radiologic diagnosis. We pursued the practical MR imaging findings available for the diagnosis of MSA-P in our study.
We performed our study with a 1.5T MR scanner and acquired T1-weighted images by using the spin-echo technique. Recently, 3T MR scanners have become available in several facilities, and our knowledge about image characteristics in a high magnetic field improved. However, few research studies have focused on T1-weighted images with the spin-echo technique in a high magnetic field. T1H in patients with MSA-P is possibly obscured on 3T T1-weighted images. Additional studies are necessary to confirm the feasibility of T1H in patients with MSA-P in a high magnetic field.
Conclusions
All putaminal findings except T2 L were useful for the diagnosis of MSA-P, and T1H was superior to T2H to differentiate MSA-P from other diseases. We conclude that T1H, in addition to T2H and putaminal atrophy, is a useful finding for the diagnosis of MSA-P.
Footnotes
indicates article with supplemental on-line figures.
References
- 1.Poewe W. Clinical features, diagnosis, and imaging of parkinsonian syndromes. Curr Opin Neurol Neurosurg 1993;6:333–38 [PubMed] [Google Scholar]
- 2.Litvan I, Agid Y, Jankovic J, et al. Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy. Neurology 1996;46:922–30 [DOI] [PubMed] [Google Scholar]
- 3.Hughes AJ, Daniel SE, Ben-Shlomo Y, et al. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 2002;125:861–70 [DOI] [PubMed] [Google Scholar]
- 4.Seppi K, Schocke MF, Esterhammer R, et al. Diffusion-weighted imaging discriminates progressive supranuclear palsy from PD, but not from the parkinson variant of multiple system atrophy. Neurology 2003;60:922–27 [DOI] [PubMed] [Google Scholar]
- 5.Schocke MF, Seppi K, Esterhammer R, et al. Diffusion-weighted MRI differentiates the Parkinson variant of multiple system atrophy from PD. Neurology 2002;58:575–80 [DOI] [PubMed] [Google Scholar]
- 6.Kraft E, Trenkwalder C, Dorothee PA. T2*-weighted MRI differentiates multiple system atrophy from Parkinson's disease. Neurology 2002;59:1265–67 [DOI] [PubMed] [Google Scholar]
- 7.Schrag A, Good CD, Miszkiel K, et al. Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology 2000;54:697–702 [DOI] [PubMed] [Google Scholar]
- 8.Watanabe H, Saito Y, Terao S, et al. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain 2002;125:1070–83 [DOI] [PubMed] [Google Scholar]
- 9.Wenning GK, Ben-Shlomo Y, Magalhaes M, et al. Clinical features and natural history of multiple system atrophy: an analysis of 100 cases. Brain 1994;117:835–45 [DOI] [PubMed] [Google Scholar]
- 10.Schrag A, Kingsley D, Phatouros C, et al. Clinical usefulness of magnetic resonance imaging in multiple system atrophy. J Neurol Neurosurg Psychiatry 1998;65:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraft E, Schwarz J, Trenkwalder C, et al. The combination of hypointense and hyperintense signal changes on T2-weighted magnetic resonance imaging sequences: a specific marker of multiple system atrophy? Arch Neurol 1999;56:225–28 [DOI] [PubMed] [Google Scholar]
- 12.Gilman S, Low PA, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci 1999;163:94–98 [DOI] [PubMed] [Google Scholar]
- 13.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 1997;48:119–25 [DOI] [PubMed] [Google Scholar]
- 14.Litvan I, Bhatia KP, Burn DJ, et al. Movement Disorders Society Scientific Issues Committee. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord 2003;18:467–86 [DOI] [PubMed] [Google Scholar]
- 15.Ito S, Shirai W, Hattori T. Evaluating posterolateral linearization of the putaminal margin with magnetic resonance imaging to diagnose the Parkinson variant of multiple systems atrophy. Mov Disord 2007;22:578–81 [DOI] [PubMed] [Google Scholar]
- 16.Rockette HE, Gur D, Metz CE. The use of continuous and discrete confidence judgments in receiver operating characteristic studies of diagnostic imaging techniques. Invest Radiol 1992;27:169–72 [DOI] [PubMed] [Google Scholar]
- 17.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74 [PubMed] [Google Scholar]
- 18.Metz CE. Basic principles of ROC analysis. Semin Nucl Med 1978;8:283–98 [DOI] [PubMed] [Google Scholar]
- 19.Cakirer S, Karaarslan E, Arslan A. Spontaneously T1-hyperintense lesions of the brain on MRI: a pictorial review. Curr Probl Diagn Radiol 2003;32:194–217 [DOI] [PubMed] [Google Scholar]
- 20.Macia F, Yekhlef F, Ballan G, et al. T2-hyperintense lateral rim and hypointense putamen are typical but not exclusive of multiple system atrophy. Arch Neurol 2001;58:1024–26 [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharya K, Saadia D, Eisenkraft B, et al. Brain magnetic resonance imaging in multiple-system atrophy and Parkinson disease: a diagnostic algorithm. Arch Neurol 2002;59:835–42 [DOI] [PubMed] [Google Scholar]
- 22.Watanabe H, Fukatsu H, Hishikawa N, et al. Field strengths and sequences influence putaminal MRI findings in multiple system atrophy. Neurology 2004;62:671. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz J, Weis S, Kraft E, et al. Signal changes on MRI and increases in reactive microgliosis, astrogliosis, and iron in the putamen of two patients with multiple system atrophy. J Neurol Neurosurg Psychiatry 1996;60:98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olanow CW. Magnetic resonance imaging in parkinsonism. Neurol Clin 1992;2:405–20 [PubMed] [Google Scholar]
- 25.Schulz JB, Skalej M, Wedekind D, et al. Magnetic resonance imaging-based volumetry differentiates idiopathic Parkinson's syndrome from multiple system atrophy and progressive supranuclear palsy. Ann Neurol 1999;45:65–74 [PubMed] [Google Scholar]
- 26.Federico F, Simone IL, Lucivero V, et al. Proton magnetic resonance spectroscopy in Parkinson's disease and atypical parkinsonian disorders. Mov Disord 1997;12:903–09 [DOI] [PubMed] [Google Scholar]