Abstract
BACKGROUND AND PURPOSE: The precise clinical characteristics of acute encephalopathy with bilateral reduced diffusion are not fully understood. We compared clinical, laboratory, and neuroimaging findings according to the patterns of brain lesions among children with reduced diffusion in the bilateral hemispheres.
MATERIALS AND METHODS: Nine patients were analyzed. The patterns of brain lesions were divided into diffuse lesions and central-sparing lesions. Diffuse lesions were defined as reduced diffusion in the whole cortex and/or subcortical white matter. Central-sparing lesions were defined as the lack of reduced diffusion in the areas around the bilateral Sylvian fissures. Clinical, laboratory, and neuroimaging findings were compared between groups.
RESULTS: Five patients showed diffuse lesions and 4 showed central-sparing lesions. Coma was significantly more common in patients with diffuse lesions, whereas a biphasic clinical course was more common in those with central-sparing lesions. Outcome was worse in patients with diffuse lesions. Maximal aspartate aminotransferase, alanine aminotransferase, and kinase levels were also significantly higher in patients with diffuse lesions. In 2 patients with diffuse lesions, diffusion-weighted images during the acute phase revealed reduced diffusion in the bilateral frontal and occipital areas, followed by diffuse lesions. No patient with central-sparing lesions showed MR imaging abnormalities during the acute phase.
CONCLUSIONS: Clinical manifestations in patients with diffuse lesions were severe, whereas those in patients with central-sparing lesions were relatively mild.
Acute encephalopathy in association with infectious disease has attracted the attention of pediatricians and pediatric neurologists in Japan since the outbreak of influenza-associated encephalopathy during the 1997/1998 winter season. Every year, it is estimated that hundreds of Japanese children die or experience neurologic sequelae due to acute encephalopathy of infectious causes, which has prompted studies on acute encephalopathy in Japan. Recent studies have revealed several patterns of neuroimaging abnormalities in children with acute encephalopathy. Acute necrotizing encephalopathy is characterized by the presence of multiple symmetric brain lesions in the bilateral thalami and other specific brain regions, such as the periventricular white matter and internal capsule.1 Clinically mild encephalitis/encephalopathy with a reversible splenial lesion is characterized by transient reduced diffusion in the splenium of the corpus callosum, with complete recovery.2
Recently, Takanashi et al3 described a form of acute encephalopathy characterized by biphasic seizures and late reduced diffusion (AESD). In patients with AESD, a seizure, especially a prolonged one, is commonly observed at onset. The following day, patients appear relatively well and seem to have recovered consciousness almost fully, though slightly reduced responsiveness, an absent-minded appearance, or subtle disorientation may be apparent. Deterioration of consciousness, clustered seizures, and involuntary movements appear 3–7 days after the first seizure. Furthermore, this subtype of acute encephalopathy is characterized by widespread reduced diffusion on MR imaging from 3 to 9 days after onset. Although the outcome of patients with AESD is reportedly very poor,3 a milder form of AESD without neurologic sequelae has also been described.4 It is interesting that all reported cases of children with AESD have been of in those of East Asian descent.5
At present, the precise clinical characteristics of AESD are not fully understood. For example, it is uncertain whether a biphasic clinical course is always observed in children with AESD. It is also unclear whether reduced diffusion is always absent on MR imaging within 2–3 days after onset. We compared the clinical manifestations, laboratory data, and MR imaging features of children with reduced diffusion in the bilateral hemispheres, according to the patterns of brain lesions, to better understand the spectrum of this subtype of acute encephalopathy associated with infection.
Materials and Methods
We identified 79 children (age, ≤15 years) with acute encephalopathy who were admitted to the Department of Pediatrics at Nagoya University Hospital, Juntendo University Hospital, and 13 affiliated hospitals between January 2000 and August 2007. Acute encephalopathy was defined as a condition characterized by decreased consciousness with or without other neurologic symptoms, lasting for >24 hours in children with infectious symptoms, such as fever, cough, and diarrhea. We carefully excluded patients with sustained decreased consciousness after a febrile seizure, and those with delirious behavior without obviously reduced consciousness. We also excluded patients who were clinically diagnosed with status epilepticus by the attending physician. In this study, a prolonged seizure was defined as one lasting for >20 minutes. Coma was defined as a condition in which the patient was not arousable with maximal painful stimulation, which is consistent with a score of 3–5 on the Glasgow Coma Scale, modified for children, or a score of 100–300 on the Japan Coma Scale.
MR imaging was performed in 65 of 79 patients, and diffusion-weighted images (DWIs) were obtained in 37 patients. Among these, 9 patients showed widespread reduced diffusion in the cortex and/or subcortical white matter of the bilateral hemispheres. These 9 patients became the subjects of this study. In these patients, DWIs were generated by using a 1.5T unit, with a spin-echo echo-planar imaging sequence with variable settings (TE, 86–109 ms; TR, 3066–4100 ms; 952- to 1445-Hz/pixel bandwidth; echo-planar factor, 53–128; section thickness, 5.0–6.0 mm). MR spectroscopy was not performed in any patient.
Two distinct patterns of brain lesions were recognized in DWI: diffuse lesions and central-sparing lesions (Figs 1 and 2). Diffuse lesions were defined as reduced diffusion in the whole cortex and/or subcortical white matter in the bilateral hemisphere during the clinical course. In some patients, reduced diffusion in the frontal and occipital areas preceded diffuse lesions. Central-sparing lesions were defined as the lack of reduced diffusion in the areas around the bilateral Sylvian fissures, though diffuse abnormalities were typically present in other areas on MR imaging. All MR imaging data were reviewed by the chief author (A.O.), with the attending physicians.
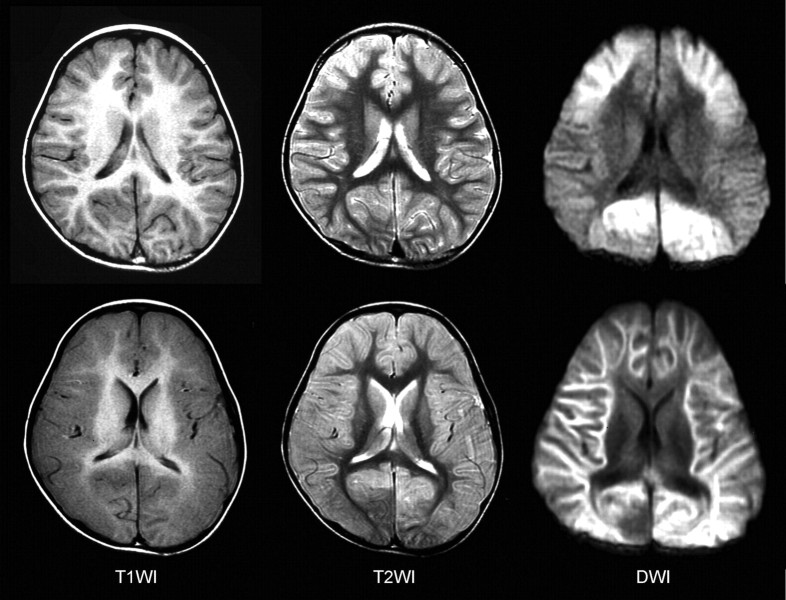
Fig 1.
MR imaging findings of a patient with diffuse lesions. Top: The day after the onset, T1-weighted (T1WI) images show mild thickening of the cortex and T2-weighted (T2WI) images reveal mildly increased intensities in the cortex of the bilateral frontal lobes. Reduced diffusivity is observed in the bilateral frontal and occipital regions on DWIs (frontal occipital lesions). Bottom: Five days after the onset, T1WI and T2WI images demonstrate marked edematous changes in the entire cortex. Reduced diffusivity is observed in the entire subcortical white matter on DWIs.
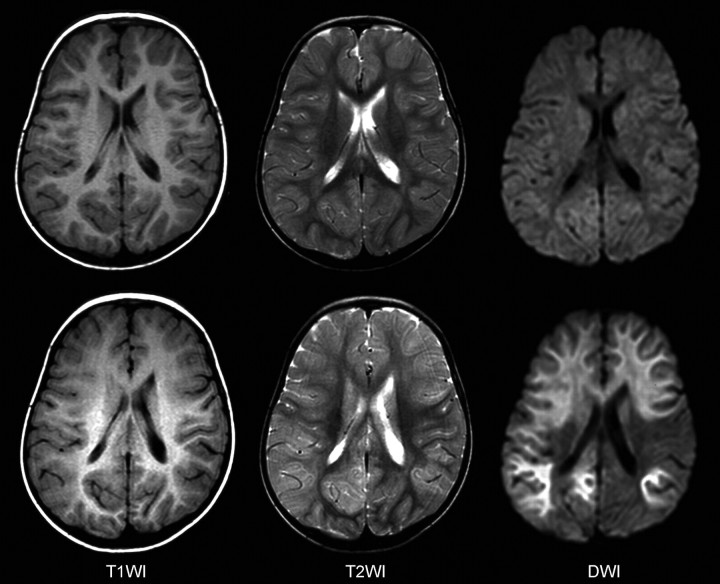
Fig 2.
MR imaging findings of a patient with central sparing lesions. Top: Two days after the onset, no abnormalities are observed. Bottom: Four days after the onset, T1-weighted (T1WI) images show unclear gray-white matter differentiation in the bilateral frontal regions. T2-weighted (T2WI) images reveal diffuse thickening of the cortex and increased signal intensities in the bilateral caudate nuclei. Reduced diffusivity is observed in the bilateral frontal and parietooccipital regions on DWIs.
Laboratory data were also assessed from medical records. We investigated the following values: platelet counts; aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase, creatinine kinase, blood urea nitrogen, creatinine, glucose, and ammonia levels; and cell counts and protein in the CSF. Some of these values were evaluated in previous studies as prognostic factors of acute encephalopathy.6,7 Nagao et al6 revealed that elevated AST levels, hyperglycemia, the presence of hematuria or proteinuria, and the use of diclofenac sodium were associated with poor outcomes in children with influenza-associated encephalopathy.
Cognitive impairment was assessed in all surviving patients 1–1.5 years after discharge. Cognitive impairment was usually measured by using the Kaufman Assessment Battery for Children, though it could not be applied in some patients with severe cognitive impairment. The severity of cognitive impairment was defined as mild when the patient's intelligence quotient (IQ) or development quotient (DQ) was between 50 and 70, moderate when IQ/DQ was between 30 and 50, and severe when IQ/DQ was below 30.
Statistical analyses between the 2 groups were performed by using the Mann-Whitney U test for numeric variables and the Fisher exact probability test for categoric variables. The outcome of patients was also analyzed by using the Mann-Whitney U test. P values < .05 were statistically significant.
Results
Patient Characteristics
Five boys and 4 girls were analyzed. The median age was 17 months (range, 3–66 months), and 6 of the children were 18 months of age or younger. One patient showed multiple congenital anomalies and developmental delay of unknown cause. Another had a history of febrile seizures. Five patients showed diffuse lesions, and 4 showed central-sparing lesions.
Patient characteristics are summarized in Table 1. Age and sex did not differ between groups. Prodromal illnesses were variable: Virologically proved influenza was observed in 3 patients; exanthem subitum, in 1; gastroenteritis, in 2; and nonspecific febrile illness, in 3.
Table 1:
Patients' characteristics, neurologic symptoms, and outcome
| Diffuse Lesions (n = 5) | Central-Sparing Lesions (n = 4) | P Value | |
|---|---|---|---|
| Age (months)* | 18 (3–52) | 15 (10–66) | NS |
| Sex (M-F) | 3:2 | 2:2 | NS |
| Prodromal illness | Not done | ||
| Influenza | 2 | 1 | |
| Subitum | 0 | 1 | |
| Gastroenteritis | 2 | 0 | |
| NSFI | 1 | 2 | |
| Coma | 5 | 1 | .048 |
| Biphasic clinical course | 1 | 4 | .048 |
| Seizure at onset | 3 | 4 | NS |
| Prolonged seizure at onset | 1 | 1 | NS |
| Seizure after the first 24 hours | 2 | 4 | NS |
| Outcome | .056 | ||
| Death | 3 | 0 | |
| Severe cognitive impairment | 1 | 1 | |
| Mild cognitive impairment | 1 | 2 | |
| Healthy | 0 | 1 |
Note:—NSFI indicates nonspecific febrile illness; NS, not significant.
Data are shown as median (range).
Neurologic symptoms are also shown in Table 1. Coma was observed within 24 hours after onset in all patients with diffuse lesions. One patient with central-sparing lesions became comatose on the fourth day of illness. Coma was significantly more common in patients with diffuse lesions than in those with central-sparing lesions (P = .048). In contrast, a biphasic clinical course was more common in patients with central-sparing lesions than in those with diffuse lesions (P = .048). All patients with central-sparing lesions showed a biphasic clinical course. The reduction of consciousness was mild within the first few days after onset, followed by seizures appearing 3–6 days after onset in association with deteriorated consciousness. One patient with diffuse lesions also showed a biphasic clinical course. In this patient, coma without seizures was the initial presentation, but the reduction of consciousness became milder thereafter. Clustered seizures and worsening of consciousness were observed 2 days after onset. Seizures at onset or after the first 24 hours were observed in all patients with central-sparing lesions, whereas seizures were observed in 3 patients at onset and in 2 after the first 24 hours among those with diffuse lesions. A prolonged seizure was observed in 1 patient with diffuse lesions and in 1 with central-sparing lesions. The duration of seizures was 60 minutes in these patients.
Statistical analyses showed marginal differences in outcome between patients with diffuse-versus-central-sparing lesions (Table 1). All except 1 patient with diffuse lesions died or had severe cognitive impairment, whereas all patients with central-sparing lesions survived and only 1 showed severe cognitive impairment (P = .056). Postmortem examination was not performed in those who died.
Laboratory Data
Maximal AST, ALT, and creatinine kinase levels were significantly higher in patients with diffuse lesions than in those with central-sparing lesions (Table 2). Although abnormalities in platelet counts and lactate dehydrogenase, blood urea nitrogen, and creatinine levels tended to be more severe in patients with diffuse lesions, these differences were not statistically significant. Elevation in ammonia levels, if present, was mild. Hyperglycemia (serum glucose >200 mg/dL) tended to be more common in patients with diffuse lesions, though this difference did not reach statistical significance. CSF analyses did not reveal pleocytosis or increased protein levels in any patient. Disseminated intravascular coagulation was observed in only 1 patient with diffuse lesions. Metabolic acidosis was observed in all except 1 patient with diffuse lesions and in none of those with central-sparing lesions (P = .048).
Table 2:
Laboratory data
| Diffuse Lesions (n = 5) | Central Sparing Lesions (n = 4) | P Value | |
|---|---|---|---|
| Minimal Plt (×104/μL)* | 14.3 (6.2–36.6) | 17.8 (11.3–46.9) | NS |
| Maximal AST (IU/L)* | 917 (189–4407) | 107 (53–239) | .028 |
| Maximal ALT (IU/L)* | 403 (48–3200) | 33 (19–77) | .028 |
| Maximal LDH (IU/L)* | 1325 (681–7758) | 815 (351–1193) | NS |
| Maximal CK (IU/L)* | 6500 (2057–128472) | 320 (62–915) | .014 |
| Maximal BUN (mg/dL)* | 19 (7.0–79.1) | 12.5 (8.3–15.2) | NS |
| Maximal Cr (mg/dL)* | 0.59 (0.20–2.2) | 0.29 (0.22–0.40) | NS |
| Maximal ammonia (μg/dL)* | 147 (35–176) | 83 (55–111) | NS |
| Blood glucose >200 mg/dL | 4 | 1 | NS |
| CSF cell >10/μL | 0 | 0 | NS |
| CSF protein >40 mg/dL | 0 | 0 | NS |
| DIC | 1 | 0 | NS |
| Metabolic acidosis | 4 | 0 | .048 |
Note:—Plt indicates platelet counts; LDH, lactate dehydrogenase; CK, creatinine kinase; BUN, blood urea nitrogen; Cr, creatinine; DIC, disseminated intravascular coagulation; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Data are shown as median (range).
Neuroimaging Findings
MR imaging during the acute phase (within the first 72 hours after onset) was obtained in 2 patients with diffuse lesions and in 2 with central-sparing lesions. In 2 of 3 patients with diffuse lesions, reduced diffusion was observed in the cortical and subcortical areas in the bilateral frontal and occipital areas (Fig 1). In addition, T1-weighted images demonstrated mild thickening of the cerebral cortex in the corresponding areas, and T2-weighted images revealed mildly increased intensities in the same areas. However, no abnormalities were observed in the remaining 3 patients.
During the subacute phase (from the fourth to the 12th day of illness), MR imaging demonstrated abnormal findings in all patients. As used in the categorization of the patients, markedly reduced diffusion and edematous changes in the entire cortical and subcortical areas were observed in 5 patients (Fig 1). Thickening of the cortex and T1 and T2 prolongation in the subcortical white matter were more remarkable compared with these findings during the acute period in all patients. Blurring of the gray-white matter junction was also prominent. In this group of patients, MR imaging was performed on the fourth day of illness in 1 patient, on the sixth in 2, on the eighth in 1, and on the 12th in 1. In 4 patients with central-sparing lesions, pre- and postcentral areas were clearly spared (Fig 2). Thickening of the cortex and T1 and T2 prolongation in the subcortical white matter were relatively mild, and blurring of the gray-white matter junction was not observed. In this group of patients, MR imaging was performed on the fifth day of illness in 1 patient, on the sixth in 1, on the eighth in 1, and on the 12th in 1.
During the late phase (>2 weeks after onset), MR imaging was conducted in all 7 surviving patients. Three of 5 patients with diffuse lesions survived. Marked cerebral atrophy was observed in 2 patients, and mild cerebral atrophy, in 1 patient on MR imaging during the late phase. Laminar necrosis and increased signal intensities in the subcortical white matter on T2-weighted images were observed in all of these patients. All 4 patients with central-sparing lesions survived. Late MR imaging revealed mild cerebral atrophy in 3 patients and no abnormality in 1. Laminar necrosis was not observed in any patient with central-sparing lesions, whereas mildly increased signal intensities in the subcortical white matter on T2-weighted images were recognized in 3 patients.
No patient showed markedly reduced diffusion in the basal ganglia, thalami, or corpus callosum throughout the clinical course. However, T2-weighted images showed increased signal intensities in the bilateral caudate nuclei in 2 patients with central-sparing lesions during the subacute period.
Discussion
This study demonstrated that acute encephalopathy with reduced diffusion in the bilateral hemispheres can be divided into 2 distinct groups according to the distribution of brain lesions: diffuse and central-sparing lesions. Clinical manifestations, laboratory data, and outcomes were markedly different between patients with diffuse-versus-central-sparing lesions. These results indicate that these 2 groups should be distinguished, though they share common MR imaging abnormalities (ie, widespread reduced diffusion in the cortex and/or subcortical white matter of the bilateral hemispheres).
Patients with diffuse lesions appear to represent a severe phenotype of acute encephalopathy. Clinical symptoms were characterized by rapid and severe deterioration of consciousness, though seizures were not always observed. A biphasic clinical course was rare. Laboratory abnormalities were prominent, including elevated AST, ALT, and creatinine kinase levels; hyperglycemia; and metabolic acidosis. The outcome for patients with diffuse lesions was very poor. Death or severe neurologic sequelae were observed in 4 of 5 patients. These findings may be explained by a systemic inflammatory response, in which multiple organ failure, shock, and disseminated intravascular coagulation are often observed. During the acute stage of acute encephalopathy, the serum and CSF levels of inflammatory cytokines, such as interleukin-6 and tumor necrosis factor-α, were markedly elevated.7-9 Several pathologic studies have suggested that vascular injury as a result of endothelial damage by inflammatory cytokines is the pathologic substrate of severe types of acute encephalopathy, such as acute necrotizing encephalopathy.1,10 Although serum and CSF levels of inflammatory cytokines were not measured, it is possible that hypercytokinemia may contribute to the pathogenesis of diffuse lesions.
In contrast, patients with central-sparing lesions appear to represent a relatively mild phenotype of acute encephalopathy. Coma was uncommon and laboratory abnormalities were mild, if present. No patient died, though various degrees of cognitive impairment were observed as neurologic sequelae. A biphasic clinical course is characteristic of this group of patients, as described previously.3 Several studies on acute encephalopathy have also reported a biphasic clinical course.11-14 Onset is often marked by a prolonged seizure followed by improved consciousness. However, clustered seizures, signs of frontal lobe dysfunction, and worsening of consciousness become apparent at 3–4 days after onset. These features were observed in our patients with central-sparing lesions. The pathogenesis of acute encephalopathy with central-sparing lesions may be different from that of acute encephalopathy with diffuse lesions. Some authors have suggested that this subtype of acute encephalopathy is caused by excitotoxicity,3,15 because prolonged seizures are often observed at the onset of AESD. MR spectroscopy has shown increased glutamate concentrations and decreased N-acetylaspartate levels in patients with AESD.16 However, a prolonged seizure at onset was rare in our patients. Further studies are necessary to clarify the pathogenesis of acute encephalopathy with central-sparing lesions.
We consider it possible to distinguish central-sparing lesions from frontal occipital lesions, which may precede diffuse lesions. First, the appearance of reduced diffusion is earlier in frontal occipital lesions than in central-sparing lesions. In this study, central-sparing lesions were not observed in any patient within the first 3 days after onset, consistent with several previous reports.3,12-14,17 In contrast, frontal occipital lesions were recognized within the first 3 days after onset and were followed by diffuse lesions. Second, the distribution of brain lesions is different in the 2 situations. In patients with central-sparing lesions, the areas without reduced diffusion were strictly limited, around the Sylvian fissures. In the occipital lobes, lesions in the lateral areas were more prominent than those in the mesial areas. In contrast, lesions were located in the anterior half of the frontal lobes and mesial areas of the occipital lobes. Diffusion abnormalities were absent in the posterior half of the frontal lobes and the parietotemporal lobes and were less prominent in the lateral areas of the occipital lobes. However, these observations were based on a small number of patients.
The results of our study are not conclusive. Neuroimaging evaluations of many patients will be necessary to clarify differences between central-sparing lesions and frontal occipital lesions. The recognition of frontal-occipital lesions is useful in the early diagnosis of acute encephalopathy with diffuse lesions and may contribute to early intensive treatment. Our previous study indicated that early steroid use was related to better outcomes in children with acute necrotizing encephalopathy without brain stem lesions.18
The DWI patterns of our patients were characteristic, though reduced diffusion in the bilateral hemispheres may be observed with other causes of brain injury, such as hypoxic-ischemic encephalopathy and shaken infant syndrome.19,20 It is possible that encephalopathy due to substance abuse or intoxication may exhibit similar DWI abnormalities. Thus, the distinction between acute encephalopathy and brain injuries due to other causes may be problematic solely on the basis of imaging findings. For this reason, a diagnosis should be made only after considering clinical manifestations, physical and neurologic examinations, and laboratory data, in combination with MR imaging abnormalities. In our patients, there was no evidence of hypoxia-ischemia, nonaccidental head injury, or substance intoxication.
To our knowledge, the subtypes of acute encephalopathy have not been sufficiently established at present. The clinical presentation and imaging features of our patients overlap partly with other acute encephalopathy syndromes, including AESD,3 acute infantile encephalopathy predominantly affecting the frontal lobes,11 human herpes virus-6 encephalopathy with clusters of convulsions during the eruptive stage,12 and subacute encephalopathy.14 These acute encephalopathy syndromes likely represent a spectrum of disorders that share a common process in terms of brain injury. Multidisciplinary studies and further clinical experience are required to clarify the relationships between these syndromes.
In conclusion, acute encephalopathy with reduced diffusion in the bilateral hemispheres can be divided according to the pattern of brain lesions. Patients with diffuse lesions were characterized by coma, severe abnormalities in laboratory test results, and poor neurologic outcome, whereas those with central-sparing lesions were characterized by a biphasic clinical course, less severe abnormalities on laboratory test results, and relatively mild neurologic sequelae. Further neuroimaging studies with larger numbers of patients are necessary to establish the subtypes of MR imaging for acute encephalopathy with reduced diffusion in the bilateral hemispheres and will contribute to clarifying its pathogenesis and effective treatments.
Footnotes
The data in this manuscript were collected from many hospitals. The first 9 authors were attending pediatric neurologists and contributed to the collection of clinical data. Dr Toshiaki Shimizu helped to integrate the clinical data. Dr Tsuneo Morishima supervised this study. These 2 coauthors also contributed to the writing of this manuscript.
This work was supported by the grant from the Japanese Ministry of Education, Culture, Sports, Science and Technology (20249053).
References
- 1.Mizuguchi M, Abe J, Mikkaichi K, et al. Acute necrotising encephalopathy of childhood: a new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatry 1995;58:555–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tada H, Takanashi J, Barkovich AJ, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology 2004;63:1854–58 [DOI] [PubMed] [Google Scholar]
- 3.Takanashi J, Oba H, Barkovich AJ, et al. Diffusion MRI abnormalities after prolonged febrile seizures with encephalopathy. Neurology 2006;66:1304–09 [DOI] [PubMed] [Google Scholar]
- 4.Takanashi J, Tsuji M, Amemiya K, et al. Mild influenza encephalopathy with biphasic seizures and late reduced diffusion. J Neurol Sci 2007;256:86–89 [DOI] [PubMed] [Google Scholar]
- 5.Traul DE, Traul CS, Matsumoto J, et al. Acute encephalopathy with biphasic seizures and late restricted diffusion on MRI in a Japanese child living in the USA. Dev Med Child Neurol 2008;50:717–19 [DOI] [PubMed] [Google Scholar]
- 6.Nagao T, Morishima T, Kimura H, et al. Prognostic factors in influenza-associated encephalopathy. Pediatr Infect Dis J 2008;27:384–89 [DOI] [PubMed] [Google Scholar]
- 7.Ichiyama T, Endo S, Kaneko M, et al. Serum cytokine concentrations of influenza-associated acute necrotizing encephalopathy. Pediatr Int 2003;45:734–36 [DOI] [PubMed] [Google Scholar]
- 8.Ichiyama T, Isumi H, Ozawa H, et al. Cerebrospinal fluid and serum levels of cytokines and soluble tumor necrosis factor receptor in influenza virus-associated encephalopathy. Scand J Infect Dis 2003;35:59–61 [DOI] [PubMed] [Google Scholar]
- 9.Aiba H, Mochizuki M, Kimura M, et al. Predictive value of serum interleukin-6 level in influenza virus-associated encephalopathy. Neurology 2001;57:295–99 [DOI] [PubMed] [Google Scholar]
- 10.Mizuguchi M. Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev 1997;19:81–92 [DOI] [PubMed] [Google Scholar]
- 11.Yamanouchi H, Kawaguchi N, Mori M, et al. Acute infantile encephalopathy predominantly affecting the frontal lobes. Pediatr Neurol 2006;34:93–100 [DOI] [PubMed] [Google Scholar]
- 12.Nagasawa T, Kimura I, Abe Y, et al. HHV-6 encephalopathy with cluster of convulsions during eruptive stage. Pediatr Neurol 2007;36:61–63 [DOI] [PubMed] [Google Scholar]
- 13.Okamoto R, Fujii S, Inoue T, et al. Biphasic clinical course and early white matter abnormalities may be indicators of neurological sequelae after status epilepticus in children. Neuropediatrics 2006;37:32–41 [DOI] [PubMed] [Google Scholar]
- 14.Okumura A, Kidokoro H, Itomi K, et al. Subacute encephalopathy: clinical features, laboratory data, neuroimaging, and outcomes. Pediatr Neurol 2008;38:111–17 [DOI] [PubMed] [Google Scholar]
- 15.Mizuguchi M, Yamanouchi H, Ichiyama T, et al. Acute encephalopathy associated with influenza and other viral infections. Acta Neurol Scand 2007. (suppl);186:45–56 [PubMed] [Google Scholar]
- 16.Takanashi J, Tada H, Terada H, et al. Excitotoxicity in acute encephalopathy with biphasic seizures and late reduced diffusion AJNR Am J Neuroradiol 2009;30:132–35. Epub 2008 Aug 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tada H, Takanashi JI, Terada H, et al. Severe form of acute influenza encephalopathy with biphasic seizures and late reduced diffusion. Neuropediatrics 2008;39:134–36 [DOI] [PubMed] [Google Scholar]
- 18.Okumura A, Mizuguchi M, Kidokoro H, et al. Outcome of acute necrotizing encephalopathy in relation to treatment with corticosteroids and gammaglobulin Brain Dev 2009;31:221–27. Epub 2008 May 5 [DOI] [PubMed] [Google Scholar]
- 19.Biousse V, Suh DY, Newman NJ, et al. Diffusion-weighted magnetic resonance imaging in shaken baby syndrome. Am J Ophthalmol 2002;133:249–55 [DOI] [PubMed] [Google Scholar]
- 20.Barkovich AJ. Brain and spine injuries in infancy and childhood. In: Barkovich AJ, ed. Pediatric Neuroimaging. 4th ed. Philadelphia: Lippincott Williams & Wilkins;2005. :190–290